
As filed with the Securities and Exchange Commission on December 17, 2021
Registration Statement No. 333-[ ]
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
(Exact name of registrant as specified in its charter)
| 2842 | ||||
| (State
or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
QSAM Biosciences, Inc.
Tel:
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Douglas R. Baum
Chief Executive Officer
QSAM Biosciences, Inc.
9442 Capital of Texas Hwy N, Plaza 1, Suite 500
Austin, TX 78759
Tel: (512) 343-4558
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Joel D. Mayersohn, Esq. Rasika A. Kulkarni, Esq. Dickinson Wright PLLC 350 East Las Olas Blvd Suite 1750, Ft. Lauderdale FL 33301 Tel. (954) 991-5426 Fax: (844) 670-6009 |
Gregory Sichenzia, Esq. Marcelle S. Balcombe, Esq. Sichenzia Ross Ference LLP 1185 Avenue of the Americas 31st Floor New York, NY 10036 Tel.: (212) 930-9700 Fax: (212) 930-9725 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Smaller
reporting company |
Emerging Growth Company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
| Title of Securities Being Registered | Proposed Maximum Offering Price (1) | Amount of Registration Fee(2) | ||||||
| Common stock, par value $0.0001 per share (3) | $ | 23,000,000 | $ | 2,132.10 | ||||
| Underwriter’s warrants to purchase common stock (4) | $ | – | $ | – | ||||
| Common stock issuable upon exercise of the Underwriter’s warrants (5) | $ | 1,250,000 | $ | 115.88 | ||||
| Total | $ | 24,250,000 | $ | 2,247.98 | ||||
| (1) | Estimated solely for purposes of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. Pursuant to Rule 416 under the Securities Act of 1933, as amended, the shares of common stock registered hereby also include an indeterminate number of additional shares of common stock as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations, or other similar transactions. |
| (2) | Calculated pursuant to Rule 457(o) under the Securities Act, as amended based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the registrant. |
| (3) | Includes shares of common stock which may be issued on exercise of a 45-day option granted to the underwriters to cover over-allotments. |
| (4) | No registration fee is required pursuant to Rule 457(g) under the Securities Act. |
| (5) | We have agreed to issue, on the closing date of this offering, warrants to the underwriters in an amount equal to 5% of the aggregate number of common stock sold by the Company at an exercise price equal to 125% of the public offering price of the common stock offered hereby. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement related to these securities filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell or a solicitation of an offer to buy these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED , 2021 |
Shares of
Common Stock

QSAM Biosciences, Inc.
We are offering shares of our common stock par value $0.0001 per share (“common stock”), at an assumed offering price of $ per share (based upon the last reported sale price of our common stock on OTCQB Venture Market on , 2021). The public offering price of the shares of common stock in this offering will be determined through negotiation between us and the underwriters in the offering and the recent market price used throughout this prospectus may not be indicative of the final offering price.
Our common stock is currently traded on OTCQB Venture Market of OTC Markets Group (“OTCQB”), under the symbol “QSAM.” On December 15, 2021, the last reported sale price of our common stock was $0.28 per share. We will complete a reverse split of our common stock prior to completion of this offering at a ratio that will be determined prior to this offering. All share numbers in this registration statement will be adjusted to give effect to this reverse split, except in the financial statements or as otherwise indicated.
We have applied to list our common stock on the Nasdaq Capital Market (“NASDAQ”) under the symbol “QSAM”. If our application is not approved, we will not complete this offering. No assurance can be given that our application will be approved or that a trading market will develop.
Investing in our securities involves risks. See “Risk Factors” beginning on page 11 of this prospectus for a discussion of the risks that you should consider in connection with an investment in our securities. Neither the Securities and Exchange Commission (the “SEC”) nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions (1) | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
| (1) | In addition, we have agreed to reimburse the underwriters for certain expenses. See “Underwriting” beginning on page 63 of this prospectus for additional disclosure regarding underwriter compensation and offering expenses. |
We have granted the underwriters an option for a period of 45 days to purchase up to additional shares of common stock solely to cover over-allotments, if any.
The underwriters expect to deliver the Company’s securities to the purchasers on or about , 2021.
ThinkEquity
The date of this prospectus is , 2021.

TABLE OF CONTENTS
| i |
You should rely only on the information contained in this prospectus and any free writing prospectus that we have authorized for use in connection with this offering. Neither we nor the underwriters have authorized anyone to provide you with information that is different. We are offering to sell, and seeking offers to buy, the securities covered hereby only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the securities covered hereby. Our business, financial condition, results of operations and prospects may have changed since that date. We are not, and the underwriters are not, making an offer of these securities in any jurisdiction where the offer is not permitted. You should also read and consider the information in the documents to which we have referred under the caption “Where You Can Find Additional Information” in the prospectus. In addition, this prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed or will be filed as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the heading “Where You Can Find Additional Information.”
For investors outside the United States: Neither we nor any of the underwriters have taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby and the distribution of this prospectus outside of the United States.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market share, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Cautionary Note Regarding Forward-Looking Statements.”
This prospectus contains references to our trademarks and service marks and to those belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
| ii |
PROSPECTUS SUMMARY
This summary highlights information about us, this offering and selected information contained elsewhere in this Prospectus and does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should carefully read this entire prospectus and the documents incorporated by reference herein, including our financial statements and the related notes and the information set forth under the sections titled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements.” Unless otherwise indicated, all share amounts and per share amounts in this prospectus have been presented on a retrospective and pro forma basis to reflect the reverse stock split of our outstanding shares of common stock at a ratio of 1-for- which we effected on , 2022.
Except where the context requires otherwise, in this prospectus the “Company,” “we,” “us” and “our” refer to QSAM Biosciences Inc., a Delaware corporation and, where appropriate, its subsidiaries.
The Company
We are developing next-generation nuclear medicines for the treatment of cancer and related diseases. Our initial technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “New Technology”), a clinical-stage bone targeting radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. We believe improvements in formulation and manufacturing from a prior FDA-approved drug utilizing the same radioisotope (Quadramet®) has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability. Samarium-153 and DOTMP form a highly stable complex, which we believe, when used either as a monotherapy or in combination with other more widely used treatments such as external beam radiation, may demonstrate meaningful disease modifying results in primary and metastatic bone cancer. Ultimately, we may seek to further develop and commercialize CycloSam® for one or more market indications or license the technology to a larger pharmaceutical partner.
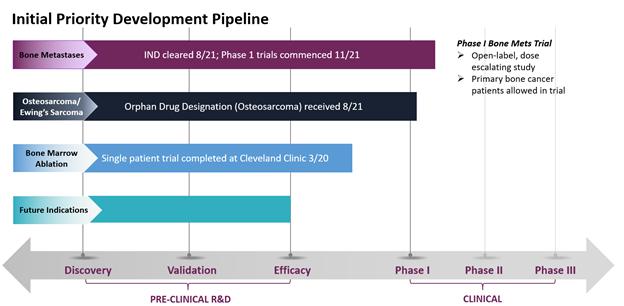
In August 2021, the Food & Drug Administration (FDA) cleared our Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas. We initiated this trial at our first site (Houston, TX) in November 2021, and we seek to commence dosing patients in this open-label, dose escalating study by the first quarter of 2022. Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults. Although patients with osteosarcoma or Ewing’s sarcoma are eligible to participate in our initial Phase 1 trials, we anticipate filing an amended protocol to our current commercial IND application in 2022 to commence clinical trials specifically for these primary, pediatric bone cancers. In May 2020, CycloSam® was also utilized in a Single Patient Investigational New Drug for Emergency Use at the Cleveland Clinic. We believe the study we conducted at the Cleveland Clinic showed promising safety results in connection with a bone marrow ablation procedure, including patient tolerability at high dosages. To date, CycloSam® has completed animal studies in both small and large animals, including treating bone cancer in patient dogs at a university veterinary clinic.
| 1 |
Clinical trials, the drug approval process, and the marketing of drugs are intensively regulated in the United States and in all major foreign countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and related regulations. Drugs are also subject to other federal, state, and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
Current Development Stage of CycloSam® for Target Indications. Our initial IND to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas has been cleared by the FDA, and we seek to commence dosing patients by the first quarter of 2022. Patients with primary bone cancer, such as osteosarcoma or Ewing’s sarcoma, are eligible to participate in our initial Phase 1 trials; however, we anticipate filing an amended protocol to our current commercial IND application in 2022 to commence clinical trials specifically for these primary, pediatric bone cancers. Our initial Phase 1 trial is an open label, dose escalating study of approximately 20 patients.
What is CycloSam®. CycloSam® is a targeted, bone seeking radiopharmaceutical that combines the beta-emitting radioisotope Samarium-153 (153Sm) with a chelating agent, DOTMP (1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetramethylenephosphonic acid). Samarium-153 is acquired from a nuclear reactor from a third party and the chelating agent is supplied in the form of kits. Chelating agents are organic compounds capable of linking together metal ions to form complex ring-like structures. This combination forms a stable complex which delivers a radioactive dose to sites of rapid bone mineral turnover such as bone cancers and tumors. CycloSam® has a physical half-life of 46 hours (radiation decreases by half in 46 hours) and emits both medium-energy beta particles that produce the therapeutic effect, and gamma photons that make it possible to take images of the skeleton and locate and characterize the size and nature of tumors. The use of radioisotopes to both diagnose and treat disease is called “theragnostics” and is a rapidly growing area of medical discovery.
| 2 |
How we believe CycloSam® Works – Mechanism of Action & Administration. CycloSam® utilizes a chelating agent called DOTMP that seeks out bone locations of high mineral turnover, typical in cancer cells and tumor growth. The DOTMP part of the molecule is taken up by calcium turnover locations in bones and carries the radioactive “payload” along with it. The radioisotope Samarium-153 emits radiation as it decomposes in the form of beta particles. Approximately 50% of the radioactivity concentrates in bone mineral with a very high lesion-to-normal bone ratio. We believe this provides a radiation dose to the adjacent tumor cells. The absorbed radiation dose produces the presumed therapeutic effect to the tumor, killing the cancer cells or slowing their growth by damaging their DNA. Our pre-clinical studies and single patient IND performed at the Cleveland Clinic demonstrates that the remaining half of the administered activity is rapidly excreted through the kidneys.
Generally, radiation therapy does not immediately kill cancer cells and more than one treatment is expected to eradicate a tumor, dramatically reduce its size, or slow its growth. CycloSam® has a short half-life of 46 hours and is rapidly eliminated from the body. This avoids an undesirable radioactive buildup in healthy tissues and organs when used in multiple treatments, which we believe, is an important feature of CycloSam® over predecessor drugs. CycloSam® has also not demonstrated saturation of the bone sites in animal studies, which supports a multi-dosage treatment regimen. Additionally, we believe that high dosages may be administered for ablating the marrow in patients that may require procedures such as stem cell transplants.
The final drug product of CycloSam® is prepared from DOTMP kits and 153SmCl in 0.1 N HCl at a nuclear pharmacy local to the patient administration site. The final drug product is then delivered to the physician for use as an intravenous (IV) injection.
What are CycloSam®’s competitive advantages. We believe CycloSam® has competitive advantages over current radiopharmaceutical offerings in the marketplace. Such potential competitive advantages include:
| ● | CycloSam®’s radioisotope, Samarium-153, emits beta particles that travel farther than alpha particles with what we believe is sufficient energy to slow the growth or decrease the size of target cancer cells. We believe beta particles penetrate bone matter deeper than the alpha emitting radiopharmaceuticals currently in the marketplace and may be more effective in treating tumors that form in or metastasize to bones. | |
| ● | CycloSam®’s delivery agent, DOTMP, compared to other chelating agents such as EDTMP used in Quadramet®, has shown in animal and other pre-clinical testing to have a high bone binding affinity allowing for the maximum delivery of the radioactive “payload” adjacent to the tumor without saturation of the bone, as observed from our pre-clinical trials. | |
| ● | Our method of manufacturing Samarium-153 compared to Quadramet®, has shown in our pharmacopeial limits studies to produce a 30-fold reduction in levels of long-lived radioactive impurities, mainly Europium-154. We believe this may mitigate toxicity issues with the patient. | |
| ● | Our initial studies show CycloSam® has fewer toxicities and a short 46 hour half-life that may allow for more frequent and repeated dosing of our radiopharmaceutical. We believe this may have a greater ability to slow or reverse tumor growth. | |
| ● | We believe we have in place an efficient and cost-effective manufacturing process and established distribution system that may in the future allow for 24/7 availability and enable the clinician to order and have the treatment delivered to the patient within approximately three days. |
The competitive advantages we believe to be important to CycloSam® are based on pre-clinical animal and other studies including our single patient IND performed at the Cleveland Clinic. We cannot be sure that our technology will perform similarly in clinical trials with multiple human patients. Failure to achieve these competitive advantages could negatively affect our ability to achieve FDA approval as a new drug, or our ability to market CycloSam® as a treatment for bone cancer.
Radiopharmaceuticals and Market Growth
Radiation is one of the most widely used treatments for cancer, with approximately 50% of all cancer patients receiving radiation therapy during their course of treatment [Source: Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci 2012; 9(3):193-199. doi:10.7150/ijms.3635]. A major limitation of some forms of radiation treatments, such as external beam therapy, is that radiation cannot be delivered with enough precision to prevent collateral damage to healthy tissue. Radiopharmaceuticals seek to overcome these limitations by delivering the tumor-killing power of radiation directly to tumor cells while sparing healthy tissue. This also expands the potential therapeutic benefit to a broader array of cancer type and stages, including metastatic disease.
| 3 |
To create radiopharmaceuticals, radiation emitting medical isotopes are typically attached to targeting molecules and administered via intravenous injection. Once administered, the radiopharmaceuticals selectively target tumor characteristics that are unique to, or preferentially expressed on, cancer cells. In the case of CycloSam®, we attach Samarium-153 to a chelating agent called DOTMP. This chelator seeks out and targets sites of high bone mineral turnover adjacent to cancer cells that have formed in or metastasized to the bone.
The radiopharmaceutical market is projected to reach $13.8 Billion by 2028 from $7.6 Billion in 2021, according to a published study by The Insight Partners: “Radiopharmaceuticals Market to 2028 – Global Analysis and Forecast – by Type, Product Type, Application, and End User.” Overall, the market is expected to grow at a CAGR of 9.0% during 2021–2028. North America dominates the global radiopharmaceuticals market, which is attributed to the prevalence of chronic disorders and the presence of supportive government plans for the development of research regarding radiopharmaceuticals. Based on type, the radiopharmaceuticals market is bifurcated into diagnostic nuclear medicine and therapeutic nuclear medicine. The diagnostic nuclear medicine segment is holding a larger share of the market and is anticipated to register a higher CAGR during 2021–2028. By application, the market is segmented into oncology, cardiology, neurology, and others, with the oncology segment holding the largest market share in 2021.
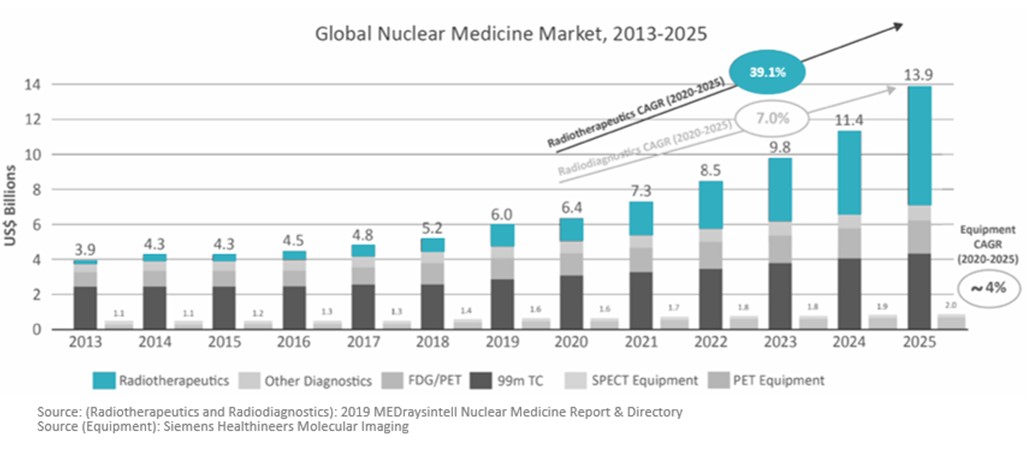
Another study published in 2019 in Radiotherapeutics and Radiodiagnostics shows similar growth, but with therapeutics experiencing the highest CAGR from 2020 to 2025, and the overall market reaching over $13.8 Billion by 2025:
Potential Market Indications for CycloSam®.
CycloSam’s therapeutic profile and presumed advantages over other radiopharmaceuticals, including Quadramet, translate to several potential key market indications as detailed in the following table:
| Market | Estimated New Cases Diagnosed Annually (US) | |||
| Bone Metastases (Breast, Prostate, Lung) | 400,000 | |||
| Other Primary Bone Cancers | 2,400 | |||
| Primary Bone Cancer – Osteosarcoma | 1,000 | |||
| Bone Marrow Ablation | 15,000 | |||
| Primary Bone Cancer – Ewing’s Sarcoma | 200 | |||
Source: American Cancer Society estimates of new cases reported each year in the United States. Data as of July 2020.
| 4 |
Bone metastases arise in about 5% of all types of cancer, 29% of patients with multiple myeloma (15,000), 16% of lung (37,000), 6% of prostate (48,000) and 7% of breast cancers (70,000). Roughly 70% of patients with bone metastases will experience bone pain, and many are at risk for skeletal-related events including fracture and spinal cord compression. The total annual cost for treatment of metastatic bone disease is approximately $12.7 billion or 17% of the total of $74 billion that was spent on direct medical costs of these cancers [Source: Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007 Jun 1;109(11):2334-42. doi: 10.1002/cncr.22678. PMID: 17450591]. In addition to metastatic bone cancers, according to the National Institute of Health SEER, there are approximately 14,000 people living with osteosarcoma in the US at any one time [Source: Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007 Jun;459:40-7. doi: 10.1097/BLO.0b013e318059b8c9. PMID: 17414166, and National Cancer Institute: Surveilance, E., and End Results Program Cancer Stat Facts: Bone and Joint Cancer, <https://seer.cancer.gov/statfacts/html/bones.html> (2020)] and their cost of care is estimated to exceed $100,000 per patient [Source: American Cancer Society. Key Statistics About Bone Cancer].
Metastatic bone cancer is currently incurable, and therefore palliation and arrest or deceleration of the progress of disease are important near-term goals. Quadramet® (Samarium-153-EDTP) and MetastronTM (89Sr chloride) were approved by the FDA for pain palliation resulting from osteoblastic bone metastases, but their widespread acceptance and use is hampered by concern about the perceived risk of myelosuppression when administered concurrently with chemotherapy. Xofigo®, an alpha particle emitter, was approved in May 2013 and initially was expected to capture significant market share rapidly; however, the product has only recently proven market success after many additional clinical trials.
Osteosarcoma is the most common childhood and adolescent/young adult (ages 15-39) primary high-grade bone malignancy [Source: Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian J Med Paediatr Oncol. 2017;38(1):33-43. doi:10.4103/0971-5851.203513]. Patients can often have metastatic cancer at diagnosis, and metastasis to the lungs is often fatal for these patients. For patients who develop or present with metastatic cancer in this diagnosis, the 5-year survival rate is 66% [Source: Osteosarcoma - Childhood and Adolescence - Statistics.” Cancer.Net, 30 Sept. 2021, https://www.cancer.net/cancer-types/osteosarcoma-childhood-and-adolescence/statistics]. Osteosarcoma standard-of-care usually involves chemotherapy which has substantial negative side effects, or drastic surgeries such as limb salvage or amputation. Osteosarcoma is relatively resistant to External Beam Radiation Therapy (EBRT), and currently approved radiopharmaceutical therapeutics fall short due to myelotoxicity and long-lived radioactive impurities. There is a tremendous unmet need for a better treatment that is more efficacious against pediatric osteosarcoma and better tolerated by patients.
| 5 |
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties, including, but not limited to, the following:
| ● | COVID-19 and the related governmental restrictions could have a material and adverse effect on our business, financial condition and results of operations. | |
| ● | Drug development is a long and inherently uncertain process with a high risk of failure at every stage of development. | |
| ● | The future of our business and operations depends on the success of our development and commercialization programs. | |
| ● | If we do not obtain regulatory approval for our product candidates or if the terms of any approval impose significant restrictions or limitations on use, our business, results of operations and financial condition will be adversely affected. | |
| ● | Setbacks in clinical development programs could have a material adverse effect on our business. | |
| ● | Our business is highly dependent on our lead product candidate, CycloSam®, and a failure to obtain regulatory approval or successfully commercialize our product could adversely affect our financial condition and results of operations. | |
| ● | Our FDA approvals are dependent upon successful clinical trials for our product candidates. Clinical trial results may be unfavorable or inconclusive, and often take longer and cost more than expected. | |
| ● | We are subject to extensive and ongoing regulation which can be costly and time consuming, may interfere with marketing approval for our product candidates, and can subject us to unanticipated limitations, restrictions, delays and fines. | |
| ● | We are increasingly dependent on information technology, and potential cyberattacks, security problems, or other disruption and expanding social media vehicles present new risks. | |
| ● | We have been and expect to continue to be dependent on collaborators for the development, manufacturing and sales of certain products and product candidates, which expose us to the risk of reliance on these collaborators. | |
| ● | Manufacturing resources could limit or adversely affect our ability to commercialize products. |
| 6 |
| ● | Failure of any manufacturer of our various product candidates to comply with applicable regulatory requirements could subject us to penalties and have a material adverse effect on supplies of our product candidates. | |
| ● | The validity, enforceability and commercial value of our patents and other intellectual property rights are highly uncertain. | |
| ● | We have a limited operating history and are operating at a loss, and there is no guaranty that we will become profitable. | |
| ● | Because our history is limited and we are subject to intense competition, any investment in us would be inherently risky. | |
| ● | If you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of your investment. | |
| ● | We have a high concentration of stock ownership and control within our executive officers and certain existing stockholders. | |
| ● | The Company has Preferred Stock with additional priority rights. | |
| ● | We are a smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to smaller reporting companies will make our common shares less attractive to investors. | |
| ● | We have historically identified certain material weaknesses in our internal control over financial reporting and if our remediation of such material weaknesses is not effective, or if we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired. |
General Information
Our common stock is currently traded on the OTCQB, under the symbol “QSAM.” On December 15, 2021, the last reported sale price of our common stock was $0.28 per share.
Our corporate headquarters is located at 9442 Capital of Texas Hwy N, Plaza 1, Suite 500 Austin, TX 78759 and our main phone number is (512) 343-4558. We maintain a website at www.qsambio.com. The information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus and is intended for informational purposes only.
The SEC also maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
| 7 |
Summary Financial Information
The following tables summarize our consolidated financial data for our business. We have derived the summary consolidated statement of operations data from (i) our interim unaudited condensed consolidated financial statements as September 30, 2021 and December 31, 2020 and (ii) unaudited condensed consolidated statements of operations for the nine months ended September 30, 2021 and 2020. Our financial statements are prepared and presented in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP. Our historical results are not necessarily indicative of our future results. You should read this data together with our consolidated financial statements and related notes appearing elsewhere in this prospectus and the information contained under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
QSAM BIOSCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| September 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 1,067,287 | $ | 8,304 | ||||
| Prepaid expenses and other assets | 19,197 | 12,896 | ||||||
| Deferred offering costs | 35,000 | - | ||||||
| TOTAL CURRENT ASSETS | 1,121,484 | 21,200 | ||||||
| TOTAL ASSETS | $ | 1,121,484 | $ | 21,200 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 241,266 | $ | 308,157 | ||||
| Accrued payroll and related expenses | 48,006 | 48,006 | ||||||
| Accrued interest – related parties | 1,478 | - | ||||||
| Notes payable - related parties | 7,500 | 63,992 | ||||||
| Debentures | 35,000 | 137,500 | ||||||
| Paycheck protection program loan - current portion | - | 34,163 | ||||||
| Convertible bridge notes, at fair value | - | 3,598,000 | ||||||
| TOTAL CURRENT LIABILITIES | 333,250 | 4,189,818 | ||||||
| Paycheck protection program loan - net of current portion | - | 108,779 | ||||||
| TOTAL LIABILITIES | 333,250 | 4,298,597 | ||||||
| Redeemable convertible preferred stock - Series A; $0.0001 par value, 1,500 designated Series A, 480 and 600 shares issued and outstanding (liquidation preference of $686,380 and $784,044) as of September 30, 2021 and December 31, 2020, respectively | 686,380 | 784,044 | ||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Preferred stock, Series B, $0.001 par value; 2,500 shares authorized, 1,509 and 281 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 2 | - | ||||||
| Preferred stock, Series E-1, $0.0001 par value; 8,500 shares authorized, 8,500 and 0 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 1 | - | ||||||
| Common stock, $0.0001 par value, 300,000,000 shares authorized, 34,958,306 and 19,512,517 shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | 3,496 | 1,947 | ||||||
| Unearned deferred compensation | (1,307,593 | ) | (148,333 | ) | ||||
| Subscription receivable | - | (25,000 | ) | |||||
| Additional paid-in capital | 27,243,381 | 11,021,840 | ||||||
| Accumulated deficit | (25,837,433 | ) | (15,911,895 | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | 101,854 | (5,061,441 | ) | |||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 1,121,484 | $ | 21,200 | ||||
| 8 |
QSAM BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited)
| For the nine months ended | ||||||||
| September 30, | ||||||||
| 2021 | 2020 | |||||||
| REVENUES | $ | - | $ | - | ||||
| OPERATING EXPENSES FROM CONTINUING OPERATIONS | ||||||||
| Compensation and related expenses | 5,555,471 | 608,029 | ||||||
| Professional fees | 1,427,703 | 327,961 | ||||||
| General and administrative | 64,887 | 121,692 | ||||||
| Research and development expenses | 385,785 | 206,943 | ||||||
| Total Operating Expenses | 7,433,845 | 1,264,625 | ||||||
| LOSS FROM CONTINUING OPERATIONS | (7,433,845 | ) | (1,264,625 | ) | ||||
| OTHER INCOME (EXPENSE) FROM CONTINUING OPERATIONS | ||||||||
| Financing costs including interest | (38,978 | ) | (431,790 | ) | ||||
| Change in fair value of convertible bridge notes | - | (1,666,422 | ) | |||||
| Other miscellaneous income | - | 5,000 | ||||||
| Gain on sale of equity method investment | 100,000 | |||||||
| Loss on debentures and accrued expenses converted to common stock | (390,068 | ) | - | |||||
| Gain on forgiveness of debt from Paycheck Protection Program | 142,942 | - | ||||||
| Gain (loss) on conversion of bridge notes including accrued interest and debt forgiveness | (744,505 | ) | (503,762 | ) | ||||
| Total Other Income (Expense) | (930,609 | ) | (2,596,974 | ) | ||||
| Loss from continuing operations before income taxes | (8,364,454 | ) | (3,861,599 | ) | ||||
| INCOME TAXES | - | - | ||||||
| Loss from continuing operations | (8,364,454 | ) | (3,861,599 | ) | ||||
| DISCONTINUED OPERATIONS: | ||||||||
| Income from discontinued operations before income taxes | - | 126,964 | ||||||
| INCOME TAXES | - | - | ||||||
| Income from discontinued operations | - | 126,964 | ||||||
| NET LOSS | (8,364,454 | ) | (3,734,635 | ) | ||||
| PREFERRED STOCK | ||||||||
| Series A and Series B preferred contractual dividends and deemed dividends | (1,583,421 | ) | (26,366 | ) | ||||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | (9,947,875 | ) | $ | (3,761,001 | ) | ||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS: BASIC AND DILUTED: | ||||||||
| CONTINUING OPERATIONS | $ | (0.36 | ) | $ | (1.69 | ) | ||
| DISCONTINUED OPERATIONS | - | 0.06 | ||||||
| $ | (0.37 | ) | $ | (1.64 | ) | |||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | 27,318,388 | 2,296,748 | ||||||
| 9 |
THE OFFERING
| Common stock offered by us | shares | |
| Common stock to be outstanding after this offering (1) | shares (or shares if the underwriters exercise in full their option to purchase additional shares to cover over-allotments, if any. | |
| Underwriter’s over-allotment option | We have granted the underwriters an option for a period of 45 days from the date of this prospectus to purchase up to an additional shares, representing 15% of shares of common stock sold in the public offering solely to cover over-allotments, if any. The purchase price to be paid per additional share of common stock shall be equal to the public offering price, less the underwriting discount. |
| Use of proceeds | We estimate that we will receive gross proceeds of approximately $ (or approximately $ if the underwriters exercise in full their option to purchase additional shares), assuming a public offering price of $ per share, before deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
|
| We currently expect to use the net proceeds from this offering for the following purposes: |
| ● | Advancement of our current Phase 1 clinical trials of CycloSam® for the indication of bone metastasis, and possible commencement of an amended protocol clinical trial for the indication of primary bone cancer, including osteosarcoma; and research and development of other indications and radiopharmaceutical assets; and | ||
| ● | Working capital and general corporate purposes. |
| See “Use of Proceeds” for additional information. |
| Proposed Nasdaq Capital Market symbol | We have applied to list the common stock to be issued in this offering on the Nasdaq Capital Market tier under the symbol “QSAM.” | |
| Reverse stock split | Prior to completion of this offering, we will complete a reverse split of our common stock, in a ratio to be determined by our board of directors. The purpose of the reverse stock split is principally to meet NASDAQ’s minimum stock price requirement. All share numbers in this registration statement will be adjusted to give effect to this reverse split, except in the financial statements or as otherwise indicated. | |
| Risk factors | See “Risk Factors” beginning on page 11 and the other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our common stock. | |
| Lock-up | We, our executive officers and directors, and holders of 5% or more of our common stock, have agreed with the underwriters not to offer for sale, issue, sell, contract to sell, encumber or otherwise dispose of any of our common stock or securities convertible into common stock for a period of 180 days in the case of officers and directors, and 90 days in the case of other stockholders, after the date of this prospectus. See “Underwriting” on page 63 of this prospectus. |
(1) The number of shares of common stock that will be outstanding after this offering as shown above is based on shares of common stock issued and outstanding as of the date of this prospectus, and the issuance and sale of shares of our common stock in this offering at a public offering price of $ per share. This number excludes:
| ● | shares of common stock issuable upon the exercise of warrants outstanding as of , 2021; | |
| ● | shares of common stock issuable upon the exercise of outstanding options to directors, employees and consultants under our 2016 Omnibus Equity Incentive Plan and grants and awards approved by the board of directors (collectively the “Equity Incentive Plan”) of which are vested as of ; and | |
| ● | shares of common stock reserved for future issuance upon conversion of our Series A preferred stock. |
Unless otherwise indicated, all information in this prospectus assumes and gives effect to:
| ● | a 1: reverse stock split effective as of ; | |
| ● | no exercise of the underwriter’s warrants; | |
| ● | the conversion of all outstanding convertible notes into an aggregate of shares of common stock upon completion of this offering; and | |
| ● | the conversion of the remaining outstanding Series B preferred stock into an aggregate of shares of common stock upon completion of this offering. |
| 10 |
RISK FACTORS
Investing in our securities involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including our financial statements and related notes, before deciding whether to purchase shares of our securities. If any of the following risks is realized, our business, operating results, financial condition and prospects could be materially and adversely affected. In that event, the price of our common stock could decline, and you could lose part or all of your investment.
General Risks Related to our Business and Technology
Drug development is a long and inherently uncertain process with a high risk of failure at every stage of development.
Drug development is a highly uncertain scientific and medical endeavor, and failure can unexpectedly occur at any stage of clinical development. Typically, there is a high rate of attrition for product candidates in preclinical and clinical trials due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. Pre-clinical studies and clinical trials are long, expensive and highly uncertain processes that can take many years. It will take us several years to complete our clinical trials and the time required for completing, testing and obtaining approvals is uncertain. The start or end of a clinical trial is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparator drug or required prior therapy, clinical outcomes, or financial constraints. The FDA and other U.S. and foreign regulatory agencies have substantial discretion, at any phase of development, to terminate clinical trials, require additional clinical development or other testing, delay, condition or withhold registration and marketing approval and mandate product withdrawals, including recalls. Additionally, we may also amend, suspend or terminate clinical trials at any time if we believe that the participating patients are being exposed to unacceptable health risks. Results attained in our single early human clinical trial may not be indicative of results in later clinical trials. Our failure to demonstrate adequately the safety and efficacy of a product under development would delay or prevent marketing approval, which could adversely affect our operating results and credibility. The failure of one or more of our product candidates could have a material adverse effect on our business, financial condition and results of operations.
The future of our business and operations depends on the success of our development and commercialization programs.
Our business and operations entail a variety of serious risks and uncertainties and are inherently risky. The development programs on which we focus involve novel approaches to treating bone cancer and related diseases. Our product candidates are in clinical development, and in some respects, involve technologies with which we have limited prior experience. We are subject to the risks of failure inherent in the development and commercialization of product candidates based on new technologies. There is some precedent for the successful commercialization of products based on our technologies, but there are still a number of technological challenges that we must overcome to complete our clinical trials and development efforts. We may not be able to successfully further develop our product candidates. We must successfully complete clinical trials and obtain regulatory approvals for potential commercial products. Once approved, if at all, commercial product sales are subject to general and industry-specific local and international economic, regulatory, technological and policy developments and trends. Delays, higher costs or other weaknesses in the manufacturing process or any of our contracted manufacturing organizations could hinder the development and commercialization of our product pipeline. The oncology space in which we operate presents numerous significant risks and uncertainties that may be expected to increase to the extent it becomes more competitive or less favored in the commercial healthcare marketplace.
We currently have no in-house sales, marketing or distribution capabilities and have limited experience in marketing products. We may in the future develop an in-house marketing and sales team, which would require significant capital expenditures, management resources and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. If we decide against absorbing marketing and sales responsibilities in-house, we will need to collaborate with third-parties. However, there can be no assurance that such collaborations will be successful or even if they are, they will be profitable for the Company after expending capital resources in fees and expenses. There can be no assurance that we will be able to develop in-house sales and distribution capabilities or establish or maintain relationships with third-party collaborators to commercialize any product.
If we do not obtain regulatory approval for our product candidates on a timely basis, or at all, or if the terms of any approval impose significant restrictions or limitations on use, our business, results of operations and financial condition will be adversely affected. Setbacks in clinical development programs could have a material adverse effect on our business.
Regulatory approvals are necessary to market product candidates and require demonstration of a product’s safety and efficacy through extensive pre-clinical and clinical trials. We may not obtain regulatory approval for product candidates on a timely basis, or at all, and the terms of any approval (which in some countries includes pricing and reimbursement approval) may impose significant restrictions, limitations on use or other commercially unattractive conditions. The process of obtaining FDA and foreign regulatory approvals often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. We have had only limited experience in filing and pursuing applications and other submissions necessary to gain marketing approvals. Products under development may never obtain marketing approval from the FDA or other regulatory authorities necessary for commercialization.
| 11 |
We or regulators may also amend, suspend or terminate clinical trials if we or they believe that the participating patients are being exposed to unacceptable health risks, and after reviewing trial results, we may abandon projects which we previously believed to be promising for commercial or other reasons unrelated to patient risks. During this process, we may find, for example, that results of pre-clinical studies are inconclusive or not indicative of results in human clinical trials, clinical investigators or contract research organizations do not comply with protocols or applicable regulatory requirements, or that product candidates do not have the desired efficacy or have undesirable side effects or other characteristics that preclude marketing approval or limit their potential commercial use if approved. In such circumstances, the entire development program for that product candidate could be adversely affected, resulting in delays in trials or regulatory filings for further marketing approval and a possible need to reconfigure our clinical trial programs to conduct additional trials or abandon the program involved. Conducting additional clinical trials or making significant revisions to a clinical development plan would lead to delays in regulatory filings. If clinical trials indicate, or regulatory bodies are concerned about, actual or possible serious problems with the safety or efficacy of a product candidate, we may stop or significantly slow development or commercialization of affected products. As a result of such concerns, the development programs for our product candidates may be significantly delayed or terminated altogether.
The results of our preclinical or initial single patient clinical studies may not be predictive of the results of clinical trials, and the results of any early-stage clinical trials we commence may not be predictive of the results of the later-stage clinical trials. In addition, initial success in clinical trials may not be indicative of results obtained when such trials are completed. There can be no assurance that any of our current or future clinical trials will ultimately be successful or support further clinical development of any of our product candidates. There is a high failure rate for drugs and biologics proceeding through clinical trials.
If the results of any of our clinical trials are not satisfactory or we encounter problems and/or delays enrolling patients, clinical trial supply issues, setbacks in developing drug formulations or in clinical trials, including raw material supply, manufacturing, stability or other difficulties, or issues complying with protocols or applicable regulatory requirements, the entire development program for our product candidates could be adversely affected in a material manner. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses and many companies that believed their product candidates performed satisfactorily in preclinical studies or clinical trials nonetheless failed to obtain FDA approval or approval from foreign regulatory authorities.
Our business is highly dependent on our lead product candidate, CycloSam®, and a failure to obtain regulatory approval or successfully commercialize our product could adversely affect our financial condition and results of operations.
In April 2020, the Company, through its wholly-owned subsidiary QSAM Therapeutics entered into an exclusive worldwide patent and technology license agreement and trademark assignment with respect to CycloSam® and obtained exclusive commercial rights to the patent portfolio developed by IGL Pharma Inc. (“IGL”) (the “Original Agreement”). The agreement was mutually amended on November 24, 2021 (collectively with the Original Agreement referred to hereinafter as the “License Agreement). The License Agreement is terminable in the event of a material breach by us that is not cured within a predefined period of time after notice of the breach is provided to us. If the License Agreement is terminated, the Company will not have further rights to CycloSam® and will be unable to continue its regulatory approval process or if already commercialized, benefit from the future sales of CycloSam®. Further, the Company may be liable for damages for the breach and may suffer additional losses, which could adversely impact our financial condition. As of the date of this prospectus, the Company’s focus is to solely develop CycloSam® and it does not possess licenses to other product candidates. If we do not obtain regulatory approval for CycloSam® or fail to successfully commercialize CycloSam®, we currently have no fall back options to continue our business operations unless we secure licenses of or develop alternative drug candidates. There is no assurance that either will occur.
We must design and conduct successful clinical trials for our product candidates to obtain regulatory approval. We rely on third parties to conduct our clinical trials, which reduces our control over their timing, conduct and expense and may expose us to conflicts of interest. Clinical trial results may be unfavorable or inconclusive, and often take longer and cost more than expected.
We have limited internal resources for conducting clinical trials, and we rely on or obtain the assistance of others to design, conduct, supervise, or monitor some or all aspects of some of our clinical trials. In relying on these third parties, we have less control over the timing and other aspects of clinical trials than if we conducted them entirely on our own. Problems with the timeliness or quality of the work of a contract research organization or clinical data management organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials and contractual restrictions may make such a change difficult or impossible. These third parties may also have relationships with other entities, some of which may be our competitors. In all events, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA and other foreign regulatory authorities require us to comply with good clinical practices for conducting and recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements.
| 12 |
To obtain regulatory approval of our product candidates we must demonstrate through preclinical studies and clinical trials that they are safe and effective. Adverse or inconclusive clinical trial results concerning any of our product candidates that regulators find deficient in scope, design or one or more other material respects, could require additional trials, resulting in increased costs, significant delays in submissions of approval applications, approvals in narrower indications than originally sought, or denials of approval, none of which we can predict. As a result, any projections that we publicly announce of commencement and duration of clinical trials are not certain. Clinical trial delays may occur as a result of slower than anticipated enrollment. Delays can be caused by, among other things, deaths or other adverse medical events; regulatory or patent issues; interim or final results of ongoing clinical trials; failure to enroll clinical sites as expected; competition for enrollment from other clinical trials; scheduling conflicts with participating clinicians and institutions; disagreements, disputes or other matters arising from collaborations; our inability to obtain necessary funding; or manufacturing problems.
A pandemic, epidemic or outbreak of an infectious disease, such as COVID-19, or coronavirus, may materially and adversely affect our business and our financial results.
The COVID-19 pandemic, including the recent surge caused by the “delta variant”, has materially affected segments of the global economy and may affect our operations by causing a period of business disruption, supply chain issues, including the potential interruption of our clinical trial activities and delays or disruptions in the supply of our products and product candidates. In addition, there could be a potential effect of COVID-19 to the business at FDA or other health authorities, which could result in delays of reviews and approvals, including with respect to our product candidates.
The continued spread of COVID-19 globally could also adversely impact our clinical trial operations, including our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to COVID-19 if an outbreak occurs in their geography. COVID-19, or another infectious disease, could also negatively affect our manufacturing operations, which could result in delays or disruptions in the supply of our product candidates.
We cannot presently predict the scope and severity of any potential business shutdowns or disruptions, but if we or any of the third parties with whom we engage were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted, which could have a material adverse effect on our business and our results of operation and financial condition.
Even if our product candidates obtain marketing approval, our ability to generate revenue will depend upon public perception of radiopharmaceuticals and will be diminished if our products are not accepted in the marketplace, or if we select pricing strategies for our products that are less competitive than those of our competitors, or fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payers or government agencies.
Adverse events in clinical trials of our product candidates or in clinical trials of others developing similar products and the resulting negative publicity, as well as any other adverse events in the field of radiopharmaceuticals that may occur in the future, could result in a decrease in demand for our products or any product candidates that we may develop. If public perception is influenced by claims that radiopharmaceuticals or specific therapies within radiopharmaceuticals are unsafe, our products or product candidates may not be accepted by the general public or the medical community.
The commercial success of our products will depend upon their acceptance by the medical community and third-party payers as clinically useful, cost effective and safe. Market acceptance of approved products is affected by a wide range of factors including the timing of regulatory approvals, product launches and the presence of generic, over-the-counter or other competitors; the pricing of the product and relative prices of competing products; product development efforts for new indications; the availability of reimbursement for the product; our ability to obtain sufficient commercial quantities of the product; success in arranging for necessary sublicense or distribution relationships; and general and industry-specific local and international economic pressures. If health care providers believe that patients can be managed adequately with alternative, currently available therapies, they may not prescribe our products, especially if the alternative therapies are viewed as more effective, as having a better safety or tolerability profile, as being more convenient to the patient or health care providers or as being less expensive. Third-party insurance coverage may not be available to patients for any products we develop. For pharmaceuticals administered in an institutional setting, the ability of the institution to be adequately reimbursed from government and health administration authorities, private health insurers and other third-party payers could also play a significant role in demand for our products. Significant uncertainty exists as to the reimbursement status of newly-approved pharmaceuticals. Government and other third-party payers increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for indications for which the FDA has not granted labeling approval. In most foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the U.S., we expect that there will continue to be a number of federal and state proposals to implement similar government control and that the emphasis on managed care in the U.S. will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we can receive for any products in the future and adversely affect our ability to successfully commercialize our products. If any of our product candidates do not achieve market acceptance, we will likely lose our entire investment in that product candidate.
| 13 |
We are subject to extensive and ongoing regulation, which can be costly and time consuming, may interfere with marketing approval for our product candidates, and can subject us to unanticipated limitations, restrictions, delays and fines.
Our business, products and product candidates are subject to comprehensive regulation by the FDA and comparable authorities in other countries, and include the Sunshine Act under the Patient Protection and Affordable Care Act (“PPACA”). These agencies and other entities regulate the pre-clinical and clinical testing, safety, effectiveness, approval, manufacture, labeling, marketing, export, storage, recordkeeping, advertising, promotion and other aspects of our products and product candidates. We cannot guarantee that approvals of product candidates, processes or facilities will be granted on a timely basis, or at all. If we experience delays or failures in obtaining approvals, commercialization of our product candidates will be slowed or stopped. In addition to these uncertainties, there have been several attempts and public announcements by members of the U.S. Congress to repeal the PPACA and replace it with a curtailed system of tax credits and dissolve an expansion of the Medicaid program. For example, Tax Cuts and Jobs Act of 2017 was enacted in 2017, which, among other things, eliminated the individual mandate requiring most Americans (other than those who qualify for a hardship exemption) to carry a minimum level of health coverage, and became effective January 1, 2019. There is considerable uncertainty regarding the future of the current PPACA framework, and any changes will likely take time to unfold. As such, we cannot predict what effect the PPACA or other healthcare reform initiatives that may be adopted in the future will have on our business.
Even if we obtain regulatory approval for a product candidate, the approval may include significant limitations on indicated uses for which the product could be marketed or other significant marketing restrictions.
If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be subject to forced removal of a product from the market, product seizure, civil and criminal penalties and other adverse consequences.
Our products may face regulatory, legal or commercial challenges even after approval.
Even if a product receives regulatory approval:
| ● | It might not obtain labeling claims necessary to make the product commercially viable (in general, labeling claims define the medical conditions for which a drug product may be marketed, and are therefore very important to the commercial success of a product), or may be required to carry warnings that adversely affect its commercial success. | |
| ● | Approval may be limited to uses of the product for treatment or prevention of diseases or conditions that are relatively less financially advantageous to us than approval of greater or different scope or subject to an FDA imposed Risk Evaluation and Mitigation Strategy (“REMS”) that imposes limits on the distribution or use of the product. While we may develop a product candidate with the intention of addressing a large, unmet medical need, the FDA or other foreign regulatory authorities may only approve the use of the drug for indications affecting a relatively small number of patients, thus greatly reducing the market size and our potential revenues. | |
| ● | Side effects identified after the product is on the market might hurt sales or result in mandatory safety labeling changes, additional pre-clinical testing or clinical trials, imposition of a REMS, product recalls or withdrawals from the market, reputational harm to us, and lawsuits (including class-action suits). | |
| ● | Efficacy or safety concerns regarding a marketed product, or manufacturing or other problems, may lead to a recall, withdrawal of marketing approval, marketing restrictions, reformulation of the product, additional pre-clinical testing or clinical trials, changes in labeling, imposition of a REMS, warnings and contraindications, the need for additional marketing applications, declining sales or other adverse events. These potential consequences may occur whether or not the concerns originate from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not they are scientifically justified. If products lose previously received marketing and other approvals, our business, results of operations and financial condition would be materially adversely affected. | |
| ● | In certain foreign jurisdictions, drug products cannot be marketed until pricing and reimbursement for the product is also approved. In the United States, reimbursement approval is not required, but if not available, that may severely limit the sales, and the Center for Medicare & Medicaid Services may require additional clinical studies, more than the FDA demands. | |
| ● | We will be subject to ongoing FDA obligations and continuous regulatory review, and might be required to undertake post-marketing trials to verify the product’s efficacy or safety or other regulatory obligations. |
| 14 |
We are increasingly dependent on information technology, and potential cyberattacks, security problems, or other disruption and expanding social media vehicles present new risks.
We rely on information technology networks and systems, including the internet, to process, transmit, and store electronic information, and to manage or support a variety of business processes, including financial transactions and records, billing, and operating data. We may purchase some of our information technology from vendors, on whom our systems will depend, and we rely on commercially available systems, software, tools, and monitoring to provide security for processing, transmission, and storage of confidential operator and other customer information. We depend upon the secure transmission of this information over public networks. Our networks and storage applications could be subject to unauthorized access by hackers or others through cyberattacks, which are rapidly evolving and becoming increasingly sophisticated, or by other means, or may be breached due to operator error, malfeasance or other system disruptions. In some cases, it will be difficult to anticipate or immediately detect such incidents and the damage they cause. Any significant breakdown, invasion, destruction, interruption, or leakage of information from our systems could harm our reputation and business.
In addition, the use of social media could cause us to suffer brand damage or information leakage. Negative posts or comments about us on any social networking website could damage our or our brands’ reputations. Employees or others might disclose non-public sensitive information relating to our business through external media channels, including through the use of social media. The continuing evolution of social media will present us with new challenges and risks.
Risks Related to our Financial Position and Operating History
We have a limited operating history and are operating at a loss, and there is no guaranty that we will become profitable.
We recently began operations under our current business model and anticipate that we will operate at a loss for some time. Since we have limited operating history and no history of profitability, we have limited financial results upon which you may judge our potential. Further, our ability to become profitable depends upon our ability to generate revenue. We have recorded no revenue from continuing operations since inception. We do not expect to generate significant product revenue unless or until we successfully complete clinical development and obtain regulatory approval of, and then successfully commercialize, at least one of our product candidates; or alternatively, out license or sell our drug candidates. Both of these scenarios are highly uncertain. In the future, we may experience under-capitalization, development delays, set-backs with our drug development programs, lack of funding options, setbacks and many of the problems, delays and expenses encountered by any early stage business, many of which are beyond our control. These include, but are not limited to:
| ● | our lack of an operating history; | |
| ● | the net losses that we expect to incur as we develop our business; | |
| ● | obtaining FDA or other regulatory approvals or clearances for our technology; | |
| ● | implementing and achieving successful outcomes for clinical trials of our products; | |
| ● | convincing physicians, hospitals and patients of the benefits of our technology and to convert from current technology; | |
| ● | the ability of users of our products (when and as developed) to obtain third-party reimbursement; | |
| ● | any failure to comply with rigorous FDA and other government regulations; and | |
| ● | securing, maintaining and defending patent or other intellectual property protections for our technology. |
Because our history is limited and we are subject to intense competition, any investment in us would be inherently risky.
Because we are a company with limited operational history and no profitability, our business activity is early-staged and subject to numerous risks. The pharmaceutical development business is highly competitive with many companies having access to the similar products and markets. Many of them have greater financial resources and longer operating histories than we have and can be expected to compete within the business in which we engage and intend to engage. There can be no assurance that we will have the necessary resources to become or remain competitive. We are subject to the risks which are common to all companies with a limited history of operations and profitability. Therefore, investors should consider an investment in us to be an extremely risky venture.
There is substantial doubt as to our ability to continue as a going concern.
The Report of our Independent Registered Public Accounting Firm issued in connection with our audited consolidated financial statements for the calendar year ended December 31, 2020 expressed substantial doubt about our ability to continue as a going concern because of our recurring operating losses and our lack of liquidity and working capital. A going concern opinion means that there is substantial doubt that the Company can continue as an ongoing business for the next 12 months. While we expect to become a going concern upon the completion of this offering, if we fail to successfully deploy our funds, fail to implement our business plan or commercialize our drug as planned, or fail to raise additional capital when required, there can be no assurance that we will not again lose our ability to continue as a going concern.
Even upon successful closing of this offering, we will require additional financing.
Pharmaceutical development is inherently costly and requires significant capital. We expect our expenses to increase significantly as we enter into the next stage of our drug development including steps such as preclinical studies, clinical trials, research and development, and FDA marketing approvals. If we do obtain FDA approval, we expect to incur substantial costs in commercialization of the product. In addition, we will continue to incur costs to operate as a public company. Accordingly, we will need to obtain additional financing in connection with our continuing operations. There can be no assurance that additional funds will be available when and if needed, or on acceptable terms to the Company. If we are unable to obtain such financing, or if the terms thereof are too costly, we may be forced to curtail or cease operations until such time as alternative financing may be arranged, which could have a materially adverse impact on our planned operations and our shareholders’ investment.
Based upon our current operating plan, we believe that the net proceeds from this offering, together with our existing cash and cash equivalents, will enable us to fund our operations through at least 2022. In particular, we expect that the net proceeds from this offering and our existing cash and cash equivalents will allow us to complete the Phase 1 portion of our planned clinical trials for CycloSam® used in connection with metastatic bone cancer, and also may allow us to commence or prepare for commencement of clinical trials for additional indications, including primary bone cancer and osteosarcoma. We have based these estimates on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we currently expect. Our operating plans and other demands on our cash resources may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other capital sources, including potentially additional collaborations, licenses and other similar arrangements. In addition, our ability to continue as a going concern is dependent on our ability to raise additional capital in order to implement our current business plan. If the market conditions are favorable or given our strategic considerations, even if we believe we have sufficient funds for our current or future operating plans, we may raise additional capital. Attempting to secure additional financing may divert our management from our day-to-day activities, which may adversely affect our ability to develop our product candidates.
| 15 |
Our success will be dependent on our management, and the continued service of key employees.
Our success is dependent upon the decision making of our directors and executive officers. We believe that our success depends on the continued service of our key employees and our ability to hire additional key employees when and as needed. Although we currently intend to retain our existing management, we cannot assure you that such individuals will remain with us. Further, we cannot assure that we will be able to find and recruit new employees on terms acceptable to the Company. We have fixed term employment agreements with our three key employees – Messrs. Baum, Piazza and Nelson — but have not obtained key man life insurance on the lives of any of them. The unexpected loss of the services of one or more of our key executives, directors and advisors, or the inability to find new key employees within a reasonable period of time could have a material adverse effect on the economic condition and results of operations of the Company.
Risks Related to Working with Third Parties
We have been and expect to continue to be dependent on collaborators for the development, manufacturing and sales of certain products and product candidates, which expose us to the risk of reliance on these collaborators.
In conducting our operations, we currently depend, and expect to continue to depend, on numerous collaborators. In addition, certain clinical trials for our product candidates may be conducted by government-sponsored agencies, and consequently will be dependent on governmental participation and funding. These arrangements expose us to the same considerations we face when contracting with third parties for our own trials.
If any of our collaborators breach or terminate its agreement with us or otherwise fail to conduct successfully and in a timely manner the collaborative activities for which they are responsible, the preclinical or clinical development or commercialization of the affected product candidate or research program could be delayed or terminated. We generally do not control the amount and timing of resources that our collaborators devote to our programs or product candidates. We also do not know whether current or future collaboration partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases or conditions targeted by our collaborative arrangements. Our collaborators are also subject to similar development, regulatory, manufacturing, cyber-security and competitive risks as us, which may further impede their ability to successfully perform the collaborative activities for which they are responsible. Setbacks of these types to our collaborators could have a material adverse effect on our business, results of operations and financial condition.
We are dependent upon third parties for a variety of functions. These arrangements may not provide us with the benefits we expect.
We rely on third parties to perform a variety of functions. We are party to numerous agreements which place substantial responsibility on clinical research organizations, consultants and other service providers for the development of our product candidates. We also rely on medical and academic institutions to perform aspects of our clinical trials of product candidates. We may not be able to enter new arrangements without undue delays or expenditures, and these arrangements may not allow us to compete successfully. Moreover, if third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct clinical trials in accordance with regulatory requirements or applicable protocols, our product candidates may not be approved for marketing and commercialization or such approval may be delayed. If that occurs, we or our collaborators will not be able, or may be delayed in our efforts, to commercialize our product candidates.
Our relationships with customers and third-party payers are or may become subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Health care providers, physicians and third-party payers play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payers and customers will or already do require us and them to comply with broadly applicable fraud and abuse and other health care laws and regulations, including both federal and state anti-kickback and false claims laws, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products that obtain marketing approval. Efforts to ensure that business arrangements comply with applicable health care laws and regulations involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If such operations are found to be in violation of any of these laws or other applicable governmental regulations, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of related operations. If physicians or other providers or entities involved with our products are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which may adversely affect us.
| 16 |
If we or our partners are unable to obtain sufficient quantities of the materials needed to make our products or product candidates, development of our products or product candidates or commercialization of our approved products could be slowed or stopped.
We have utilized Missouri University Research Reactor (“MURR”) to procure Samarium-153, a primary ingredient used in manufacturing of CycloSam®, for our recently conducted clinical studies. Samarium-153 is critical to manufacturing of CycloSam® and to our supply-chain process both during our clinical trials and if the product is commercialized. MURR has verbally committed to supply us Samarium-153 in the future and we are expecting to enter into definitive agreements with MURR in 2022. We also plan to qualify additional suppliers in 2022 as part of our supply chain and general business risk diversification strategy. However, if we fail to partner with MURR or secure other partners for supply of Samarium-153 or if our arrangements with a supplier do not satisfy our requirements in the future, it will directly and adversely impact the production of CycloSam®.
We or our partners may not be able to obtain the materials necessary to make a particular product or product candidate in adequate volume and quality. If any materials needed to make a product or product candidate is insufficient in quantity or quality, if a supplier fails to deliver in a timely fashion or at all or if these relationships terminate, we or our partners may not be able to fulfill manufacturing obligations for our products or product candidates, either on our own or through third-party suppliers. A delay or disruption of supplies of our products or product candidates would have a material adverse effect on our business as a whole. Our existing arrangements with suppliers may result in the supply of insufficient quantities of our product candidates needed to accomplish our clinical development programs or commercialization, and we may not have the right and in any event, do not currently have the capability to manufacture these products if our suppliers are unable or unwilling to do so. Some of these raw materials or their starting materials, components or ingredients may come from foreign countries, which can present significant supply chain issues. We currently arrange for supplies of critical raw materials used in production of our product candidates from single sources. We do not have long-term contracts with any of these suppliers. Any delay or disruption in the availability of materials would slow or stop product development and commercialization of the relevant product.
Manufacturing resources could limit or adversely affect our ability to commercialize products.
We or our partners may engage third parties, including nuclear reactor sites, to manufacture our product candidates. We or our partners may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturing organizations or CMOs at acceptable costs.
In order to commercialize our product candidates successfully, we need to be able to manufacture or arrange for the manufacture of products in commercial quantities, in compliance with regulatory requirements, at acceptable costs and in a timely manner. Manufacture of our product candidates can be complex, difficult to accomplish even in small quantities, difficult to scale-up for large-scale production and subject to delays, inefficiencies and low yields of quality products. The manufacture of radiopharmaceuticals is relatively complex and requires significant capital expenditures. We continue to rely on CMOs for our product candidates. The cost of manufacturing our product candidates may make them prohibitively expensive. If adequate supplies of any of our product candidates or related materials are not available on a timely basis or at all, our clinical trials or commercialization of our product candidates could be seriously delayed, since these materials are time consuming to manufacture and cannot be readily obtained from third-party sources. We continue to be dependent on a limited number of highly specialized manufacturing and development partners, including single source manufacturers for certain of our product candidates. If we were to lose one or more of these key relationships, it could materially adversely affect our business. Establishing new manufacturing relationships, or creating our own manufacturing capability, would require significant time, capital and management effort, and the transfer of product-related technology and know-how from one manufacturer to another is an inherently complex and uncertain process.
Failure of any manufacturer of our various product candidates to comply with applicable regulatory requirements could subject us to penalties and have a material adverse effect on supplies of our product candidates.
Third-party manufacturers are required to comply with current goods manufacturing practice regulations or cGMP or similar regulatory requirements outside of the U.S. If manufacturers of our product candidates cannot successfully manufacture material that conforms to the strict regulatory requirements of the FDA and any applicable foreign regulatory authority, they may not be able to supply us with our product candidates. If these facilities are not approved for commercial manufacture, we may need to find alternative manufacturing facilities, which could result in delays of several years in obtaining approval for a product candidate. We do not control the manufacturing operations and are completely dependent on our third-party manufacturing partners or contractors for compliance with the applicable regulatory requirements for the manufacture of some of our product candidates. Manufacturers are subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMP and similar regulatory requirements. Failure of any manufacturer of any of our product candidates to comply with applicable cGMP or other regulatory requirements could result in sanctions being imposed on our collaborators or us, including fines, injunctions, civil penalties, delays, suspensions or withdrawals of approvals, operating restrictions, interruptions in supply and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates and have a material adverse impact on our business, financial condition and results of operations.
| 17 |
If the use of hazardous and biological materials by us or third parties, such as CROs or CMOs, in a manner that causes injury or violates applicable law, we may be liable for damages.
Our research and development activities may involve the controlled use of potentially hazardous substances, including radioactive, chemical and biological materials, by us or third parties, such as contract research organizations or CROs and CMOs. Exposure to high levels of radiation can cause acute health effects such as skin burns and acute radiation syndrome (“radiation sickness”). It can also result in long-term health effects such as cancer and cardiovascular disease. We and such third parties are subject to federal, state, and local laws and regulations in the United States governing the use, manufacture, storage, handling, and disposal of medical and hazardous materials. Although we believe that our and such third-parties’ procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. In the event of any such contamination or injury, we may incur liability or local, city, state, or federal authorities may curtail the use of these materials and interrupt our business operations. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development and manufacturing efforts, which could harm our business prospects, financial condition, or results of operations. We plan to maintain insurance coverage upon completion of this offering for injuries resulting from the hazardous materials we use; however, future claims may exceed the amount of our coverage. Also, we do not have insurance coverage for pollution cleanup and removal. Currently the costs of complying with such federal, state, provincial, local and foreign environmental regulations are not significant, and consist primarily of waste disposal expenses. However, they could become expensive, and current or future environmental laws or regulations may impair our research, development, production and commercialization efforts. Further, although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
Unexpected disruptions could seriously harm our future revenue and financial condition and increase our expenditures.
Our operations, and those of our CROs, CMOs and other contractors and consultants, could be subject to events like earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce and process our product candidates to meet our demands. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption.
Risks Relating to Our Intellectual Property
The validity, enforceability and commercial value of our patents and other intellectual property rights are highly uncertain.
We license a number of issued patents and other patent applications that have not yet been issued. We must obtain, maintain and enforce patent and other rights to protect our intellectual property. The patent position of biotechnology and pharmaceutical firms is highly uncertain and involves many complex legal and technical issues. There are many laws, regulations and judicial decisions that dictate and otherwise influence the manner in which patent applications are filed and prosecuted and in which patents are granted and enforced, all of which are subject to change from time to time. There is no clear policy involving the breadth of claims allowed, or the degree of protection afforded, under patents in this area. Accordingly, patent applications owned by or licensed to us may not result in patents being issued. Even if we own or license a relevant issued patent, we may not be able to preclude competitors from commercializing drugs that may compete directly with one or more of our products or product candidates, in which event such rights may not provide us with any meaningful competitive advantage. In the absence or upon successful challenge of patent protection, drugs may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products.
It is generally difficult to determine the relative strength or scope of a biotechnology or pharmaceutical patent position in absolute terms at any given time. The issuance of a patent is not conclusive as to its validity or enforceability, which can be challenged in litigation or via administrative proceedings. The License Agreement from which we derive or license intellectual property provide for various royalty, milestone, sublicensing and other payments, and include other provisions like patent prosecution and enforcement, insurance, indemnification and other obligations and rights, and is subject to certain reservations of rights. While we generally have the right to defend and enforce patents licensed to or by us, either in the first instance or if the licensor or licensee chooses not to do so, we must usually bear the cost of doing so.
Patents have a limited life and expire by law.
In addition to uncertainties as to scope, validity, enforceability and changes in law, patents by law have limited lives. Upon expiration of patent protection, our drug candidates and/or products may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products.
We depend on intellectual property licensed from third parties and unpatented technology, trade secrets and confidential information. If we lose any of these rights, including by failing to achieve milestone requirements or to satisfy other conditions, our business, results of operations and financial condition could be harmed.
Our core product candidate is derived from intellectual property licensed from a third party. We could lose the right to patents and other intellectual property licensed to us if the related License Agreement is terminated due to a breach by us or otherwise. Our ability to commercialize products incorporating licensed intellectual property would be impaired if the related License Agreements were terminated. In addition, we are required to make substantial cash payments, achieve milestones and satisfy other conditions, including filing for and obtaining marketing approvals and introducing products, to maintain rights under our intellectual property license. Due to the nature of this agreement and the uncertainties of development, we may not be able to achieve milestones or satisfy conditions to which we have contractually committed, and as a result may be unable to maintain our rights under the license. If we do not comply with our License Agreement, the licensor may terminate it, which could result in our losing our rights to, and therefore being unable to commercialize, related products.
| 18 |
We also rely on unpatented technology, trade secrets and confidential information. Third parties may independently develop substantially equivalent information and techniques or otherwise gain access to our technology or disclose our technology, and we may be unable to effectively protect our rights in unpatented technology, trade secrets and confidential information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with us. These agreements may, however, not provide effective protection in the event of unauthorized use or disclosure of confidential information. Any loss of trade secret protection or other unpatented technology rights could harm our business, results of operations and financial condition.
If we infringe third-party patent or other intellectual property rights, we may need to alter or terminate a product development program.
There may be patent or other intellectual property rights belonging to others that require us to alter our products, pay licensing fees or cease certain activities. If our products infringe patent or other intellectual property rights of others, the owners of those rights could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. We may not prevail in any action brought against us, and any license required under any rights that we infringe may not be available on acceptable terms or at all.
Research, development and commercialization of a biopharmaceutical product often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes may depend on subsequent discoveries and test results and cannot be predicted with certainty at the outset. There are numerous third-party patents in our field, and we may need to obtain a license under a patent in order to pursue the preferred development route of one or more of our products or product candidates. The need to obtain a license would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate the program altogether.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to take legal action to enforce our patents or our licensors’ patents against such infringing activity. Such enforcement proceedings can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that one or more of our patents is invalid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the compositions or activities in question. An adverse result could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense against these assertions, non-infringement, invalidity or unenforceability regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Post-grant proceedings provoked by third parties or brought by the United States Patent and Trademarks Office may be brought to determine the validity or priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or post-grant proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as those within the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
| 19 |
Risks Related to our common stock and this Offering
Liquidity risks associated with our common stock.
There is a limited trading market for our shares of common stock and while the Company’s common stock may be approved for listing on NASDAQ Capital Market, there can be no assurance that (1) an active trading market will be developed or sustained, (2) the liquidity of such market will increase, (3) our stockholders will be able sell their shares of common stock, or (4) the price that our stockholders may obtain for their common stock will be greater than the public offering price. If an active market for our common stock with meaningful trading volume does not develop or is not maintained, the market price of our common stock may decline materially below the offering price and you may not be able to sell your shares. Further, prior to NASDAQ listing, our stock traded on OTCQB and our shares of common stock were thinly traded. Therefore, prospective stockholders who require immediate liquidity in their investments should consider these risks before investing in our common stock.
Our failure to meet the continued listing requirements of the NASDAQ Capital Market could result in a delisting of our common stock.
If we are successful in having our shares of common stock listed on the NASDAQ Capital Market, we will be required to satisfy the continued listing requirements. If we fail to satisfy the continued listing requirements of the NASDAQ Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, NASDAQ may take steps to delist our common stock. Such a delisting would likely have a negative effect on the price of our common stock, and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we would take actions to restore our compliance with NASDAQ’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ’s listing requirements.
If you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of your investment.
The public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. You will experience immediate dilution of $ per share, representing the difference between our pro forma net tangible book value per share after giving effect to this offering and the public offering price. This includes automatic conversion of all shares of Series B preferred stock and outstanding convertible notes, into shares of our common stock as of the closing of this offering. See “Dilution” for more detail.
The price of our common stock may fluctuate significantly, which could lead to losses for stockholders.
The securities of public companies can experience extreme price and volume fluctuations, which can be unrelated or out of proportion to the operating performance of such companies. We expect our common stock price will be subject to similar volatility. Any negative change in the public’s perception of the prospects of our Company or companies in our market could also depress our common stock price, regardless of our actual results. Factors affecting the trading price of our common stock may include:
| * | Regulatory actions; | |
| * | Variations in our operating results; | |
| * | Announcements of technological innovations, new products or product enhancements, strategic alliances or significant agreements by us or by our competitors; |
| 20 |
| * | Recruitment or departure of key personnel; | |
| * | Litigation, legislation, regulation or technological developments that adversely affect our business; | |
| * | Changes in the estimates of our operating results or changes in recommendations by any securities analysts that elect to follow our common stock; and | |
| * | Market conditions in our industry, the industries of our customers and the economy as a whole. |
The offering price per share of our common stock offered under this prospectus may not accurately reflect the value of your investment.
Prior to this offering, our common stock was trading on OTCQB. The offering price per share of our common stock offered by this prospectus was based upon following factors:
| ● | Our latest trading price on OTCQB and trading volumes; | |
| ● | Required bid and closing prices for listing on NASDAQ; | |
| ● | Our capital structure, including a reverse split of our common stock in the amount of for , effected on ; | |
| ● | General conditions of the capital markets at the time of this offering; and | |
| ● | Other factors deemed relevant. |
The offering price may not accurately reflect the value of our common stock and may not be realized upon any subsequent disposition of the shares.
The application of the “penny stock” rules could adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
The open-market trading of our common stock is currently subject to, and may also be subject upon trading on NASDAQ, to the “penny stock” rules. The penny stock rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1 million or annual income exceeding $200,000 or $300,000 together with their spouses). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser’s written consent to the transaction before the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability or decrease the willingness of broker-dealers to sell our common stock, and may result in decreased liquidity of our common stock and increased transaction costs for sales and purchases of our common stock as compared to other securities. Therefore, as long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
We do not intend to pay dividends.
We have not paid any cash dividends on our common stock since inception and we do not anticipate paying any cash dividends in the foreseeable future. Earnings, if any, that we may realize will be retained in the business for further development and expansion.
Concentration of Stock Ownership and Control.
Our executive officers and directors currently control approximately 44.1% of the common stock of the Company, and Checkmate Capital and its affiliates, one of our former debt holders and lead investors in the Series B round, control approximately 13.4% of the common stock of the Company. The Company has employee options, incentive stock warrants, preferred stock and convertible notes that could result in further dilution. We may conduct funding rounds in the future, much of which may utilize our common stock. In this regard, management, prior investors and future investors may control a significantly large amount of equity, and as a result, these stockholders acting together will be able to influence many matters requiring stockholder approval including the election of directors and other significant corporate transactions. This concentration of ownership may have the effect of delaying, preventing or deterring a change in control, and could deprive our stockholders of an opportunity to receive a premium for their shares of common stock as part of a sale of our company and may affect the market price of our stock.
| 21 |
The Company has Preferred Stock with additional priority rights.
The Company has two classes of preferred stock, which provide voting, approval, liquidation, conversion, and other rights that are senior to the common stock of the Company. The Company may also issue up to an additional 4,998,071 shares of blank-check preferred stock in the future. As a result, the preferred stockholders can exert significant influence over the Company and can dilute the financial interests of the common stockholders.
Series A preferred stockholders have rights to approve certain transactions, including additional debt, payment of dividends in certain scenarios, and other similar items, all voting together as a separate class. There are currently 480 Series A shares outstanding held by two institutional holders, convertible into approximately 4.2 million shares of common stock inclusive of accrued dividends. Series B preferred stock has liquidation preferences senior to the common stock; however, all such shares and accrued dividends will automatically convert into shares of common stock in connection with this offering. This will lead to dilution of your shares. On December 6, 2021, all holders of Series E-1 preferred stock agreed to exchange their preferred shares for 28,839,428 million shares of common stock, and Series E-1 preferred stock was retired prior to this offering.
As long as shares of preferred stock are outstanding, whether now or in the future, common stockholders may have reduced control over certain affairs of the Company, lower priority at the time of liquidation, and continued dilution of their voting and economic rights in the shares of common stock of the Company.
Our management will have broad discretion in the use of the net proceeds we receive in this offering and may not apply the proceeds in ways that increase the value of your investment.
Our management will have broad discretion in the application of the net proceeds we receive in this offering, including for any of the purposes described in the section titled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. If we do not use the net proceeds that we receive in this offering effectively, our business, results of operations, financial condition and prospects could be harmed, and the market price for our common stock could decline.
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As of December 17, 2021, we had four full-time employees and one part-time employee. As our drug development and commercialization plans and strategies develop, and as we transition into operating as a NASDAQ listed company, we expect to need additional operational, sales, marketing, managerial, financial and other personnel, as well as additional resources to expand our operations.
We currently rely, and for the foreseeable future will continue to rely, in large part on certain third party organizations, advisors and consultants to provide certain services, including substantially all aspects of regulatory approval, clinical trial management and manufacturing. There can be no assurance that the services of independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. In addition, if we are unable to manage our outsourced activities effectively or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all. If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, or we are not able to effectively build out new facilities to accommodate this expansion, we may not be able to successfully implement our business plan to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
We are a smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are a smaller reporting company as defined in the Exchange Act, and we will remain a smaller reporting company until the fiscal year following the determination that our voting and non-voting common stock held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenue is more than $100 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter. Smaller reporting companies are able to provide simplified executive compensation disclosure and have certain other reduced disclosure obligations, including, among other things, being required to provide only two years of audited financial statements and not being required to provide selected financial data, supplemental financial information or risk factors.
| 22 |
We may choose to take advantage of the available exemptions for smaller reporting companies. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our shares price may be more volatile.
If we fail to comply with the rules and regulations under the Sarbanes-Oxley Act, our operating results, our ability to operate our business and investors’ views of us may be harmed.
Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls. Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock. In addition, our efforts to comply with the rules and regulations under the Sarbanes-Oxley or new or changed laws, regulations, and standards may differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice. Regulatory authorities may investigate transactions disclosed in our “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and if legal proceedings are initiated against us, it may harm our business.
We have identified certain material weaknesses in our internal control over financial reporting and if our remediation of such material weaknesses is not effective, or if we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired.
In the course of preparing our financial statements, we have historically identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses related to limited accounting personnel and resources resulting into lack of segregation of duties, lack of experience in accounting for equity transactions, and lack of internal controls. We have concluded that these material weaknesses in our internal control over financial reporting occurred because, prior to this offering, we did not have a chief financial officer and our principal accounting officer has not had the necessary business processes, systems, personnel and related internal controls necessary to satisfy the accounting and financial reporting requirements of a public company.
On December 1 2021, we recruited an interim chief financial officer who has previously served in similar capacities at publicly traded companies and we believe that his expertise and oversight over preparation of financial statements and an understanding of SEC’s rules and regulations will allow for better segregation of duties in our financial reporting processes and improve our procedure for disclosure controls. The Company intends to recruit a permanent CFO in the future and continues to take appropriate measures with the goal to establish robust systems and processes for better internal control over financial reporting.
We believe we are taking necessary actions to substantially address each of the material weaknesses discussed above, and we plan to take additional steps to improve our accounting function. However, we may not be able to fully remediate these material weaknesses until it can be confirmed that such remedial measures have been operating effectively for a sufficient period of time. Further, we cannot assure you that any such actions will be sufficient to remediate the control deficiencies that led to our material weaknesses in our internal control over financial reporting or that they will prevent or avoid potential future material weaknesses. Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls and internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods.
Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until after we become an “accelerated” or “large accelerated” filer as those terms are defined in the Exchange Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed or operating. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we will eventually be required to include in our periodic reports that are filed with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on the NASDAQ.
Our financial statements may be materially affected if our estimates prove to be inaccurate as a result of our limited experience in making critical accounting estimates.
Financial statements prepared in accordance with GAAP require the use of estimates, judgments, and assumptions that affect the reported amounts. Actual results may differ materially from these estimates under different assumptions or conditions. These estimates, judgments, and assumptions are inherently uncertain, and, if they prove to be wrong, then we face the risk that charges to income will be required. In addition, because we have limited to no operating history and limited experience in making these estimates, judgments, and assumptions, the risk of future charges to income may be greater than if we had more experience in these areas. Any such charges could significantly harm our business, financial condition, results of operations, and the price of our securities. See “Note 3 – Summary of Significant Accounting Policies” under notes to our consolidated financial statements. For a discussion of the accounting estimates, judgments, and assumptions that we believe are the most critical to an understanding of our business, financial condition, and results of operations.
| 23 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective products, product approvals, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated products are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties and assumptions described under the sections in this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, including those set forth below:
| ● | our lack of an operating history; | |
| ● | the net losses that we expect to incur as we develop our business; | |
| ● | obtaining Federal Drug Administration (“FDA”) or other regulatory approvals or clearances for our technology; | |
| ● | implementing and achieving successful outcomes for clinical trials of our products; | |
| ● | convincing physicians, hospitals and patients of the benefits of our technology and to convert from current technology or other standards of care; | |
| ● | the ability of users of our products (when and as developed) to obtain third-party reimbursement; | |
| ● | any failure to comply with rigorous FDA and other government regulations; | |
| ● | securing, maintaining and defending patent or other intellectual property protections for our technology; and | |
| ● | availability of nuclear reactors to manufacture our products, transportation, handling, and disposal of radioactive drugs, changes in regulations of the Nuclear Regulatory Commission (NRC) or the Environmental Protection Agency (EPA) or similar state regulatory agencies. |
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time to time, and it is not possible for us to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
| 24 |
USE OF PROCEEDS
We estimate that the net proceeds from our issuance and sale of shares of our common stock in this offering will be approximately $ (or approximately $ if the underwriters exercise the over-allotment option in full), based upon an assumed public offering price of $ per share, and after deducting underwriting discounts and commissions and offering expenses payable by us.
A $1.00 increase or decrease in the assumed public offering price of $ per share would increase or decrease the net proceeds from this offering by approximately $ million, assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of 150,000 in the number of shares of common stock offered by us would increase or decrease our net proceeds by approximately $ million, assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for:
| ● | Approximately $ million for advancement of our current Phase 1 clinical trials of CycloSam® for the indication of bone metastasis, and possible commencement of an amended protocol clinical trial for the indication of primary bone cancer, including osteosarcoma; and research and development of other indications and radiopharmaceutical assets; and | |
| ● | the remainder for working capital and general corporate purposes. |
Changing circumstances may cause us to consume capital significantly faster than we currently anticipate. The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our development efforts and clinical trials, the commencement of clinical trials for additional indications or for new assets, FDA review and approvals of our drug candidate(s), economic and political effects of COVID-19 and government responses to it, and the overall economic environment. Therefore, our management will retain broad discretion over the use of the proceeds from this offering. We may ultimately use the proceeds for different purposes than what we currently intend. Pending any ultimate use of any portion of the proceeds from this offering, if the anticipated proceeds will not be sufficient to fund all the proposed purposes, our management will determine the order of priority for using the proceeds, as well as the amount and sources of other funds needed.
Pending our use of the net proceeds from this offering, we may invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and U.S. government securities.
| 25 |
DIVIDEND POLICY
We have never declared or paid any dividends on our common stock. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on various factors, including applicable laws, our results of operations, financial condition, future prospects and any other factors deemed relevant by our board of directors.
| 26 |
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of , 2021:
| ● | on an actual basis as of , 2021; | |
| ● | on a pro forma basis after giving effect to (i) the conversion of Series B preferred stock into an aggregate of million shares of common stock upon completion of this offering, and (ii) conversion of all outstanding convertible notes into an aggregate of shares of common stock upon completion of this offering; and | |
| ● | on a pro forma as adjusted basis after giving effect to (i) the pro forma adjustments set forth above; and (ii) the issuance and sale of shares of our common stock in this offering at an assumed public offering price of $ per share, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, as if the sale of the common stock had occurred on , 2021. |
The pro forma basis and pro forma as adjusted information set forth in the table below is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table in conjunction with the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| (In thousands, except share and per share data) | Actual | As
of , 2021 Pro Forma | Pro Forma As Adjusted | |||||||||
| Cash, cash equivalents and short-term investments | $ | $ | $ | |||||||||
| Debt: | ||||||||||||
| Convertible Notes | $ | $ | - | $ | - | |||||||
| Total Debt | ||||||||||||
| Convertible Series B preferred stock, USD $0.001 par value per share, issued and outstanding, actual; no shares issued and outstanding pro forma and pro forma as adjusted | ||||||||||||
| Stockholders’ equity (deficit): | ||||||||||||
| Common stock, par value USD $0.0001 per share; 305,000,000 shares authorized, shares issued and outstanding actual, shares issued and outstanding, pro forma, and shares issued and outstanding, pro forma as adjusted | ||||||||||||
| Additional paid-in capital | ||||||||||||
| Accumulated comprehensive income | ||||||||||||
| Accumulated gains (losses) | ||||||||||||
| Total stockholders’ equity (deficit) | ||||||||||||
| Total Capitalization | ||||||||||||
| (1) | A $1.00 increase or decrease in the assumed public offering price of $ per share of common stock would increase or decrease the amount of each of cash and cash equivalents and total stockholders’ equity by approximately $ , assuming that the number of shares of common stock offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase or decrease of in the number of shares of common stock offered by us would increase or decrease each of cash and cash equivalents and total stockholders’ equity by approximately $ million, assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and commissions and any estimated offering expenses payable by us. |
The number of shares of our common stock to be outstanding immediately after this offering is based on shares of common stock outstanding as of assuming the conversion of all outstanding Series B preferred stock and convertible notes into an aggregate of shares of common stock upon completion of this offering and excludes:
| ● | shares of common stock issuable upon the exercise of warrants outstanding as of ; | |
| ● | shares of common stock issuable upon the exercise of options to directors, employees and consultants under our 2016 Omnibus Equity Incentive Plan and other grants and awards approved by the board of directors (collectively the “Equity Incentive Plan”) of which are vested as of ; and | |
| ● | shares of common stock reserved for future issuance upon conversion of our Series A preferred stock. |
| 27 |
DILUTION
If you invest in our common stock, you will experience immediate and substantial dilution to the extent of the difference between the public offering price of our common stock and the as adjusted net tangible book value per share of common stock immediately after the offering. Net tangible book value per share of common stock represents the amount of our total tangible assets less our total liabilities, divided by the total number of common stock outstanding. Our historical net tangible book deficit as of , 2021 was $ or $ per share of common stock.
Our pro forma negative net tangible book value as of , 2021 would have been $ representing approximately $ per share of common stock. Our pro forma net tangible book value per share of common stock represents the amount of our total tangible assets less our total liabilities, divided by , the total number of common stock outstanding on , 2021, after giving effect to (i) the conversion of Series B preferred stock into an aggregate of shares of common stock upon completion of this offering, and (ii) the conversion of all of our outstanding convertible notes into an aggregate of shares of common stock upon completion of this offering.
After giving effect to the (i) pro forma adjustments set forth above, and (ii) our sale of shares of our common stock offered by us in this offering and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, assuming no exercise of the Underwriter’s over-allotment option, our pro forma as adjusted net tangible book value estimated at , 2021 would have been approximately $ million, representing $ per share of common stock. At the assumed public offering price for this offering of $ per share of common stock, this represents an immediate increase in historical net tangible book value of $ per share of common stock to existing stockholders and an immediate dilution in net tangible book value of $ per share of common stock to purchasers of common stock in this offering. Dilution for this purpose represents the difference between the price per share of common stock paid by these purchasers and as adjusted net tangible book value per share of common stock immediately after the completion of this offering.
The following table illustrates this dilution on a per share basis to new investors:
| Assumed public offering price per share | $ | |||
| Historical adjusted net tangible book value per share as of , 2021 | $ | |||
| Increase in net tangible book value per share attributable to new investors in this offering | $ | |||
| As adjusted net tangible book value per share after offering | $ | |||
| Dilution in tangible book value per share to new investors | $ |
The dilution information discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering to be determined at pricing. A $1.00 increase or decrease in the assumed initial public offering price of $ per share of common stock would increase or decrease our as adjusted net tangible book value per share of common stock after this offering by $ and the dilution per share of common stock to new investors by $ , assuming the number of common stock offered by us, as set forth on the cover page of this prospectus, remains the same, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of common stock we are offering.
An increase or decrease of in the number of common stock offered by us would increase or decrease our as adjusted net tangible book value after this offering by approximately $ million and the as adjusted net tangible book value per share of common stock after this offering by $ per share of common stock and would increase or decrease the dilution per share of common stock to new investors by $ , assuming the assumed public offering price remains the same, after deducting estimated underwriting discounts and estimated offering expenses payable by us.
The following table summarizes, on an as adjusted basis as of , 2021, the number of shares of our common stock, the total consideration and the average price per share by (i) existing stockholders and (ii) new investors acquiring our common stock in this offering at an assumed public offering price of $ per share of common stock, indicated on the cover page of this prospectus.
| Shares | Total Consideration | Average Price Per Share of Common | ||||||||||||||||||
| Number | Percent | Amount | Percent | Stock | ||||||||||||||||
| Existing stockholders | % | $ | % | $ | ||||||||||||||||
| New investors | % | $ | % | $ | ||||||||||||||||
| Total | % | $ | $ | % | $ | |||||||||||||||
The total number of common stock that will be outstanding after this offering is based on shares of common stock issued and outstanding as of , 2021 and includes (i) the conversion of Series B preferred stock into an aggregate of shares of common stock upon completion of this offering, and (ii) the conversion of all of our outstanding convertible notes into an aggregate of shares of common stock upon completion of this offering, but excludes:
| ● | shares of common stock issuable upon the exercise of warrants outstanding as of ; | |
| ● | shares of common stock issuable upon the exercise of options to directors, employees and consultants under our 2016 Omnibus Equity Incentive Plan and other grants and awards approved by the board of directors (collectively the “Equity Incentive Plan”) of which are vested as of ; and | |
| ● | shares of common stock reversed for future issuance upon conversion of our Series A preferred stock. |
To the extent that new options are granted under our equity benefit plans, there will be further dilution to investors purchasing common stock in this offering.
Except as otherwise indicated, the above discussion and tables assume no exercise of the underwriters’ option to purchase additional shares. If the underwriters exercise in full their option to purchase additional shares, our existing stockholders would own % and our new investors would own % of the total number of shares of our common stock outstanding upon the completion of this offering.
| 28 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which we have prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate estimates and judgments, including those described in greater detail below. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
As used in this “Management’s Discussion and Analysis of Financial Condition and Results of Operation,” except where the context otherwise requires, the term “we,” “us,” “our,” or “the Company,” refers to the business of QSAM Biosciences, Inc.
Overview
The Company’s business is the development of next-generation nuclear medicines for the treatment of cancer and related diseases. Our initial technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “New Technology”), a clinical-stage bone targeting radiopharmaceutical. The FDA cleared our IND in August 2021 to commence clinical trials of CycloSam® on patients with metastatic bone cancer, an open-label, dose escalating trial that is expected to last approximately two years and may result in sufficient data to move subsequently into a pivotal trial.
On April 20, 2020, we executed a Patent and Technology License Agreement and Trademark Assignment with IGL Pharma Inc. (“IGL”), through a newly created, wholly-owned subsidiary called QSAM Therapeutics Inc. (“QSAM Therapeutics”), which was mutually amended on November 24, 2021. The License Agreement provides QSAM Therapeutics with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and knowhow with respect to CycloSam®. The License Agreement also transfers to QSAM Therapeutics the rights to the product name CycloSam® for the technology, and provides QSAM Therapeutics a first right of refusal to license other technologies from IGL in the future.
On November 6, 2020, the Company entered into an Omnibus Separation Agreement (the “Separation Agreement”) with its unconsolidated investee, Earth Property Holdings LLC (“EPH”). Our board of directors approved the Separation Agreement in furtherance of its previously disclosed plan to secure new technologies and business opportunities in the broader biosciences sector, and to significantly reduce debt and liabilities of the Company and eliminate under-performing assets and agreements.
The Separation Agreement marked a discontinuance of the Company’s soil health operations (the “Legacy Business”) to focus solely on the development of its exclusively licensed radiopharmaceutical drug candidate, Samarium-153-DOTMP, aka CycloSam®, as well as other drug candidates that it may license or otherwise secure in the future. Revenue and expenses from the Legacy Business are presented on our consolidated financial statements as “Discontinued Operations.”
COVID-19
In March 2020, the World Health Organization declared COVID-19 a global pandemic and recommended containment and mitigation measures worldwide. We are monitoring this closely, and although operations have not been materially affected by the COVID-19 outbreak to date, the ultimate duration and severity of the outbreak and its impact on the economic environment and our business is uncertain. Accordingly, while we do not anticipate an impact on our operations, we cannot estimate the duration of the pandemic and potential impact on our business. In addition, a severe or prolonged economic downturn could result in a variety of risks to our business, including a possible delay in our ability to raise money. At this time, the Company is unable to estimate the impact of this event on its operations.
Results of Operations for the nine month period ended September 30, 2021 and 2020
For the nine month period ended September 30, 2021 and 2020, we recorded no revenue from continuing operations. Revenue from a management agreement and other Legacy Business operations have been reclassified in our Statement of Operations on our unaudited Condensed Consolidated Financial Statements as “discontinued operations.”
Our operating expenses for the nine months ended September 30, 2021 was $7,433,845 as compared to $1,264,625 for the nine months ended September 30, 2020. The difference of $6,169,220 was primarily due to non-cash stock-based compensation expense of approximately $6,292,531 related to stock issued for services and Series E-1 preferred stock and options to employees and directors, and $101,366 due to a warrant modification pursuant to a service contact that has been included in professional fees, and an increase in research and development expenses of $178,842, offset by a decrease in wages and related expenses of $305,158 due to a reduction in employees. Our other income (expense) changed as follows:
| For the nine months ended | ||||||||
| September 30, | ||||||||
| 2021 | 2020 | |||||||
| OTHER INCOME (EXPENSE) FROM CONTINUING OPERATIONS | ||||||||
| Financing costs including interest | (38,978 | ) | (431,790 | ) | ||||
| Change in fair value of convertible bridge notes | - | (1,666,422 | ) | |||||
| Other miscellaneous income | - | 5,000 | ||||||
| Gain on sale of equity method investment | 100,000 | - | ||||||
| Loss on debentures and accrued expenses converted to common stock | (390,068 | ) | - | |||||
| Gain on forgiveness of debt from Paycheck Protection Program | 142,942 | |||||||
| Loss on conversion of bridge notes including accrued interest and debt forgiveness | (744,505 | ) | (503,762 | ) | ||||
| Total Other Expense | $ | (930,609 | ) | $ | (2,596,974 | ) | ||
| 29 |
NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS
Net loss attributable to common shareholders for the nine months ended September 30, 2021 was $9,947,875 as compared to $3,761,001 for the nine months ended September 30, 2020. The net loss attributed to common stockholders includes a loss from discontinued operations that has been described in the unaudited condensed financial statements for the nine months ended September 30, 2021. In addition, the net loss attributed to common stockholders for the nine months ended September 30, 2021 includes a Series A and Series B preferred contractual dividends and deemed dividends, and in the 2020 period, Series A preferred contractual dividends and deemed dividends. The net loss attributable to common stockholders, basic and diluted, including continuing operations and discontinued operations for the nine months ended September 30, 2021 was a loss of $0.37 per share as compared to a loss of $1.64 per share in the 2020 period.
Financial Condition, Liquidity and Capital Resources
Net cash used in operating activities was $1,128,525 for the nine months ended September 30, 2021, which reflected our net loss during the period of $8,364,454, non-cash adjustments of $7,320,145, and a net increase in operating assets and a net decrease in liabilities of $84,216. The majority of non-cash adjustments consists of $6,292,531 of stock-based compensation, a gain from the Paycheck Protection Plan, the loss on conversion into common stock of the Bridge Notes and accrued interest of $744,505, and the loss on conversion into common stock of debentures and promissory notes with unrelated parties of $390,068. Net cash used by operating activities was $620,118 for the nine months ended September 30, 2020, which reflected our net loss during the period of $3,734,635, offset by the non-cash adjustments of $2,845,693 and the net increase in operating assets and liabilities of $268,823. The majority of non-cash adjustments consists of $1,666,422 in the fair value change of convertible bridge notes and the paid-in-kind interest on the convertible bridge notes of $427,360, in addition to the $503,762 loss incurred on the conversion of the bridge notes to common stock.
There was no cash provided by or used in investing activities during nine months ended September 30, 2021 or September 30, 2020.
Net cash provided by financing activities during the nine months ended September 30, 2021 was $2,187,508. This was primarily from the proceeds of $ 2,221,000 from the issuance of Series B preferred stock. Net cash provided by financing activities during the nine months ended September 30, 2020 was $621,315. The Company received proceeds from convertible notes payable and notes payable with related parties of $335,873, proceeds from notes payable of $142,500 and proceeds of $142,942 from the Paycheck Protection Program.
As of September 30, 2021, we had cash of $1,067,287 held at a large U.S. bank.
Based on our current strategy and operating plan, at the end of 2020 we needed to raise additional capital to support operations. We were able to raise over $3.3 million in equity and debt offerings in 2021 (approximately $1.1 million after the end of the third quarter of 2021); however, we expect our expenses to increase over the coming year as our technology enters into clinical trials, and we will need to raise additional capital in 2022. There is still substantial doubt about our ability to operate as a going concern. See “Note 2 – Basis of Presentation and Going Concern” in our unaudited Condensed Consolidated Financial Statements.
Series B Financing. In January 2021, the Company closed a Series B Convertible Preferred Stock private placement (the “Series B Offering”) and issued a total of 2,500 shares at a price of $1,000 per share, raising an aggregate amount of $2.2 million, inclusive of $156,000 in debt conversion and $23,000 from conversion of notes payable to directors. The Series B Offering, which commenced in 2020, was led by Checkmate Capital Group, LLC, a California based investment firm focused on biotechnology and other technology investments. The Company completed the offering primarily to advance its business of drug development including funding the Company’s upcoming clinical trials for its drug candidate CycloSam, as well as for general working capital and overhead.
| 30 |
The shares of Series B Preferred Stock are convertible into an aggregate of approximately 15.6 million shares of common stock of the Company, exclusive of accrued dividends, and have voting rights alongside common stockholders on an as-converted basis. In 2021, our Board approved a modification to the offering and issued to investors six-month, non-registered warrants to purchase an aggregate of up to 6.27 million shares of Common Stock at $0.35 per share; the expiration date which was extended on June 17, 2021 by board resolution from July 8, 2021 until September 30, 2021; and then again on September 22, 2021, the board extended expiration date to October 15, 2021, and reduced the exercise price from $0.35 per share to $0.25 per share. For the performance of its lead investor commitment in 2021, Checkmate Capital also earned a 12 month warrant convertible into 475,000 shares of Common Stock at $0.45 per share, which was not amended.
Funds received from the Series B Offering are expected to support operations through the end of 2021.
In the third quarter of 2021, 15 holders of an aggregate of 991 shares of Series B preferred stock converted their preferred shares into 6,525,378 shares of common stock, which included $53,061 of accrued dividends. All remaining Series B preferred shares automatically convert into common stock upon the completion of a $5 million qualified offering or the listing of our common shares on Nasdaq.
Cash and Working Capital
We have incurred negative cash flows from operations since inception. As of September 30, 2021, we had an accumulated deficit of $25,837,433 and working capital surplus of $788,234.
Critical Accounting Policies and Estimates
Our financial statements are prepared in conformity with U.S. generally accepted accounting principles (GAAP). Disclosures regarding our Critical Accounting Policies are provided in “Note 3 – Summary of Significant Accounting Policies” under our notes to our consolidated financial statements.
Results of Operations for the years ended December 31, 2020 and 2019
For the years ended December 31, 2020 and 2019, we recorded no revenue from continuing operations. Revenue from a management agreement and other Legacy Business operations have been reclassified in our Statement of Operations on our Consolidated Financial Statements as “discontinued operations.”
For the year ended December 31, 2020, we recorded a net loss from continuing operations of $4,898,123, an increase of $4,180,144 (582%) from our net loss from continuing operations of $717,979 for the same period in 2019. Basic and diluted net loss per share was $0.88 and $0.34 for the years ended December 31, 2020 and 2019, respectively. The primary reasons for the increase in the net loss for 2020 compared to 2019 were an increase of $194,702 in operating expenses, as well as an increase in the loss recognized due to the change in the fair value of the convertible bridge notes of $4,228,113 and the loss of $834,903 on convertible debt and other liabilities converted to equity; offset slightly by a decrease of $50,475 in financing costs. Expenses from the Legacy Business operations have been reclassified in our Statement of Operations on our Consolidated Financial Statements as “discontinued operations.”
| 31 |
The majority of the $194,702 increase in operating expenses was due to the research and development expenses of $362,456 and higher general and administrative expenses of $103,955 (246%); offset by lower payroll and related expenses of $277,222 (43%) to $372,938 in the year ended December 31, 2020 from $650,160 for the year ended December 31, 2019.
Financial Condition, Liquidity and Capital Resources
For year ended December 31, 2020, cash increased by $7,826 from $478 as of December 31, 2019 to $8,304 as of December 31, 2020. This increase was primarily the result of cash provided by financing activities of $750,725, offset by cash used in operating activities of $742,899.
Net cash used by operating activities was $742,899 for the year ended December 31, 2020, which reflected our net loss during the period of $4,862,683, non-cash adjustments of $3,741,254, and a net increase in operating liabilities of $378,530. The majority of non-cash adjustments consists of a $3,170,236 loss on change in fair value of the Bridge Notes primarily as a result of our higher stock price and proximity to maturity, $484,031 in paid-in-kind interest related to the Bridge Notes, the loss on conversion into common stock of the Bridge Notes and accrued interest of $495,320, the loss on conversion of accrued salary and bonus, director fees and promissory notes with related parties of $271,210 and $258,667 of stock based compensation; offset by the gain on forgiveness of promissory notes and accrued expenses.
Our net loss resulted largely from the non-cash items of the change in fair value of the Bridge Notes and loss on convertible debt and other liabilities that were converted to equity.
Net cash used in investing activities during year ended December 31, 2020 consisted of $0.
Net cash provided by financing activities during the year ended December 31, 2019 consisted of additional notes from EPH, a related party, of $788,500 and additional convertible Bridge Note of $30,000.
At December 31, 2020, our cash totaled $8,304. Our cash is currently held at large U.S. banks.
Based on our current strategy and operating plan, at the end of 2020 we needed to raise additional capital to support operations. This was accomplished in the first quarter of 2021; however, at the end of 2020 there was still substantial doubt about our ability to operate as a going concern. See “Note 2 – Basis of Presentation and Going Concern” in our consolidated financial statements.
Bridge Note Financing. The Company issued a total of $2,851,908 in Convertible Promissory Notes (the “Bridge Notes”) during 2017, 2018 and 2019. Proceeds from the Bridge Notes were used to for the Company’s legacy business. As of December 31, 2020, a total of $1,965,030 in principal, as well as $964,525 in accrued interest on the Bridge Notes were converted into approximately 13.3 million shares of common stock. As of March 31, 2021, the remaining $1,447,312 of principal and interest was converted into 6,578,702 shares of common stock, and no Bridge Notes currently remain outstanding.
Additional Debt Settlement. As of December 31, 2020, officers, directors and related parties of the Company converted $346,867 in accrued compensation, unpaid bonuses and other notes payable into 1,576,668 shares of common stock. This is in addition to the $993,985 in liabilities owed to EPH that were forgiven pursuant to the Separation Agreement, and additional $117,659 note principal and accrued interest converted into 534,814 shares of common stock with an unrelated party.
| 32 |
Company’s Prior Series A Preferred Stock Financing
We sold $600,000 of our Series A 6% Convertible Preferred Stock (the “Series A Preferred Stock”) to two separate accredited investors in November 2015 and January 2016, respectively. The Series A Preferred Stock bears a 6% dividend per annum, calculable and payable per quarter in cash or additional shares of common stock as determined in the Certificate of Designation. The Series A Preferred Stock was originally convertible at $6.50 per share at the discretion of the holders and contains price protection provisions in the instance that we issue shares at a lower price, subject to certain exemptions. The price has been reset several times since. Most currently, as a result of the closing of the Series B preferred stock offering in January 2021, the conversion price was reset to $0.16 per share. Series A Preferred Stock holders also received other rights and protections including piggy-back registration rights, rights of first refusal to invest in subsequent offerings, security over our assets (secondary to our debt holders), and certain negative covenant guaranties that we will not incur non-ordinary debt, enter into variable pricing security sales, redeem or repurchase stock or make distributions, and other similar warranties. The Series A Preferred Stock was redeemable on July 1, 2019 per a March 2019 modification and is currently in technical default. The holders hold the right in default to foreclose on assets of the Company. The Series A Preferred Stock has no voting rights until converted to common stock. The Series A Preferred Stockholders also received warrants in connection with their investment, all of which had expired in January 2021.
All promissory notes and shares in these offerings were sold pursuant to an exemption from the registration requirements of the Securities Exchange Commission under Regulation D to accredited or sophisticated investors who completed questionnaires confirming their status. Unless otherwise described in this Quarterly Report, reference to “restricted” common stock means that the shares have not been registered and are restricted from resale pursuant to Rule 144 of the Securities Act of 1933, as amended.
Cash and Working Capital
We have incurred negative cash flows from operations since inception. As of December 31, 2020, we had an accumulated deficit of $15,911,895 and working capital deficit of $4,168,618.
Critical Accounting Policies
Our financial statements are prepared in conformity with U.S. generally accepted accounting principles (GAAP). Disclosures regarding our Critical Accounting Policies are provided in “Note 3 – Summary of Significant Accounting Policies” under notes to our consolidated financial statements.
Off-Balance Sheet Arrangements
The Company did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of December 31, 2020.
| 33 |
OUR BUSINESS
Overview
We are developing next-generation nuclear medicines for the treatment of cancer and related diseases. Our initial technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “New Technology”), a clinical-stage bone targeting radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. We believe improvements in formulation and manufacturing from a prior FDA-approved drug utilizing the same radioisotope (Quadramet®) has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability. Samarium-153 and DOTMP form a highly stable complex, which we believe, when used either as a monotherapy or in combination with other more widely used treatments such as external beam radiation, may demonstrate meaningful disease modifying results in primary and metastatic bone cancer. Ultimately, we may seek to further develop and commercialize CycloSam® for one or more market indications or license the technology to a larger pharmaceutical partner.
In August 2021, the Food & Drug Administration (FDA) cleared our Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas. We initiated this trial at our first site (Houston, TX) in November 2021 and we seek to commence dosing patients in this open-label, dose escalating study by the first quarter of 2022. Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults. Although patients with osteosarcoma or Ewing’s sarcoma are eligible to participate in our initial Phase 1 trials, we anticipate filing an amended protocol to our current commercial IND application in 2022 to commence clinical trials specifically for these primary, pediatric bone cancers. In May 2020, CycloSam® was also utilized in a Single Patient Investigational New Drug for Emergency Use at the Cleveland Clinic. We believe the study we conducted at the Cleveland Clinic showed promising safety results in connection with a bone marrow ablation procedure, including patient tolerability at high dosages. To date, CycloSam® has completed animal studies in both small and large animals, including treating bone cancer in patient dogs at a university veterinary clinic.
Current Development Stage of CycloSam® for Target Indications. Our initial IND to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas has been cleared by the FDA, and we seek to commence dosing patients by the first quarter of 2022. Patients with primary bone cancer, such as osteosarcoma or Ewing’s sarcoma, are eligible to participate in our initial Phase 1 trials; however, we anticipate filing an amended protocol to our current commercial IND application in 2022 to commence clinical trials specifically for these primary, pediatric bone cancers. Our initial Phase 1 trial is an open label, dose escalating study of approximately 20 patients.
| 34 |
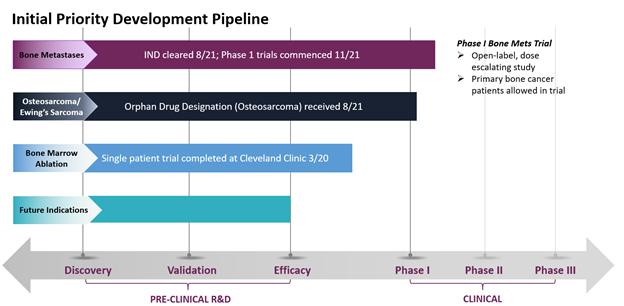
What is CycloSam®. CycloSam® is a targeted, bone seeking radiopharmaceutical that combines the beta-emitting radioisotope Samarium-153 (153Sm) with a chelating agent, DOTMP (1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetramethylenephosphonic acid). Samarium-153 is acquired from a nuclear reactor from a third party and the chelating agent is supplied in the form of kits. Chelating agents are organic compounds capable of linking together metal ions to form complex ring-like structures. This combination forms a stable complex which delivers a radioactive dose to sites of rapid bone mineral turnover such as bone cancers and tumors. CycloSam® has a physical half-life of 46 hours (radiation decreases by half in 46 hours) and emits both medium-energy beta particles that produce the therapeutic effect, and gamma photons that make it possible to take images of the skeleton and locate and characterize the size and nature of tumors. The use of radioisotopes to both diagnose and treat disease is called “theragnostics” and is a rapidly growing area of medical discovery.
How we believe CycloSam® Works – Mechanism of Action & Administration. CycloSam® utilizes a chelating agent called DOTMP that seeks out bone locations of high mineral turnover, typical in cancer cells and tumor growth. The DOTMP part of the molecule is taken up by calcium turnover locations in bones and carries the radioactive “payload” along with it. The radioisotope Samraium-153 emits radiation as it decomposes in the form of beta particles. Approximately 50% of the radioactivity concentrates in bone mineral with a very high lesion-to-normal bone ratio. We believe this provides a radiation dose to the adjacent tumor cells. The absorbed radiation dose produces the presumed therapeutic effect to the tumor, killing the cancer cells or slowing their growth by damaging their DNA. Our pre-clinical studies and single patient IND performed at the Cleveland Clinic has demonstrated that the remaining half of the administered activity is rapidly excreted through the kidneys.
Generally, radiation therapy does not immediately kill cancer cells and more than one treatment is expected to eradicate a tumor, dramatically reduce its size, or slow its growth. CycloSam® has a short half-life of 46 hours and is rapidly eliminated from the body. This avoids an undesirable radioactive buildup in healthy tissues and organs when used in multiple treatments, which we believe, is an important feature of CycloSam® over predecessor drugs. CycloSam® has also not demonstrated saturation of the bone sites in animal studies, which supports a multi-dosage treatment regimen. Additionally, we believe that high dosages may be administered for ablating the marrow in patients that may require procedures such as stem cell transplants.
The final drug product of CycloSam® is prepared from DOTMP kits and 153SmCl in 0.1 N HCl at a nuclear pharmacy local to the patient administration site. The final drug product is then delivered to the physician for use as an intravenous (IV) injection.
| 35 |
How is CycloSam® Made – Method of Manufacturing. CycloSam® uses a patented, low-specific-activity Samarium-153 which is produced in the lower flux region (beryllium reflector) of the nuclear reactor and can be accessed with a pneumatic tube on a daily basis. Once prescribed by radiation oncologists and nuclear medicine physicians, we order the radioisotopes from Missouri University Research Reactor (MURR) to be sent overnight to an onsite or nearby (to the patient) nuclear pharmacy to be compounded with a DOTMP “cold kit” and delivered to the treating physician for administration. While CycloSam® is still in clinical development, we believe that we have already established an efficient and cost-effective manufacturing process and supply chain, allowing the clinician to treat the patient within approximately three days from order.
The DOTMP “cold kit” is patent pending, and developed by IsoTherapeutics LLC, the inventors of CycloSam®. Although we believe the IsoTherapeutics cGMP manufacturing facility has the capacity to manufacture sufficient supply for our initial rollout, we are concurrently securing secondary manufacturing partners for the kits. MURR has been our source of Samarium-153 used in both our animal studies and the Single Patient IND for Emergency Use at the Cleveland Clinic, and MURR has verbally committed to supply us Samarium-153 in the future. Although we expect MURR to be our primary supplier of Samarium-153 in the U.S. subject to definitive agreements, and we believe they have the capability to produce our requirements of Samarium-153 on a commercial scale for U.S. distribution, we plan to qualify additional suppliers in 2022 as part of our supply chain and general business risk diversification strategy.
What are CycloSam®’s competitive advantages. We believe CycloSam® has competitive advantages over current radiopharmaceutical offerings in the marketplace. Such potential competitive advantages include:
| ● | CycloSam®’s radioisotope, Samarium-153, emits beta particles that travel farther than alpha particles with what we believe is sufficient energy to slow the growth or decrease the size of target cancer cells. We believe beta particles penetrate bone matter deeper than the alpha emitting radiopharmaceuticals currently in the marketplace and may be more effective in treating tumors that form in or metastasize to bones. | |
| ● | CycloSam®’s delivery agent, DOTMP, compared to other chelating agents such as EDTMP used in Quadramet®, has shown in animal and other pre-clinical testing to have a high bone binding affinity allowing for the maximum delivery of the radioactive “payload” adjacent to the tumor without saturation of the bone, as observed from our pre-clinical trials. | |
| ● | Our method of manufacturing Samarium-153 compared to Quadramet®, has shown in our pharmacopeial limits studies to produce a 30-fold reduction in levels of long-lived radioactive impurities, mainly Europium-154. We believe this may mitigate toxicity issues with the patient. | |
| ● | Our initial studies show CycloSam® has fewer toxicities and a short 46 hour half-life that may allow for more frequent and repeated dosing of our radiopharmaceutical. We believe this may have a greater ability to slow or reverse tumor growth. | |
| ● | We believe we have in place an efficient and cost-effective manufacturing process and established distribution system that may in the future allow for 24/7 availability and enable the clinician to order and have the treatment delivered to the patient within approximately three days. |
| 36 |
The competitive advantages we believe to be important to CycloSam® are based on pre-clinical animal and other studies including our single patient IND at the Cleveland Clinic. We cannot be sure that our technology will perform similarly in clinical trials with multiple human patients. Failure to achieve these competitive advantages could negatively affect our ability to achieve FDA approval as a new drug, or our ability to market CycloSam® as a treatment for bone cancer.
Background of Radiopharmaceuticals and Medical Isotopes
Radiation is one of the most widely used treatments for cancer, with approximately 50% of all cancer patients receiving radiation therapy during their course of treatment [Source: Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and Radiation Therapy: Current Advances and Future Directions. Int J Med Sci 2012; 9(3):193-199. doi:10.7150/ijms.3635]. A major limitation of some forms of radiation treatments, such as external beam therapy, is that radiation cannot be delivered with enough precision to prevent collateral damage to healthy tissue. Radiopharmaceuticals seek to overcome these limitations by delivering the tumor-killing power of radiation directly to tumor cells while sparing healthy tissue. This also expands the potential therapeutic benefit to a broader array of cancer type and stages, including metastatic disease.
To create radiopharmaceuticals, radiation emitting medical isotopes are typically attached to targeting molecules and administered via intravenous injection. Once administered, the radiopharmaceuticals selectively target tumor characteristics that are unique to, or preferentially expressed on, cancer cells. In the case of CycloSam®, we attach Samarium-153 to a chelating agent called DOTMP. This chelator seeks out and targets sites of high bone mineral turnover adjacent to cancer cells that have formed in or metastasized to the bone.
Isotopes are atoms of the same element that have a different number of neutrons, but the same number of electrons and protons. Every isotope has its own unique chemical and physical properties that determine the best application for use in radiopharmaceuticals. These properties include half-life, or the time over which half of the radioactive element has transformed to a non-radioactive state, the type of radiation emitted, such as alpha particles or beta particles, the energy of the emitted radiation, and the decay chain, which is the process by which the isotope turns into other radioisotopes before becoming a stable isotope. Another important consideration in making radiopharmaceuticals is the accessibility and availability of the desired isotope.
One of the key differences between radioisotopes is the method in which energy is released during decay from the unstable radioactive form to a stable, non-radioactive isotope. Some radioisotopes decay by releasing beta particles, which are very small electrons that can travel relatively far distances. Samarium-153 releases beta particles. Other radioisotopes decay by releasing alpha particles, which are much larger and heavier, and are higher in energy compared to beta particles, but travel much shorter distances. Although, both alpha and beta particles cause damage to the DNA of tumor cells resulting in tumor cell death, there are distinct differences to each type of decay.
Because beta particles can travel to more distant tumor cells not in direct contact with the delivery point of the radiopharmaceutical, we believe that beta-emitters may be better suited to the tumor environment, specifically those that have formed in or metastasized to bones. This is often the case with tumors that have infiltrated deep into the bone. Alpha-emitting radioisotopes can deposit two to three times more energy to tumor cells compared to beta-emitters, but can only travel two to three cell lengths. This quality may make alpha-emitters more suitable for non-tumor cancers, such as leukemia. With the option of several different alpha- and beta-emitting isotopes for radiopharmaceutical development and the emergence of additional promising radioisotopes, each with its own unique properties, we believe that both alpha-emitting and beta-emitting radioisotopes will continue to be at the forefront of radiopharmaceutical treatment.
Two of the earliest targeted radiopharmaceuticals, Bexxar and Zevalin, are beta-emitting therapies connected to antibodies for the treatment of CD20 positive lymphomas. We believe that despite receiving approval from the FDA, Bexxar and Zevalin struggled commercially. Both required specialized, lead-lined rooms for administration which limited the number of sites that could deliver the treatment. In addition, because medical oncologists had to manage the patients while nuclear medicine physicians administered the therapies, clinical uptake was hampered by burdensome reimbursement, logistics, and supply chain issues. These challenges limited the commercial success of these first-generation radiopharmaceuticals.
| 37 |
Since then, several successful radiopharmaceuticals were approved by the FDA. The first and only approved alpha-emitting therapy is Xofigo® (Bayer), a salt of radium that naturally localizes to regions where cancer cells are infiltrating bone. Xofigo® was approved in 2013 for the treatment of bone metastases associated with prostate cancer. Unlike some of the first-generation targeted radiopharmaceutical therapies, Xofigo® utilizes centralized manufacturing, can be administered in typical oncology suites and has overcome reimbursement challenges. Xofigo® has been widely adopted and used in over 1,000 sites in the United States alone and achieved peak sales of close to $500 million in 2017 according to Bayer’s 2018 annual report, although the sales dropped to $299 million in 2020 per the same report. Another next-generation targeted radiopharmaceutical therapy that was recently approved is Lutathera® (Novartis), a beta-emitting therapy. Lutathera® was the first FDA-approved radiopharmaceutical to use lutetium-177. Annual worldwide sales of Lutathera® reached $445 million in 2020, as reported in a third party article, just two years after its initial FDA approval for only a subset of neuroendocrine cancers.
Radiopharmaceutical Market
The radiopharmaceutical market is projected to reach $13.8 Billion by 2028 from $7.6 Billion in 2021, according to a published study by The Insight Partners: “Radiopharmaceuticals Market to 2028 – Global Analysis and Forecast – by Type, Product Type, Application, and End User.” Overall, the market is expected to grow at a CAGR of 9.0% during 2021–2028. North America dominates the global radiopharmaceuticals market, which is attributed to the prevalence of chronic disorders and the presence of supportive government plans for the development of research regarding radiopharmaceuticals. Based on type, the radiopharmaceuticals market is bifurcated into diagnostic nuclear medicine and therapeutic nuclear medicine. The diagnostic nuclear medicine segment is holding a larger share of the market and is anticipated to register a higher CAGR during 2021–2028. By application, the market is segmented into oncology, cardiology, neurology, and others, with the oncology segment holding the largest market share in 2021.
Another study published in 2019 in Radiotherapeutics and Radiodiagnostics shows similar growth, but with therapeutics experiencing the highest CAGR from 2020 to 2025, and the overall market reaching over $13.8 Billion by 2025:
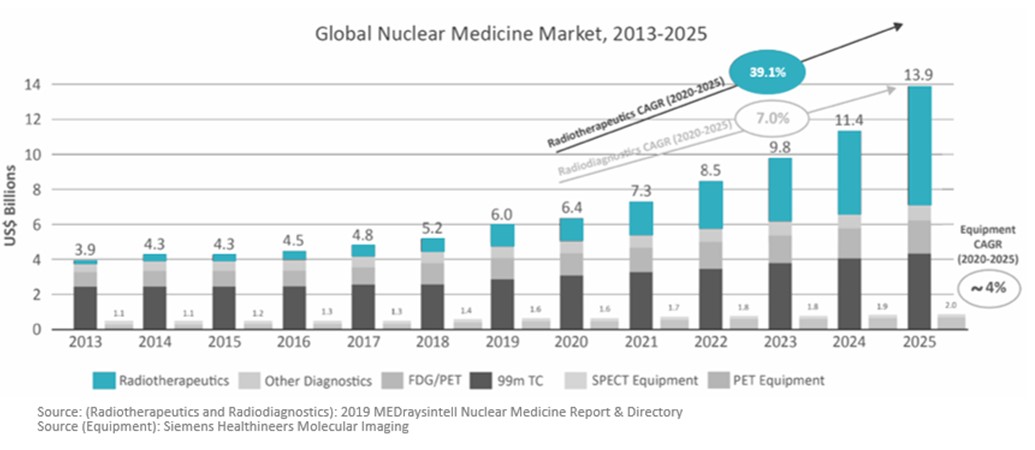
Overall, we believe that the recent technological developments in radiopharmaceuticals, including promising new alpha-emitting candidates, will continue to provide supportive tailwinds to this sector. As a result, management believes that it is well positioned to capitalize on these future opportunities.
History of CycloSam® development and past studies and trials
The Company has an exclusive worldwide patent and technology License Agreement for CycloSam®. The New Technology was developed at IsoTherapeutics Group, LLC (“IsoTherapeutics”) by its founders Jim Simone, PhD and R. Keith Frank, PhD (the “Inventors”). The Inventors also developed one of the first commercial radiopharmaceuticals on the market, Quadramet®, approved by the FDA in 1997 for pain palliation. Drs. Simone and Frank each have over 30 years of experience in radiopharmaceuticals publishing more than 100 papers and authoring over 60 patents in the field. The Inventors spent much of their careers at Dow Chemical Company prior to divestiture of its radiopharmaceutical business. According to the Inventors, CycloSam® was developed to address the shortcomings of other radiopharmaceuticals, including Quadramet, such as toxicity, saturation effects, long-lived impurities, and supply-chain complexities.
| 38 |
Prior generation Quadramet vs Improvements in CycloSam®. CycloSam® is a second-generation bone-seeking radiopharmaceutical based on Quadramet. Although Quadramet was clinically proven to be effective for pain palliation associated with metastatic bone cancer, the toxicity of the drug made repeat doses undesirable. Therefore, Quadramet’s use has been limited to pain control, not tumor reduction, elimination or disease modification. The loose binding affinity of Quadramet’s chelating agent, EDTMP, means the ratio of chelant to Samarium-153 is extremely high (~300:1). This resulting problem of bone saturation prohibits usage of Quadramet in high doses required for treatment of bone cancer or bone marrow ablation. Additionally, high levels of impurities from the Samarium-153 production, namely Europium-154, made repeated dosing of Quadramet undesirable. Lastly, Quadramet faced supply-chain and distribution limitations because the Samarium-153 it uses could only be accessed from the reactor once per week. We believe that because of these challenges, Quadramet demonstrated limited market success, and to our knowledge, was recently discontinued by its manufacturer and distributor. We believe CycloSam® overcomes these inherent limitations of Quadramet in terms of toxicity, usage, and availability.
The Vienna Protocol – Precedent of Efficacy. In August 2011, Dr. Helmut Sinzinger published a study in the Quarterly Journal of Nuclear Medicine and Molecular Imaging, which demonstrated that despite the described limitations of Samarium-153 EDTMP (Quadramet®), it could still be used to effectively treat bone metastasis.
The Vienna Protocol, as it was labeled, was based on a 550 patient study developed by Dr. Sinzinger to deliver therapeutic doses of Quadramet® on a periodic low dose basis balancing hematological toxicity and europium buildup with clinical results. The specific regimen used very low doses of the predecessor drug (30 mCi) on an outpatient basis. The treatment was administered at three month intervals during the first year, followed by another five treatments at six month intervals, then five therapies at nine month intervals, and then annually indefinitely. The dosing schedule was driven by hematological concerns and constant monitoring was required.
During Dr. Sinzinger’s trials, a wide range of positive clinical responses were seen including arrested tumor growth and even regression of the cancer in the bone. Some patients were treated for over five years exhibiting significant clinical response. While effective, this regimen required significant time on the part of both the physician and patient both of which were considered overly burdensome. Although this study was well published and the efficacy results were promising, wide clinical adoption did not occur due to the overall effort that was required to deliver a true therapeutic dose while avoiding the toxicity issues. Quadramet was never approved by the FDA for the treatment of bone cancer, but rather, just for pain management associated with the disease.
Improvements of CycloSam® over Predecessor Drug
CycloSam® is a new, advanced generation Samarium-153 drug with a dramatically different clinical profile than Quadramet®. By producing the Samarium-153 in a different part of the nuclear reactor, the decay by-product Europium has shown in studies to be nearly non-existent, thus eliminating long-term buildup concerns [Source: IsoTherapeutics Group. (2021). Preparation and Stability of CycloSam® Sm-153-DOTMP. (Report No. QSM-1)]. Secondly, the superior binding affinity of the new chelating agent, DOTMP, means more energy can be delivered to the target, thus minimizing off-target concerns. Further, the method of harvesting the patented low specific activity Samarium-153 means it can be accessed on a daily basis, compared to weekly for Quadramet®, at a reduced cost. We believe that all of these clinical and manufacturing improvements were achieved without any reduction in either the tumor killing power of Samarium-153 or its ability to travel deep into the bone tumor.
The following table illustrates the expected improved clinical profile of CycloSam® over Quadramet®:
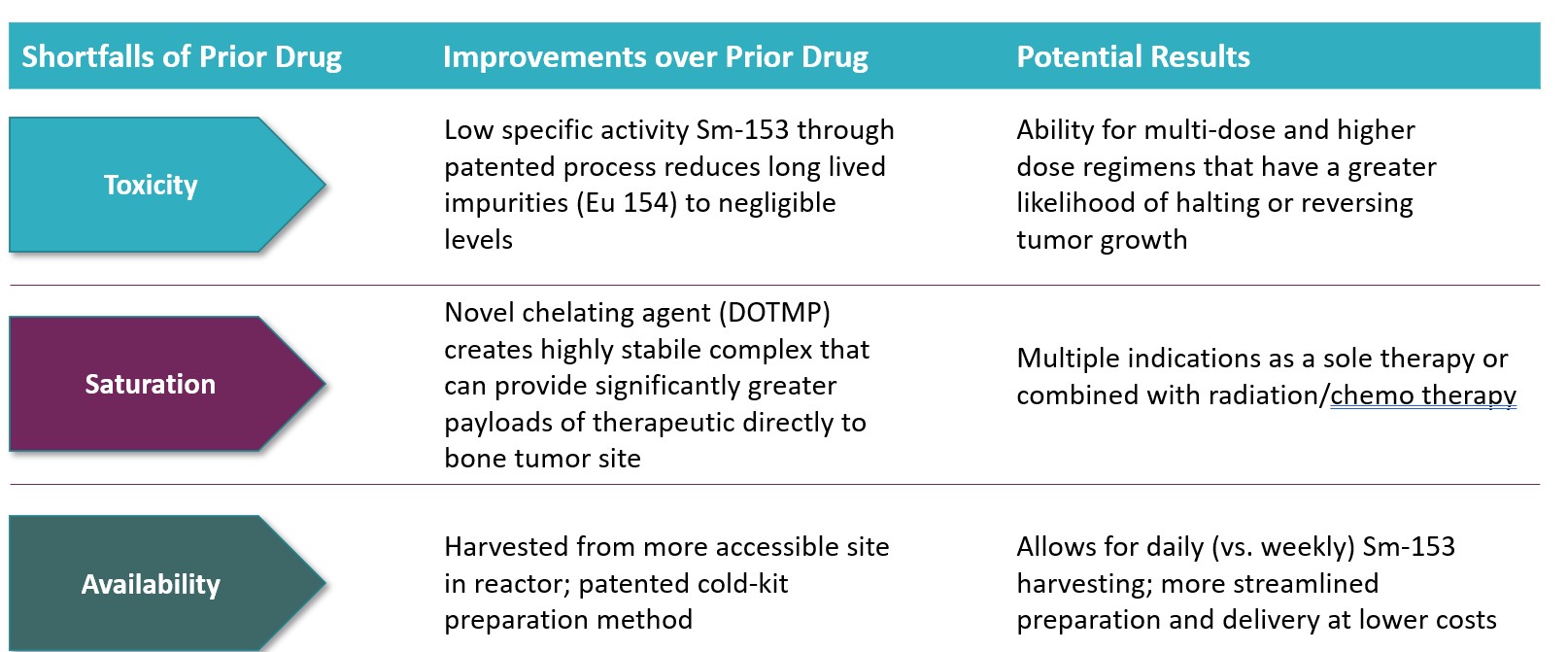
Potential Market Indications for CycloSam®.
CycloSam’s therapeutic profile and presumed advantages over other radiopharmaceuticals, including Quadramet, translate to several potential key market indications as detailed in the following table:
| Market | Estimated New Cases Diagnosed Annually (US) | |||
| Bone Metastases (Breast, Prostate, Lung) | 400,000 | |||
| Other Primary Bone Cancers | 2,400 | |||
| Primary Bone Cancer – Osteosarcoma | 1,000 | |||
| Bone Marrow Ablation | 15,000 | |||
| Primary Bone Cancer – Ewing’s Sarcoma | 200 | |||
Source: American Cancer Society estimates of new cases reported each year in the United States. Data as of July 2020.
Bone metastases arise in about 5% of all types of cancer, 29% of patients with multiple myeloma (15,000), 16% of lung (37,000), 6% of prostate (48,000) and 7% of breast cancers (70,000). Roughly 70% of patients with bone metastases will experience bone pain, and many are at risk for skeletal-related events including fracture and spinal cord compression. The total annual cost for treatment of metastatic bone disease is approximately $12.7 billion or 17% of the total of $74 billion that was spent on direct medical costs of these cancers [Source: Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007 Jun 1;109(11):2334-42. doi: 10.1002/cncr.22678. PMID: 17450591]. In addition to metastatic bone cancers, according to the National Institute of Health SEER, there are approximately 14,000 people living with osteosarcoma in the US at any one time [Source: Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007 Jun;459:40-7. doi: 10.1097/BLO.0b013e318059b8c9. PMID: 17414166, and National Cancer Institute: Surveilance, E., and End Results Program Cancer Stat Facts: Bone and Joint Cancer, <https://seer.cancer.gov/statfacts/html/bones.html> (2020)] and their cost of care is estimated to exceed $100,000 per patient [Source: American Cancer Society. Key Statistics About Bone Cancer].
| 39 |
Metastatic bone cancer is currently incurable, and therefore palliation and arrest or deceleration of the progress of disease are important near-term goals. Quadramet® (Samarium-153-EDTP) and MetastronTM (89Sr chloride) were approved by the FDA for pain palliation resulting from osteoblastic bone metastases, but their widespread acceptance and use is hampered by concern about the perceived risk of myelosuppression when administered concurrently with chemotherapy. Xofigo, an alpha particle emitter, was approved in May 2013 and initially was expected to capture significant market share rapidly; however, the product has only recently proven market success after many additional clinical trials.
Osteosarcoma is the most common childhood and adolescent/young adult (ages 15-39) primary high-grade bone malignancy [Source: Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian J Med Paediatr Oncol. 2017;38(1):33-43. doi:10.4103/0971-5851.203513]. Patients can often have metastatic cancer at diagnosis, and metastasis to the lungs is often fatal for these patients. For patients who develop or present with metastatic cancer in this diagnosis, the 5-year survival rate is 66% [Source: Osteosarcoma - Childhood and Adolescence - Statistics.” Cancer.Net, 30 Sept. 2021, https://www.cancer.net/cancer-types/osteosarcoma-childhood-and-adolescence/statistics]. Osteosarcoma standard-of-care usually involves chemotherapy which has substantial negative side effects, or drastic surgeries such as limb salvage or amputation. Osteosarcoma is relatively resistant to External Beam Radiation Therapy (EBRT), and currently approved radiopharmaceutical therapeutics fall short due to myelotoxicity and long-lived radioactive impurities. There is a tremendous unmet need for a better treatment that is more efficacious against pediatric osteosarcoma and better tolerated by patients.
Preclinical and Clinical Studies
Preclinical Studies. Preclinical toxicology studies of CycloSam® in rats and dogs have shown that a single intravenous dose of non-radioactive Samarium-153 DOTMP elicited no significant systemic toxicity. Skeletal uptake has also been studied in rats over a wide range of doses to determine whether Samarium-153 DOTMP displays a similar saturation effect which has been observed in studies of Samarium-153 EDTMP (aka Quadramet). In the rat saturation study, no statistically significant difference was found in uptake as a function of increased dosage of Samarium-153 DOTMP.
In addition to rat and dog toxicological studies, a proof-of-concept study was conducted in ten dogs with spontaneously occurring bone cancer treated with 1-2 mCi/kg of Samarium-153 DOTMP. Treatment was well tolerated with seven dogs treated at a dose of 1 mCi/kg and one dog treated with 2 mCi/kg who did not experience a dose limiting toxicity. One dog treated with 2 mCi/kg and one dog treated with 2.3 mCi/kg experienced grade 4 asymptomatic thrombocytopenia and neutropenia; which refers to a manageable depressed level of platelets and neutrophils in the blood. Results from these preclinical studies suggested Samarium-153 DOTMP has potential as a therapeutic agent in the treatment of primary bone cancer and metastatic bone disease.
Safety/Tox Studies. Non-radioactive CycloSam® has been through a full-scale 14-day, acute toxicological study in both rats and dogs. This study was designed to determine the toxicokinetics of the product at four different dose levels that are higher than expected to be used in the current clinical trials. The studies showed no systemic toxicity in either of the species with a single intravenous administration of CycloSam® (non-radioactive). Some mild to moderate allergic-like responses were seen in dogs at the highest dose, which is much higher than would be expected for clinical use.
Non-clinical testing. Rat and rabbit pharmacology and targeted bone pharmacology studies have been undertaken and published in both patents and in the literature for Samarium-153-DOTMP and their preclinical results demonstrated significant skeletal uptake fractions. We believe these studies suggest Samarium-153-DOTMP has promise as a bone seeking radiopharmaceutical.
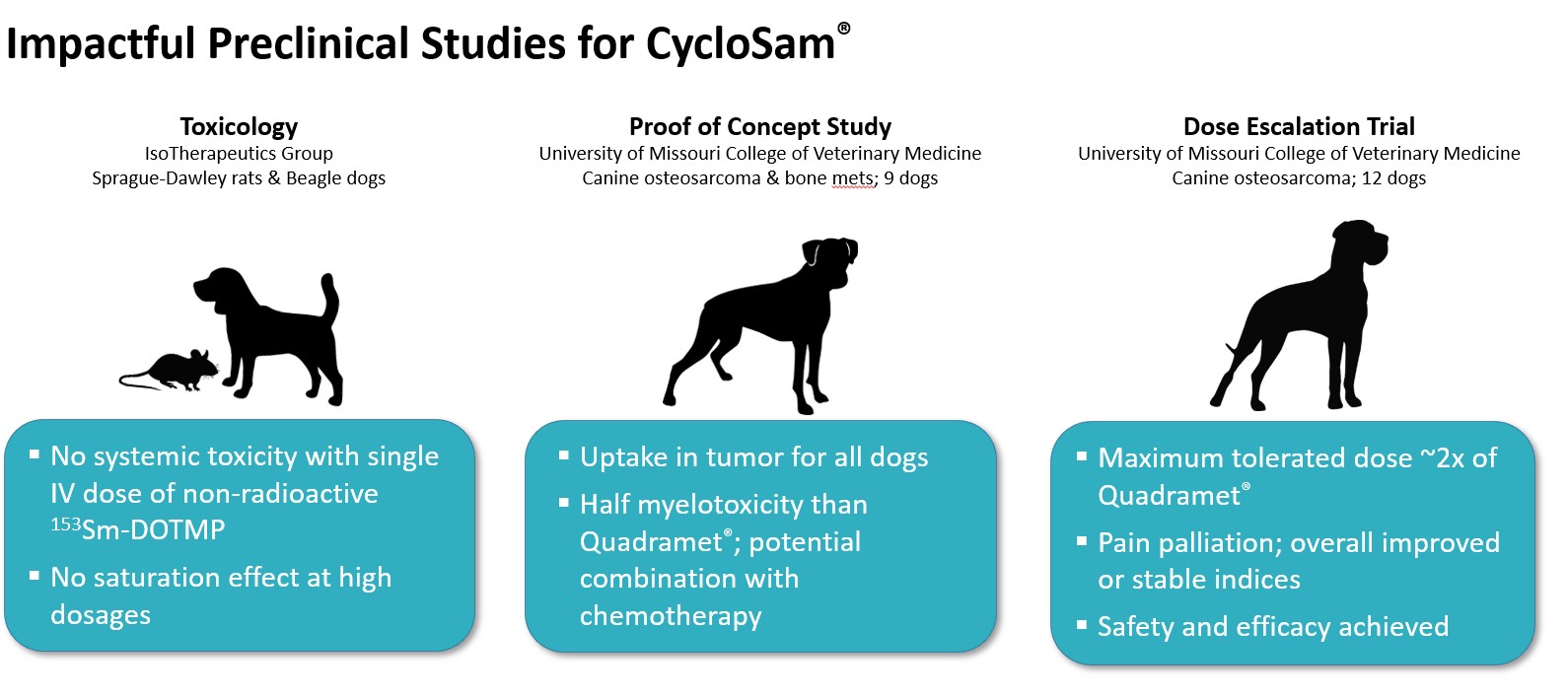
Clinical Pharmacology. The majority of non-clinical pharmacology studies with CycloSam® have been done in rats. When administered through the tail vein, much of the Samarium-153-DOTMP binds to the bone; the half-life is 46.3 hours, but the portion of the drug not bound to bone or calcified tissue is completely eliminated through the kidneys within 6 hours of administration. Two additional studies in dogs with osteosarcoma have also elicited promising results, and confirm bone uptake, preliminary safety, and preliminary clinical benefit against bone tumor. Preclinical results demonstrated significant skeletal uptake fractions.
Clinical Studies. Samarium-153 DOTMP was recently studied for the first time in humans under a Single Patient Investigational New Drug (IND) for Emergency Use at the Cleveland Clinic. The patient, a 25 year-old male who suffered from myelodysplastic syndrome (MDS) and high-risk osteosarcoma, received a single low dose of 1 mCi/kg of Samarium-153 DOTMP on March 24, 2020 for dosimetry. This was followed seven days later on March 31, 2020 by a single high dose of 32 mCi/kg (1919 mCi) of Samarium-153 DOTMP. No injection site effects were noted at the time of injection. At 48 hours post-injection of the second dose there was no renal toxicity observed. The estimated dose delivered to the skeleton was 40 Gy with bone lesion uptake of 60 Gy. The abbreviation Gy stands for “gray”, which is a measurement of radiation reaching the target. In this instance, a 45 Gy is considered required to deliver the radiation to the target, and therefore, 60 Gy was considered very good. The patient received an allogeneic stem cell transfusion two weeks following high dose injection of Samarium-153 DOTMP; however, the stem cell transplant failed to fully engraft. The patient, who was terminally ill prior to the treatment, passed away on August 18, 2020, a month after bone marrow ablation and after additional procedures not using a radiopharmaceutical were performed, from complications of an infection unrelated to the infusion of Samarium-153 DOTMP according to the investigator.
| 40 |
The investigator concluded that high-dose Samarium-153 DOTMP can be given safely with no apparent renal toxicity and no unexpected adverse events attributable to Samarium-153 DOTMP. Skeletal targeting with sparing of other tissues was observed after the high dose. This was only a single patient human clinical trial, and the patient did not survive long enough for full observation, so additional safety and efficacy clinical trial data will have to be developed.
License Agreement, Collaborations and Partnerships
Collaborations, partnerships and similar agreements, including license agreements, are a key component of the Company’s corporate strategy. As a clinical stage biotechnology company without revenue, partnerships are an essential part of our future development.
License Agreement. The Company, through its wholly-owned subsidiary QSAM Therapeutics, entered into an exclusive worldwide patent and technology License Agreement with IGL Pharma, Inc. (“IGL”) on April 20, 2020 with respect to the innovative work of Jim Simone, PhD and R. Keith Frank, PhD, at IsoTherapeutics on Samarium-153 DOTMP. IGL is an affiliated company with IsoTherapeutics, and the President of IGL, Mr. Richard C. Piazza, also serves as our Executive Chairman. Mr. Piazza was appointed to that position pursuant to a right granted to IGL to nominate a member to our board of directors which right is granted for the term of the agreement. We amended the License Agreement on November 24, 2021.
Our License Agreement, as amended, with IGL is for 20 years or until the expiration of the multiple patents covered under the license, and requires multiple milestone-based payments including: up to $410,000 as CycloSam® advances through Phase 3 of clinical trials, and $2 million upon commercialization. IGL has also received 500,000 shares of the Company as additional compensation. Upon commercialization, IGL will receive an on-going royalty equal to 4.5% of Net Sales, as defined in the License Agreement, and 5% of any consideration we receive pursuant to a sublicense, sale of the asset, or sale of QSAM Therapeutics. We will also pay for ongoing patent filing and maintenance fees, and we have certain requirements to defend the patents against infringement claims. The parties have agreed to mutual indemnification.
Either party may terminate the License Agreement 30 days after notice in the event of an uncured breach, or immediately in the case of bankruptcy or insolvency of the other party. QSAM Therapeutics may terminate for any reason upon 30 days’ notice. In the case IGL terminates due to an uncured breach, IGL will repay to us 25% of our direct clinical costs to assume ownership of data and other information gained in that process.
| 41 |
In connection with the License Agreement, QSAM Therapeutics signed a two-year Consulting and Confidentiality Agreement (the “Consulting Agreement”) with IGL, which provides IGL with payments of $8,500 per month continuing through April 2022. The Consulting Agreement is to provide us with additional consulting and advisory services from the technology’s founders to assist in the clinical development of CycloSam®. Our Executive Chairman serves as President of IGL, receives a $500 per month fee, and holds options to acquire less than 1% equity stake in IGL.
Contracted Research Organization. In January 2020, our licensor, IGL Pharma, entered into a Master Services Agreement (MSA) with a full-service Contract Research Organization (CRO) with over a 30 year history of service to pharmaceutical and biotechnology clients. The MSA was amended in February 2021 to add QSAM as a party, and includes a fixed monthly retainer for regulatory and clinical trial consulting services as well as specific work orders for clinical trial execution services. The CRO has a full-time staff of project managers, statisticians, physicians, nurses and other regulatory and operational personnel to support our FDA interactions, filings and preclinical and clinical trial activities. Specifically, the CRO provides clinical trial management services, clinical study monitoring services, medical coding services, electronic data capture services, data management services, medical monitoring services, safety reporting and medical writing services.
Intellectual Property
Pursuant to the License Agreement, our IP estate includes 14 total patents issued and pending across three distinct patent families that we believe provide protection for the use of CycloSam® as a radiopharmaceutical in the U.S. and internationally. Under the License Agreement, the Company holds two issued patents in the US, two issued patents in Japan, one issued patent in Canada, one allowed patent in Europe, and seven pending patents in international jurisdictions. Notably, the CycloSam® kit that will be commercialized is protected by the extensive patent estate that broadly protects DOTMP kit formulations for radioisotopes, potentially allowing for efficient distribution of the product and widespread use. Additionally, the patents cover the use of low-specific activity Samarium-153 allowing for daily supply of the highly toxicity-reduced isotope, and methods relating to repeat dosing regimens for therapeutic radiopharmaceutical agents, which suggest increased efficacy based on prior research. Taken together, management believes that the patent family provides for a significant barrier to entry for a competitor as it is expected to prevent a generic product from being developed; however, we cannot guarantee that a competitor will not or cannot challenge our patents or otherwise circumvent our patents, or that we would have the resources to defend any patent infringement.
| 42 |
A list of our patents and status of prosecution is included in the following table:
| Country/ Region | Status | App No | Filing Date | Pub No | Pub Date | Patent No | Issue Date | Expiration Date | ||||||||||||||
| “High purity therapeutic bone agents” | ||||||||||||||||||||||
| ITG-16 CA | Canada | Granted | 2,926,652 | 6-Apr-2016 | CA 2926652 | Apr-2015 | 2,926,652 | Jul-2021 | Oct-2034 | |||||||||||||
| ITG-16 EP | Europe | Allowed* | 14852866.4 | 5-May-2016 | EP3054996 | Aug-2016 | ||||||||||||||||
| ITG-16 JP | Japan | Granted | 2016-521278 | 7-Apr-2016 | 2016-532652 | Oct-2016 | 6787781 | Nov-2020 | Oct-2034 | |||||||||||||
| ITG-16 JP 1 | Japan | Pending | 2019-061398 | 27-Mar-2019 | ||||||||||||||||||
| ITG-16 US | United States | Granted | 15/027,280 | 5-Apr-2016 | US2016/0250359 | Sep-2016 | 10,172,965 | Jan-2019 | Nov-2034 | |||||||||||||
| ITG-16 US 1 | United States | Granted | 16/194,324 | 17-Nov-2018 | US2019/0083661 | Mar-2019 | 110,596,277 | Mar-2020 | Nov-2034 | |||||||||||||
| “DOTMP kit formulations for radioisotopes” | ||||||||||||||||||||||
| ITG-17 CA | Canada | Pending | 2987242 | 23-Nov-2017 | ||||||||||||||||||
| ITG-17 EP | Europe | Granted | 16800631 | 20-Nov-2017 | EP3302496 | Apr-2018 | 3302496 | Jan-2021 | May-2036 | |||||||||||||
| ITG-17 JP | Japan | Granted | 2017-561326 | 24-Nov-2017 | 2018-515585 | Jun-2018 | May-2036 | |||||||||||||||
| ITG-17 US 1 | United States | Pending | 16/866,001 | 04-May-2020 | US2020/0261607 A1 | Aug-2020 | ||||||||||||||||
| “Method of use for therapeutic bone agents” | ||||||||||||||||||||||
| ITG-18 CA | Canada | Pending | 05782981 | 7-Aug-2019 | ||||||||||||||||||
| ITG-18 EP | Europe | Pending | 18751017.7 | 22-Aug-2019 | ||||||||||||||||||
| ITG-18 JP | Japan | Pending | 2019-563340 | 7-Aug-2019 | 2020-506239 | Feb-2020 | ||||||||||||||||
| ITG-18 US | United States | Pending | 16/484,706 | 8-Aug-2019 | US 2021-0138095 A1 | May-2021 | ||||||||||||||||
Pursuant to the License Agreement, the Company also has the right to use the registered trademark “CycloSam®” for the marketing and sale of the drug candidate.
| 43 |
Competition
The biotechnology and pharmaceutical industries are characterized by the rapid evolution of technologies and understanding of disease etiology, a strong emphasis on intellectual property and intense competition. We face substantial potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic research institutions, governmental agencies and public and private research institutions.
In addition to the current methods of care for cancer patients, the field of radiopharmaceuticals is deeply studied and many parties are pursuing commercial and academic clinical trials. Early results from these trials have fueled continued interest in radiopharmaceuticals, which are pursued by several biotechnology companies as well as by large pharmaceutical companies.
We consider our direct competitors to be companies developing targeted alpha radiopharmaceuticals for the treatment of cancer. There are several companies developing targeted alpha-based radiopharmaceuticals for the treatment of cancer, including Bayer AG, or Bayer, Novartis AG, or Novartis, Actinium Pharmaceuticals, Inc., RadioMedix, Inc, Orano Med, Telix Pharmaceuticals Limited, and Fusion Pharmaceuticals Inc., as well as several early-stage companies who recently entered the field such as RayzeBio, Inc. These companies are targeting a wide range of solid and hematologic malignancies using various alpha emitting isotopes, including Radium-223, Actinium-225 and Thorium-227. The first and only approved alpha particle-based therapy is Bayer’s Xofigo, a salt of radium that is not currently attached to a targeting molecule, but naturally localizes to regions where cancer cells are infiltrating bone. Xofigo was approved in the United States by the FDA in 2013 for the treatment of bone metastases associated with prostate cancer.
There are several companies with approved or late clinical stage beta-based radiopharmaceuticals, including Progenics Pharmaceuticals, Inc., Novartis, Bayer and Q BioMed Inc., which we also consider competitors. Another competitive company, POINT Biopharma Global Inc., has two indications using beta-emitting particles in Phase 3 trials. The beta emitting isotopes used by these companies include Iodine-131, Lutetium-177, Strontium-89 and Yttrium-90. There are other beta particle-based radiopharmaceuticals in various stages of clinical development by companies including Novartis AG, Ipsen S.A., Y-mAbs Therapeutics, Inc. and Clovis Oncology, Inc.
Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies and materials complementary to, or necessary for, our programs.
We could see a reduction or elimination in our commercial opportunity if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient to administer, are less expensive or with a more favorable label than our product candidates. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. The key competitive factors affecting the success of all of our product candidates, if approved, are likely to be their efficacy, safety, convenience, price, the effectiveness of imaging diagnostics, the level of generic competition and the availability of reimbursement from government and other third-party payors.
| 44 |
Legacy Business and Separation Agreement
Until November 2020, our primary business was acquiring and managing companies in the soil health sector (the “Legacy Business”), through an affiliated company, Earth Property Holdings LLC (“EPH”). In January 2020, our board of directors authorized a strategic plan comprised of: (1) securing new technologies and business opportunities in the broader biosciences sector, and (2) significantly reducing debt and liabilities of the Company, both of which have been accomplished. The first action under this plan included appointing Douglas R. Baum to our board of directors. Mr. Baum has over 28 years of experience in the bioscience and biotech industries, including development, commercialization and marketing of multiple drugs and medical devices. After his appointment to our board, Mr. Baum secured for the Company an Exclusive Dealing Option Agreement to provide us a 90-day period to negotiate with IGL Pharma Inc. (“IGL”) for a license to CycloSam®. IGL is an affiliated entity of IsoTherapeutics Group LLC, whose founders created Quadramet® (Samarium-153-EDTMP) while working at Dow Chemical Company, one of the first effective commercial radiopharmaceuticals.
On April 20, 2020, we exercised our option and executed a Patent and Technology License Agreement and Trademark Assignment with IGL, through a newly created, wholly-owned subsidiary called QSAM Therapeutics Inc. (“QSAM Therapeutics”). The License Agreement, which was amended on November 24, 2021, provides us with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and knowhow with respect to CycloSam®. The License Agreement also transfers to us the rights to the product name CycloSam® for the technology, and provides us a first right of refusal to license other IsoTherapeutics’ technologies in the future. See “License Agreement” above.
Our board of directors determined in the fourth quarter of 2020 that the opportunities presented through the development of the New Technology presented shareholders with the best path forward in terms of long term value creation. As a result, on November 6, 2020, we entered into an Omnibus Separation Agreement (the “Separation Agreement”) with EPH. Under the terms of the Separation Agreement, all remaining assets for the Legacy Business were transferred to EPH in return for the release and termination of $993,985 in debt and other liabilities owed to EPH. An additional $114,700 in promissory notes plus accrued interest owed to an affiliate of EPH were converted into Company common stock. Under the Separation Agreement, the Management Agreement between the Company and EPH was terminated, and former employees of the Company engaged in the Legacy Business were released from non-compete agreements. We have presented revenue and expenses related to the Management Agreement, as well as other expenses, assets and liabilities related to the Legacy Business, as “discontinued operations” in our consolidated financial statements. Our sole business focus at this time is the development of radiopharmaceuticals for the treatment of cancer and other diseases.
| 45 |
Government Regulation and Product Approval
Clinical trials, the drug approval process, and the marketing of drugs are intensively regulated in the United States and in all major foreign countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and related regulations. Drugs are also subject to other federal, state, and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of biopharmaceutical products. These agencies and other federal, state, and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising, and promotion of product we develop in the future.
The FDCA and/or FDA’s policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of any candidate drug product or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
| 46 |
Marketing Approval
The process required by the FDA before new drugs may be marketed in the United States generally involves the following:
| ● | nonclinical laboratory and animal tests; | |
| ● | chemistry, manufacturing, and control testing (CMC), validation and documentation of all synthesis, preparation and production processes for all kit ingredients and finished products; | |
| ● | submission of an Investigational New Drug (IND) application, which must become effective before clinical trials may begin; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; | |
| ● | pre-approval inspection of manufacturing facilities and clinical trial sites; and | |
| ● | FDA approval of a New Drug Application (NDA) which must occur before a drug can be marketed or sold. |
The testing and approval process requires substantial time and financial resources, and we cannot be certain that any approvals will be granted on a timely basis if at all.
We will need to successfully complete additional clinical trials in order to be in a position to submit an NDA to the FDA. Future trials may not begin or be completed on schedule, if at all. Trials can be delayed for a variety of reasons, including delays in:
| ● | obtaining regulatory approval to commence a study; | |
| ● | reaching agreement with third-party clinical trial sites and vendors and their subsequent performance in conducting accurate and reliable studies on a timely basis; | |
| ● | obtaining institutional review board approval to conduct a study at a prospective site; | |
| ● | recruiting subjects to participate in a study; and | |
| ● | supply of the drug. |
We must reach an agreement with the FDA on the proposed protocols for our future clinical trials, post-market safety monitoring, and on a Pediatric Development Plan in the United States. All new drugs now require the presentation to the FDA after Phase II clinical trials have ended of a Pediatric Development Plan outlining the strategy and steps to be taken by us to study CycloSam® in children as appropriate. A separate submission to the FDA must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each study subject. Regulatory authorities, an IRB, a data safety monitoring board, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk. Such risks may include unexpected or serious adverse events, or increased severity or occurrence rate of know potential adverse events.
FDA Post-Approval Requirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping and reporting of adverse experiences with the drug and/or additional post-market clinical trials. Drug manufacturers are required to register their facilities with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain quality processes, manufacturing controls, and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality and purity characteristics that it purports to have. Under the federal Prescription Drug Marketing Act, the sampling and distribution and tracking of drugs is regulated. It is designed to discourage the sale of counterfeit, adulterated, misbranded, subpotent, and expired prescription drugs. Certain states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, fail to approve any NDA or other application, require us to recall a drug from distribution, shut down manufacturing operations or withdraw approval of the NDA for that drug. Noncompliance with cGMP or other requirements can result in issuance of warning letters, civil and criminal penalties, seizures, and injunctive action.
| 47 |
Labeling, Marketing and Promotion
The FDA closely regulates the labeling, marketing, and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to the safety and efficacy of a drug that are consistent with FDA approval and may only actively market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions, and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of drugs.
Pediatric Research Equity Act
The Pediatric Research Equity Act (“PREA”) amended the FDCA to authorize the FDA to require certain research into drugs used in pediatric patients. The intent of the PREA is to compel sponsors whose drugs have pediatric applicability to study those drugs in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The Secretary of Health and Human Services may defer or waive these requirements under specified circumstances. The FDA may decide that an NDA will be approved only following completion of additional pediatric studies.
Anti-Kickback and False Claims Laws
In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing, and scientific/educational grant programs must comply with the Anti-Kickback Statute, the False Claims Act, as amended, the privacy regulations promulgated under HIPAA, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
In the United States, we are subject to complex laws and regulations pertaining to healthcare “fraud and abuse,” including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act, and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multi-billion-dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. In addition, the federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws. The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), also created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the Anti-Kickback Statute a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation.
| 48 |
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, a similar federal requirement Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the “Affordable Care Act”) commonly referred to as the “Physician Payments Sunshine Act” requires manufacturers to track and report to the federal government certain payments and “transfers of value” made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, made in the previous calendar year. There are a number of states that have various types of reporting requirements as well. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
Patient Protection and Affordable Health Care Act
Historically in the United States, policy makers have attempted several healthcare reforms regarding the healthcare system that could expand access to healthcare, improve quality of healthcare, contain healthcare costs, prevent or delay approval of product candidates, restrict or regulate post-approval activities, and affect the profitable sale of drugs.
In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the ACA, was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. Since its enactment, there have been judicial and political challenges to certain aspects of the ACA. Most recently on June 17, 2021, the U.S. Supreme Court dismissed a judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how the healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact the ACA.
Congress and the Biden administration have generally indicated that they will continue to seek new legislative and/or administrative measures to control drug costs and improve access. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
Other Regulations
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
The CycloSam® radioactive product is regulated by the federal Nuclear Regulatory Commission (NRC), and also by similar state regulatory agencies. The handling, packaging, shipping, transportation, and disposal of radioactive materials is highly regulated, and those regulations can change, and the Company would have to comply with all requirements, which could be costly. Additionally, if overnight delivery failures occur, the radioactive compounds cannot be used, and that can result in substantial increased costs and liabilities. The disposal of radioactive materials is also regulated by the Environmental Protection Agency (EPA), and also by similar state regulatory agencies and the Company would have to comply with all of those requirements, which could also be costly.
Facilities
The Company currently conducts its business from offices in Austin, Texas and Palm Beach, Florida. The Company’s office space in Austin is leased month-to-month at a rate of $216 per month. The Company has no formal agreement for its Florida space which is leased by Greenblock Capital, a company for which our General Counsel is affiliated.
Legal Proceedings
From time to time, we may be involved in legal proceedings arising in the ordinary course of our business. We are not presently a party to any legal proceedings that, in the opinion of management, would have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us due to defense and settlement costs, diversion of management resources, negative publicity and reputation harm, and other factors.
Human Capital
As of the date of this prospectus, we have four full-time employees and one part-time employee who handles our accounting and financial reporting operations.
Reports to Security Holders
The Company files periodic reports and current reports like Form 10-K and Form 10-Q and current reports like Form 8-K with the Securities and Exchange Commission pursuant to Securities Exchange Act of 1934. It is required to make additional filings like proxy statements for solicitation of proxies from stockholders or information statements for obtaining written consent in lieu of meetings, and other forms as required by the Exchange Act from time to time. You may read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. You may also find all of the reports that we have filed electronically with the SEC at their Internet site www.sec.gov. These reports are also available on Company’s website www.qsambio.com, the content of which is not incorporated by reference in this prospectus.
| 49 |
MANAGEMENT
The current Directors and Officers of the Company are as follows:
| Name | Age | Position (s) and Offices Held | ||
| C. Richard Piazza (1) | 73 | Executive Chairman | ||
| Douglas R. Baum (2) | 54 | Chief Executive Officer & Director | ||
| Christopher Nelson | 51 | General Counsel & Director | ||
| Adam King | 36 | Interim Chief Financial Officer | ||
| Joel Mayersohn (1)(2) | 63 | Director | ||
| Charles J. Link, Jr. (2) | 61 | Director |
| (1) | Audit committee member |
| (2) | Compensation committee member |
Biographies
C. Richard Piazza, Ph.D. – Executive Chairman. Mr. Piazza was appointed as a member and the Executive Chairman of the board of the Company in November 2020. Mr. Piazza has also served since 2017 as President and CEO of IGL Pharma Inc., the licensor of CycloSam®, and a consultant to IsoTherapeutics Group, LLC, the inventors of the technology. Mr. Piazza also currently serves on the board of directors of NovaScan LLC, a privately-held cancer detection and diagnostics company. Prior to his work with IGL Pharma, from 2014 to 2016, he was CEO of SynVivo, Inc., a cancer diagnostics company. Mr. Piazza has more than 48 years of healthcare experience in both medical devices and pharmaceutical/biotech and has led several technology companies to market success including numerous FDA approvals in both sectors. Previously, he served in general management positions in both public and private international companies including Ohmeda, Smith & Nephew Pharmaceuticals, Marquest and VitaGen (world’s first bioartifical liver). Over his career, he has provided advisory services to some of world’s leading institutions including MD Anderson Cancer Center, Baylor College of Medicine, University of California San Diego, University of Chicago and Kings College Hospital (London). In 2019, he co-founded QSAM Therapeutics, Inc. with Douglas Baum, our CEO. Mr. Piazza obtained a BS in Economics and a BS in Speech Pathology from the State University of New York and MA & PhD in Economics from the University of Buffalo and Leeds University.
We believe Mr. Piazza is qualified to serve on our board of directors due to his significant experience in the pharmaceutical and biotech industry, and his experience serving of the boards and in senior management positions in several publicly traded companies.
| 50 |
Douglas R. Baum – Chief Executive Officer & Director. Mr. Baum was appointed to the board of the Company in January 2020 and to the position of CEO in November 2020. He brings to the Company over 30 years of experience in the bioscience and biotech industries, including development, FDA/EMA approval and commercialization of multiple drugs and medical devices. Over his long senior executive tenure, he has overseen 15 product approvals through the FDA and EMA and raised over $85 million in capital to fund breakthrough technologies. Between 2017 and 2020, Mr. Baum consulted with multiple medical schools, biotech and pharmaceutical companies; and between 2012 and 2017, he served as President, Chief Executive Officer and Director of Xeris Pharmaceuticals Inc. (currently, NASDAQ: XERS). Previously, he served as Executive Vice President and Chief Operating Officer of Macuclear Inc., and other executive level positions with clinical trial research firms including SCIREX and Premier Research Group, Inc. He holds a Masters of Science in Technology Commercialization and BBA in International Business and Marketing from the University of Texas.
Mr. Baum’s extensive experience in the biotech industry as a senior level executive and vast exposure to the lifecycle of healthcare products from trials to commercialization makes him qualified to serve on our board of directors.
Christopher Nelson – General Counsel & Director. Mr. Nelson has been General Counsel and director of the Company since 2015, and was also its President from 2016 to November 2020. In these roles, he has overseen corporate and governance legal matters, finance and business development for the Company. He has also served since 2016 as Managing Director of GreenBlock Capital LLC in Palm Beach, Florida, a boutique mergers and acquisitions advisory firm specializing in bio-technology, ag-technology and similar sector business combination transactions; and since 2019 as General Counsel for Earth Property Holdings, LLC, a private equity-backed company engaged in soil health and compost manufacturing in Texas and Florida. Mr. Nelson has practiced law in Florida for over 26 years, and during that time has represented many start-up, early stage and established businesses seeking financing, acquisitions and general growth management counseling. Between 1997 and 2000, Mr. Nelson was an associate with Greenberg Traurig PA, and between 1995 and 1997 an associate with Akerman Senterfitt PA, both in Miami, Florida. At both firms he served in their corporate and securities practice, representing NYSE and NASDAQ companies. Mr. Nelson received a BA from Princeton University, and JD from University of Miami School of Law, and is a member of the Florida Bar.
Mr. Nelson’s extensive experience representing public companies in capital markets finance, mergers and acquisitions, and general corporate and governance matters, makes him qualified to serve on our board of directors.
Adam King, CPA – Interim Chief Financial Officer. Mr. King currently serves as Interim CFO for the Company, a position he has held since December 6, 2021. Mr. King is the founder and CEO of King Consulting Group, where he provides a range of financial and reporting services for clients that include Vice President of Finance for large private equity-backed international companies to CFO of small start-ups. Before founding King Consulting Group in January 2021, Mr. King was the CFO for Netsertive, a venture-backed digital marketing company in Research Triangle Park, North Carolina. From 2016 to 2018, he was the Office Managing Audit Director for BDO’s Greenville, SC office, in addition to Audit Director in Raleigh, NC, and Boston. While at BDO, Mr. King worked with various clients, from Tech and Life Science start-ups to large billion-dollar publicly traded companies. Before his time at BDO, he served as the Director of Revenue Assurance and Internal Controls at Bandwidth.com and an Audit Manager at Ernst & Young. Mr. King holds a Bachelor of Science in Accounting from Elon University and is a CPA in Raleigh, NC. In October 2019, Mr. King and his wife filed for personal bankruptcy under chapter 13 of the United States Bankruptcy Code due to excessive medical expenses incurred by the family in connection with their child’s medical diagnosis and treatment. The final payment under their bankruptcy plan is scheduled to end in September 2024.
Joel Mayersohn – Director. Mr. Mayersohn has been a director of the Company since 2015. Mr. Mayersohn is a Partner in the Ft. Lauderdale, FL office of Dickinson Wright PLLC since 2016, where he specializes in corporate, securities and business law. Over the last 30 years, he has advised a diversified client base in private placements, public offerings, mergers and acquisitions, financing transactions and general securities law matters. He also has experience in venture capital, bridge loans and pipe financings. He is a member of the Florida and New York Bars. Mr. Mayersohn received a Bachelor of Arts and Juris Doctorate from State University of New York at Buffalo.
Mr. Mayersohn’s almost 40 years of experience of advising public and private companies in all areas of corporate and securities law, including representing technology and healthcare companies make him qualified to serve on our board of directors.
Charles J. Link, Jr. – Director. Dr. Link was appointed to the Board in February 2021, and brings decades of biotech and drug development experience to the Company. He currently serves on the executive committee of the Board of Directors at NovaScan Inc., a clinical-stage company focused on cancer detection. Dr. Link also serves on the Board of Directors for Viewpoint Molecular Targeting, a clinical stage company developing alpha-particle radiopharmaceuticals; and is the founder and Executive Director of biotech startup Syncromune. He is also a founder of biotech startup ChainLink Pharma. Previously, Dr. Link was the CEO, CSO, Chairman, and founder of NewLink Genetics, a NASDAQ-listed immunotherapy company focused on developing novel immuno-oncology product candidates, from 1999 until his retirement in 2019. During his tenure at NewLink, Dr. Link led a series of collaborative transactions totaling hundreds of millions of dollars with Merck, Roche and the United States government. He also oversaw the collaboration with Merck to develop EVERBO, the first Ebola vaccine to receive FDA approval. Prior to founding NewLink Genetics, Dr. Link was an attending physician at the National Cancer Institute. He has authored more than 100 peer-reviewed papers. Dr. Link received an M.D. from Stanford University, and he attended the U.S. Air Force Academy.
Dr. Link’s experience as CEO and Chairman of a NASDAQ-listed biotechnology company where he led several multi-party drug development programs and successfully raised significant funding in the capital markets, as well as his service as an oncology physician and senior government researcher, qualifies him to serve on our board of directors.
Other Key Employees and Advisors
Namrata Chand – VP Operations. Age 41. Ms. Chand has held the position of VP - Operations with the Company since November 2020. Ms. Chand brings 20 years of experience and a diverse foundation in administration, marketing, operations and business development within non-profit and for-profit sectors. Initially focusing her career in large organizations, she held leadership positions in marketing and corporate relations at Nestle, Beam Suntory, Aetna and BFAS, a leading national animal welfare organization, to build brand awareness, improve operational efficiency and drive overall growth. Ms. Chand later entered the life science space and joined La Jolla Capital Partners in 2011 where she assisted small to midsize healthcare companies through various stages of development with special emphasis on capital formation, operations, supply chain, clinical trials, marketing and business development. Most recently, Ms. Chand led Investor Relations and Business Development for Ryca International, an innovative dental product company that entered into a joint venture with a leading global medtech company. In addition to leading operations at the Company, Ms. Chand serves on the Advisory Board of a number of early-stage biotech companies.
| 51 |
Barry Sugarman – Scientific Advisor. Age 63. Mr. Sugarman has held the position of senior regulatory and scientific advisor with the Company since November 2020. Mr. Sugarman has over 30 years of experience spanning public and private companies in the pharmaceutical, medical device, dietary supplement and cosmetic industries. Mr. Sugarman has considerable direct experience in pharmaceutical product development, manufacturing, clinical trials, regulatory affairs, FDA and government relations, marketing, and distribution; as well as Good Manufacturing Practices (GMP’s), Good Clinical Practices (GCP’s), Good Laboratory Practices (GLP’s), and International Conference for Harmonization (ICH) requirements. He is an author and co-author of numerous FDA filings and approvals including Investigational New Drug Applications, New Drug Applications, Abbreviated New Drug Applications, and Medical Device Applications 510(k)’s. Mr. Sugarman is a member of the Regulatory Affairs Professional Society, American Association of Pharmaceutical Scientists, Association of Clinical Research Professionals, and the National Association of Corporate Directors. He is a co-author of “Prompt, Accurate Diagnosis of Pediatric Cancer and Leukemia for Pediatricians, Orthopedists, and Family Practitioners” (2007).
Richard “Keith” Frank – Scientific Advisor. Age 65. Dr. Frank has served as scientific advisor to the Company since April 2020. Since 2006, he has served as CEO, President and co-founder of IsoTherapeutics LLC, a radiopharmaceutical R&D and contract manufacturing company that invented CycloSam® and provides services for both large and small biotechnology companies. He also serves as Chairman of IGL Pharma, Inc., the Company’s licensor of CycloSam®. Prior to these positions, Dr. Frank spent over 20 years in numerous senior scientific positions at Dow Chemical Company. At Dow, he was a collaborator in the development of bone-seeking radiopharmaceuticals Quadramet (Sm-153-EDTMP) and STR (Ho-166-DOTMP). Additionally, Dr. Frank was the lead inventor of Iotrex™ for use in the GliaSite® Radiation Therapy System. He also initiated and was the technical leader of Dow’s ChelaMedSM Radiopharmaceutical Services offering.
Jaime “Jim” Simon – Scientific Advisor. Age 68. Dr. Simon has served as scientific advisor to the Company since April 2020. Since 2006, he has served as Vice President and Chief Science Officer, and co-founder of IsoTherapeutics LLC, a radiopharmaceutical R&D and contract manufacturing company that invented CycloSam® and provides services for both large and small biotechnology companies. Prior to co-founding IsoTherapeutics, Dr. Simon spent over 25 years as a senior scientist at Dow Chemical Company where his initial proposals led to the creation of Dow’s radiopharmaceutical group. At Dow, Dr. Simon was the lead inventor for all bone agent patents including Sm-153-EDTMP and Ho-166-DOTMP. Dr. Simon has been involved in numerous FDA submissions for clinical trials, and has coordinated the radioisotope activities at the University of Missouri Research Reactor (isotope production), the University of Missouri Veterinary School (dog studies), and The Harry S. Truman Veterans Administration Hospital (human clinical studies).
Director Independence
The Company’s common stock trades on OTCQB Venture Market of OTC Markets Group, but as a company that is required to file reports with the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended, the Company is not required under the rules mandated by OTC Markets for US companies to comply with the director independence standard, which requires certain companies to maintain a board that has at least two independent directors and an audit committee, a majority of the members of which are independent directors.
However, the Company will be applying, concurrently with the filing of this registration statement of which this prospectus forms a part or shortly thereafter, for admission to listing on NASDAQ and accordingly will be required to comply with NASDAQ’s corporate governance standards applicable to director independence upon listing. Rule 5605 therein requires companies listed on NASDAQ to maintain a majority independent board. In addition, the rules of NASDAQ require that each member of a listed company’s audit, compensation, and corporate governance and nominating committees be independent. An independent director under these rules is defined as a person other than an executive officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Further, the rules state that each of the aforementioned committees should have at least two independent members, except for audit committee that must have at least three members. Compensation committee members must not have a relationship with the Company that is material to the director’s ability to be independent from management in connection with the duties of a compensation committee member. Similarly, audit committee members should each be able to read and understand financial statements, one of whom should be “financially sophisticated” (i.e. have past employment experience in finance or accounting, professional certification in accounting, or any other comparable experience or background). Further, SEC rules require that at least one member of the audit committee be a “financial expert”. A person who qualifies as an audit committee financial expert automatically meets the financially sophisticated requirement under NASDAQ’s rules. Audit committee members must also satisfy the additional independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors has undertaken a review of the independence of each director and determined that all of our directors, other than Mr. Baum our Chief Executive Officer, Mr. Piazza our Executive Chairman, and Mr. Nelson our General Counsel, are “independent directors” as defined under the applicable listing requirements and rules of NASDAQ. In making these determinations, our board of directors reviewed and discussed information provided by the directors with regard to each director’s business and personal activities and relationships as they may relate to us and our management, including the beneficial ownership of our common stock by each non-employee director.
Board Committees
Committees established by the Board
Our board of directors currently has audit and compensation committees and will have nominating and corporate governance committee prior to the closing of this offering. Information concerning the composition and function of each board committee is as follows.
Audit Committee
The Company’s audit committee currently comprises of Joel Mayersohn and Richard Piazza. Following the closing of this offering, the audit committee will consist of three members, each of whom will meet the requirements of independence under NASDAQ’s listing standards and SEC’s rules and regulations. The audit committee will be responsible for overseeing management’s implementation of effective internal accounting and financial controls, supervising matters relating to audit functions, reviewing and setting internal policies and procedures regarding audits, accounting and other financial controls, reviewing the results of our audit performed by the independent public accountants, and evaluating and selecting the independent public accountants. The members of audit committee will be , , and , each of whom is “financially sophisticated” per NASDAQ’s listing rules, and has been designated as “audit committee financial expert” as such term is defined in Item 407(d)(5) of Regulation S-K. Following the completion of this offering, Mr. Mayersohn will no longer qualify as an audit committee member pursuant to NADSAQ’s rules and therefore will not serve on the audit committee.
Compensation Committee
The Company’s compensation committee currently comprises of Douglas Baum, Joel Mayersohn, and Charles J. Link Jr. Following the closing of this offering, the compensation committee will consist of two members, each of whom will meet the requirements of independence under NASDAQ’s listing standards and SEC’s rules and regulations. The compensation committee will determine matters pertaining to the compensation of our named executive officers and administer our stock option and incentive compensation plans, or equity incentive plans. The members of compensation committee will be and .
Nominating and Governance Committee
The Company did not have a nominating and governance committee (“NGC”) prior to this offering. Upon closing of this offering, the Company’s NGC will consist of two members, each of whom will meet the requirements of independence under NASDAQ’s listing standards and SEC’s rules and regulations. The committee will be responsible for considering potential board members, nominating directors for election to the board, implementing the Company’s corporate governance policies, for all other purposes outlined in the nominating and governance committee charter. The members of NGC will be and .
| 52 |
Nomination of Directors
As provided in its charter, the NGC is responsible for identifying individuals qualified to become directors. The NGC seeks to identify director candidates based on input provided by a number of sources including (1) the NGC members, (2) our other directors, (3) our stockholders, and (4) our Chief Executive Officer or Chair of the board. In evaluating potential candidates for director, the NGC considers the entirety of each candidate’s credentials.
Qualifications for consideration as a director nominee may vary according to the particular areas of expertise being sought as a complement to the existing composition of the board of directors. However, at a minimum, candidates for director must possess:
| ● | high personal and professional ethics and integrity; | |
| ● | the ability to exercise sound judgment; | |
| ● | the ability to make independent analytical inquiries; | |
| ● | a willingness and ability to devote adequate time and resources to diligently perform board and committee duties; and | |
| ● | the appropriate and relevant business experience and acumen. |
Legal Proceedings
There are currently no legal proceedings, and during the past 10 years there have been no legal proceedings, as described in Item 401(f) of Regulation S-K, that are material to the evaluation of the ability or integrity of any of our directors or executive officers. See Adam King’s biography disclosed on page 51 of this prospectus pertaining to Mr. King’s and his wife’s filing of personal bankruptcy under the United States Bankruptcy Code in 2019.
Family Relationships
There are no family relationships among our directors and executive officers. There is no arrangement or understanding between or among our executive officers and directors pursuant to which any director or officer was or is to be selected as a director or officer.
Code of Ethics
We have adopted a Code of Business Conduct and Ethics Policy (the “Code of Ethics”) that applies to all of our directors, officers, and employees. The Code of Ethics describes the legal, ethical and regulatory standards that must be followed by the directors, officers, and employees of the Company and sets forth high standards of business conduct applicable to each of them. As adopted, the Code of Ethics sets forth written standards that are designed to deter wrongdoing and to promote, among other things:
| ● | honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; | |
| ● | compliance with applicable governmental laws, rules and regulations; | |
| ● | the prompt internal reporting of violations of the Code of Ethics to the appropriate person or persons identified in the code; and | |
| ● | accountability for adherence to the Code of Ethics. |
| 53 |
EXECUTIVE AND DIRECTOR COMPENSATION
The following table sets forth information regarding compensation earned in or with respect to our fiscal years ended 2020 and 2019 for the following (“Named Executive Officers” or NEOs):
| (i) | our principal executive officer, or other individual serving in a similar capacity during the fiscal year 2020 and 2019; | |
| (ii) | our two most highly compensated executive officers other than our principal executive officers who were serving as executive officers at December 31, 2020 and 2019 whose compensation exceed $100,000; and | |
| (iii) | up to two additional individuals for whom disclosure would have been required but for the fact that the individual was not serving as an executive officer at December 31, 2020 and 2019. |
Summary Executive Compensation Table
Name and Principal Position | Year | Salary
($) (a) | Bonus
($) (b) | Stock
Awards ($) (c) | Option
Awards ($) (d) | Non-Equity Incentive Plan Compensation ($) (e) | Nonqualified
Deferred Compensation ($) (f) | All
Other Compensation ($) (g) | Total
Earnings ($) (h) | |||||||||||||||||||||||||||
| C. Richard Piazza, | 2020 | - | - | |||||||||||||||||||||||||||||||||
| Executive Chairman (1) | 2019 | |||||||||||||||||||||||||||||||||||
| Douglas Baum, | 2020 | - | 100 | 100 | ||||||||||||||||||||||||||||||||
| CEO and Director (2) | 2019 | |||||||||||||||||||||||||||||||||||
| Christopher Nelson, | 2020 | 107,895 | 50,000 | 43,194 | 11,743 | 212,832 | ||||||||||||||||||||||||||||||
| General Counsel and Director (former President) (3) | 2019 | 245,000 | 16,626 | 261,626 | ||||||||||||||||||||||||||||||||
| Kevin Bolin, | 2020 | 132,105 | 50,000 | 97,796 | 14,151 | 294,052 | ||||||||||||||||||||||||||||||
| Former Chairman & CEO (4) | 2019 | 295,000 | 17,853 | 312,853 | ||||||||||||||||||||||||||||||||
| (a) | Salaries include those amounts paid and accrued as an expense on the books of the Company. | |
| (c)(d) | This amount reflects the aggregate grant-date fair value of stock awards computed in accordance with FASB ASC Topic 718. | |
| (g) | All Other Compensation is comprised of healthcare costs. |
| (1) | Mr. Piazza signed his employment agreement with the Company on November 1, 2020; however, per the understanding of the parties, no salary or other compensation accrued under that agreement in 2020 until after the completion of the Company’s Series B offering in January 2021. This agreement was amended and restated on December 6, 2021. | |
| (2) | Mr. Baum signed his employment agreement with the Company on November 1, 2020; however, per the understanding of the parties, no salary or other compensation accrued under that agreement until after the completion of the Company’s Series B offering in January 2021. This agreement was amended and restated on December 6, 2021. Mr. Baum’s 8,000 options issued in 2020 are reflected in the table. | |
| (3) | Mr. Nelson received $43,194 of his annual salary paid in 196,336 shares of common stock, and his deferred bonus of $50,000 paid in 227,273 shares of common stock; both issuances at $0.22 per share. Mr. Nelson also waived $13,911 of compensation due to him in 2020. Mr. Nelson had 12,600 options outstanding as of December 31, 2020. | |
| (4) | Mr. Bolin’s employment with the Company terminated by mutual agreement on November 6, 2020. Mr. Bolin received $97,796 of his deferred annual salary paid in 444,527 shares of common stock, and his deferred bonus of $50,000 paid in 227,273 shares of common stock; both issuances at $0.22 per share. Mr. Bolin also waived $32,598 of deferred compensation due to him in 2020. Mr. Bolin had 88,000 options outstanding as of December 31, 2020. |
| 54 |
Executive Employment Agreements
C. Richard Piazza – Executive Chairman. Mr. Piazza signed his employment agreement with the Company on November 1, 2020, with an effective date of November 6, 2020. This agreement was amended and restated on December 6, 2021. The term is three years, with extensions at the agreement of the parties. His base salary is $400,000 per year, however, he is currently receiving a partial salary of $112,500 until the Company’s next financing is completed. Under the amended agreement, Mr. Piazza is entitled to receive regular company benefits, including annual bonuses and salary increases, participation in management incentive plans involving cash or stock bonuses ranging from 25% and 125% of base salary, vacations, sick leave and PTO. Mr. Piazza is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 1.75% of the consideration received by the Company. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Piazza shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Piazza will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately, and base salary and healthcare benefits will continue for 24 months. Mr. Piazza also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
Douglas Baum, CEO. Mr. Baum signed his employment agreement with the Company on November 1, 2020, with an effective date of November 6, 2020. This agreement was amended and restated on December 6, 2021. The term is three years, with extensions at the agreement of the parties. His base salary is $400,000 per year, however, he is currently receiving partial salary of $125,000 until the Company’s next financing is completed. Under the amended agreement, Mr. Baum is entitled to receive regular company benefits, including annual bonuses and salary increases, participation in management incentive plans involving cash or stock bonuses ranging from 25% and 125% of base salary, vacations, sick leave and PTO. Mr. Baum is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 1.75% of the consideration received by the Company. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Baum shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Baum will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately and salary and healthcare benefits will continue for 24 months. Mr. Baum also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
Christopher Nelson, General Counsel. Mr. Nelson initially signed his employment agreement in 2017, which renewed on a year-to-year basis on April 1 each year. On December 6, 2021, Mr. Nelson signed an amended and restated employment agreement with the Company providing for a term of three years, with extensions at the agreement of the parties. His base salary is $300,000 per year; however, he is currently receiving partial salary of $100,000 until the Company’s next financing is completed. Under the amended agreement, Mr. Nelson is entitled to receive regular company benefits, including annual bonuses and salary increases, vacations, sick leave and PTO. Mr. Nelson is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 0.5% of the consideration received by the Company. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Nelson shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Nelson will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately and salary and healthcare benefits will continue for 18 months. Mr. Nelson also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
Proprietary Information and Inventions Agreement
All officers, directors (except Joel Mayersohn), and other key employees and consultants have signed a Proprietary Information and Invention Agreement (“PIIA”) with the Company that provides in material part the following:
| ● | All inventions and discoveries made by them during their employment or using Company resources shall be assigned to the Company. | |
| ● | They will not interfere in customer relationships during the term of employment plus 12 months after termination for any reason. | |
| ● | They will not solicit other employees of the Company during the term of employment plus 12 months after termination for any reason. | |
| ● | They will not compete with the business of the Company during the term of employment plus 12 months after termination for any reason. |
| 55 |
Outstanding Option and Stock Awards
The following table presents information concerning unexercised options and unvested restricted stock awards for the named executive officer outstanding as of December 31, 2020.
Outstanding Option and Stock Awards
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||||
| Name (a) | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | Equity Incentive Plan Awards Number of Securities Underlying Unexercised Unearned Options (#) (d) | Option Exercise Price ($) (e) | Option Expiration Date (f) | Number of Shares or Units of Stock That Have Not Vested (#) (g) | Market Value of Shares or Units of Stock That Have Not Vested ($) (h) | Equity Incentive Plan Awards: Number of Unearned Shares, Vested Units or Other Rights That Have Not Vested (#) (i) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) (j) | |||||||||||||||||||||||||||
| C. Richard Piazza (1) | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
| Executive Chairman | ||||||||||||||||||||||||||||||||||||
| Douglas Baum (2) | 4,000 | 4,000 | - | $ | 0.50 | 1/14/2025 | - | - | - | - | ||||||||||||||||||||||||||
| CEO and Director | ||||||||||||||||||||||||||||||||||||
| Christopher Nelson (3) | 10,200 | - | - | $ | 0.50 | 7/30/24 | - | - | - | - | ||||||||||||||||||||||||||
| General Counsel and Director | 2,400 | - | - | $ | 0.50 | 11/17/25 | - | - | - | - | ||||||||||||||||||||||||||
| Kevin Bolin | 16,000 | - | - | $ | 0.50 | 2/29/26 | - | - | - | - | ||||||||||||||||||||||||||
| Former Chairman & CEO | 72,000 | - | - | $ | 0.50 | 12/28/26 | - | - | - | - | ||||||||||||||||||||||||||
| (1) | In December 2020, Mr. Piazza received a grant of 2,975 shares of Series E-1 Preferred Stock. All of these shares vested on July 1, 2021. On December 6, 2021, all of these shares were exchanged for 10,093,800 shares of common stock pursuant to an exchange agreement and plan of reorganization. | |
| (2) | In December 2020, Mr. Baum received a grant of 2,975 shares of Series E-1 Preferred Stock. All of these shares vested on July 1, 2021. On December 6, 2021, all of these shares were exchanged for 10,093,800 shares of common stock pursuant to an exchange agreement and plan of reorganization. | |
| (3) | In December 2020, Mr. Nelson received a grant of 850 shares of Series E-1 Preferred Stock. These shares vested in 2021. On December 6, 2021, all of these shares were exchanged for 2,883,943 shares of common stock pursuant to an exchange agreement and plan of reorganization. |
Director Compensation
In June 2019, the board approved payment to the independent directors of $500 per month plus an additional $500 per month for committee chairs. These fees were accrued but not paid in 2019 and 2020. In December 2020, all directors converted their respective accrued fees totaling $40,000 into 181,818 shares of common stock of the Company at a conversion price of $0.22 per share. These fees terminated in the fourth quarter of 2020 and were not paid in 2021 unless re-established by the board in the future.
In 2020, two of our independent directors (Mr. Mayersohn and another director who is no longer on the board) received a grant of 40,000 stock options each, one director received a grant of 20,000 stock options, and a newly appointed director (Mr. Baum) received a grant of 8,000 stock options, each option exercisable into one share. No option grants were made in 2019. Directors can also be reimbursed for reasonable travel expenses incurred in connection with meetings of the board of directors, but no such expenses were incurred in 2020 or 2019.
In February 2021, in connection with the appointment of Dr. Link to our board, he received as compensation 850 shares of our Series E-1 Preferred Stock, which were exchanged into 2,883,943 shares of common stock on December 6, 2021.
In August 2021, our board of directors received the following Incentive Stock Options under the Company’s Omnibus Equity Incentive Plan, vesting half six months after grant and the balance 12 months after grant, exercisable at $0.36 per share, and terminating 10 years from grant: Mr. Piazza and Mr. Baum, 125,000 options each; Mr. Nelson, 115,000 options; Dr. Link, 55,000 options, and Mr. Mayersohn, 54,000 options.
The following table provides all fees paid to all directors in 2020, but excludes fees paid to the inside directors, Messrs. Baum, Piazza and Nelson, for their services in 2020 in their respective officer capacities. It also excludes fees paid to Mr. Bolin for his services as Chairman and CEO, which terminated in November 2020.
| Name | Fees Earned or Paid in Cash ($) (1) | Stock Awards ($) | Option Awards ($) (4) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Joel Mayersohn | $ | 12,000 | - | $ | 5,319 | - | - | - | $ | 17,319 | ||||||||||||||||||
| Scott Whitney(2) | $ | 12,000 | - | $ | 5,319 | - | - | - | $ | 17,319 | ||||||||||||||||||
| Tristan Peitz (3) | $ | 6,000 | - | $ | 2,660 | - | - | - | $ | 8,660 | ||||||||||||||||||
| (1) | As of December 2020, all accrued fees earned in cash were converted to common stock at a price of $0.22 per share. | |
| (2) | Mr. Whitney resigned from the board on February 15, 2021. | |
| (3) | Mr. Peitz resigned from the board on December 9, 2020. | |
| (4) | This amount reflects the aggregate grant-date fair value of stock awards computed in accordance with FASB ASC Topic 718. Messers. Mayersohn, Whitney, and Peitz each had 77,800, 72,000, and 52,000 options outstanding as of December 31, 2020, respectively. |
| 56 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Related Persons
Joel Mayersohn, our director, is also a partner in the law firm of Dickinson Wright PLLC, which provides legal services for the Company. The Company received services from Dickinson Wright PLLC of $30,355 and $67,147 during the period ended September 30, 2021 and year ended December 31, 2020, respectively, of which $2,070 and $32,716 remains unpaid as of September 30, 2021 and December 31, 2020, respectively.
C. Richard Piazza, our Executive Chairman, also serves as President of IGL Pharma Inc., the licensor of the Company’s drug technology, CycloSam®, and a consultant to IsoTherapeutics Group, LLC, the inventors of CycloSam®. Mr. Piazza holds an option to acquire less than 1% of the equity of IGL Pharma, and receives a fee of $500 per month from that company. IGL also has a 24-month consulting agreement with the Company terminating in April 2022 wherein the Company pays consulting fees of $8,500 per month to IGL.
The Company currently maintains an executive office in Florida, which is leased by an investment firm in which the Company’s General Counsel serves as an officer but does not hold any equity or voting rights. The Company has no formal agreement for this space and pays no rent.
Promoters and Certain Control Persons
See the heading “Transactions with Related Persons” above.
Parents of the Smaller Reporting Company
We do not have a parent company.
Director Independence
We had two independent directors serving on our board of directors as of the end of 2020 – Messrs. Whitney and Mayersohn. While Mr. Mayersohn’s firm also serves as the Company’s corporate counsel, it was determined that the payments made for services from the Company to his firm were not material enough to create an issue with Mr. Mayersohn’s independence. In 2021, Mr. Whitney resigned from the board and was replaced with another independent director, Dr. Charles J. Link Jr.
| 57 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the beneficial ownership of our issued and outstanding common stock as of December 17, 2021 by the following persons:
| ● | each person, or group of affiliated persons, known to us to beneficially own more than 5% of our common stock; | |
| ● | each of our directors; | |
| ● | each of our named executive officers; and | |
| ● | all of our directors and executive officers as a group. |
Beneficial ownership of our common stock is determined under the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power, or of which a person has a right to acquire ownership at any time within 60 days from December 17, 2021. Except as indicated by footnote, and subject to applicable community property laws, we believe the persons identified in the table have sole voting and investment power with respect to all shares of common stock beneficially owned by them.
Unless otherwise indicated, the address of each of the following persons is c/o QSAM Biosciences, Inc., 9442 Capital of Texas Hwy N, Plaza 1, Suite 500, Austin, TX 78759.
Except as otherwise noted, the persons named in the table have sole voting and dispositive power with respect to all shares beneficially owned, subject to community property laws where applicable.
Executive Officers and Directors
| Name of beneficial owner | Amount
beneficially owned(1) | Percent
of Class Beneficially Owned(7) | ||||||
| C. Richard Piazza | 10,093,800 | (2) | 15.3 | % | ||||
| Douglas Baum | 10,120,550 | (3) | 15.3 | % | ||||
| Christopher Nelson | 3,734,107 | (4) | 5.6 | % | ||||
| Joel Mayersohn | 908,456 | (5) | 1.4 | % | ||||
| Charles J. Link, Jr. | 2,883,943 | (6) | 4.4 | % | ||||
| Adam King | - | - | ||||||
| All Officers and Directors (as a group, 6 persons) | 27,740,856 | 41.9 | % | |||||
(1) The number of shares beneficially owned includes any shares over which the person has sole or shared voting power or investment power and also any shares that the person can acquire within 60 days of December 17, 2021 through the exercise of any stock options or other right. Unless otherwise indicated, each person has sole investment and voting power (or shares such power with his or her spouse) over the shares set forth in the table. For each person, the number of shares that is included in the table because the person has options to acquire the shares is set forth below.
| Name | Shares | |||
| Douglas Baum | 8,000 | |||
| Christopher Nelson | 12,600 | |||
| Joel Mayersohn | 79,840 | |||
(2) Excludes 125,000 options that vest half on February 24, 2022 and the balance on August 24, 2022.
(3) Includes 18,750 shares of common stock receivable upon the exercise of Series B preferred stock. Excludes 125,000 options that vest half on February 24, 2022 and the balance on August 24, 2022.
(4) Includes 125,000 shares of common stock receivable upon exercise of Series B preferred stock. Excludes 115,000 options that vest half on February 24, 2022 and the balance on August 24, 2022. Mr. Nelson’s address is 420 Royal Palm Way, Suite 100, Palm Beach, FL 33480.
(5) Includes 400,000 shares of restricted stock scheduled to vest on January 15, 2021. Excludes 54,000 options that vest half on February 24, 2022 and the balance on August 24, 2022. Mr. Mayersohn’s address is 350 East Las Olas Blvd., Suite 1750, Ft. Lauderdale, FL 33301.
(6) Excludes 55,000 options that vest half on February 24, 2022 and the balance on August 24, 2022.
(7) The percentages shown are based on the 66,169,164 shares of our common stock outstanding as of December 17, 2021, plus the number of shares that the named person or group has the right to acquire within 60 days of December 17, 2021. For purposes of computing the percentages of outstanding shares of common stock held by each person, any shares that the person has the right to acquire within 60 days after December 17, 2021 are deemed to be outstanding with respect to such person but are not deemed to be outstanding for the purpose of computing the percentage of ownership of any other person.
| 58 |
More than 5% Beneficial Holders
| Name of beneficial owner | Amount beneficially owned | Percent
of Class Beneficially Owned(4) | ||||||
| Checkmate Capital Group LLC, Checkmate Strategic Capital 2, LLC, Checkmate Strategic Capital Holdings LLC, and Charles Thomas Paschall | 9,169,193 | (1) | 13.4 | % | ||||
| GSB Holdings, Inc. | 4,107,911 | (2) | 6.0 | % | ||||
| Strategic Assets Planning Ltd. | 3,430,632 | (3) | 5.1 | % | ||||
| (1) | The information is based on a Schedule 13D filed by Checkmate Strategic Capital 2, LLC with the SEC on January 26, 2021, reporting its beneficial ownership along with Checkmate Capital Group LLC, Checkmate Strategic Capital Holdings LLC, and Charles Thomas Paschall, members of its “group” as that term is defined in Section 13(d) of the Exchange Act. Includes 475,000 and 1,912,500 shares of common stock receivable by certain members of the group upon exercise of warrants and conversion of Series B preferred stock, respectively. Mr. Paschall and Checkmate Strategic Capital 2, LLC each have disclaimed beneficial ownership with respect to certain securities of the Company as reported on Schedule 13D except to the extent of their pecuniary interest therein. Their address is 595 E. Colorado Blvd., Suite 530, Pasadena, CA 91101. |
| (2) | The address for GSB Holdings, Inc. is: 14179 Laurel Trail, Wellington, FL 33414. Includes 166,667, 1,562,500, and 500,000 shares of common stock receivable upon exercise of warrants, and conversion of Series B preferred stock and convertible notes, respectively. |
| (3) | The address for Strategic Assets Planning Ltd. is: Suite 122, 12/F, Wing On Centre, 111 Connaught Road Central, Hong Kong. Includes 1,562,500 shares of common stock receivable upon conversion of Series B preferred stock. |
| (4) | The percentages shown are based on the 66,169,166 shares of our common stock outstanding as of December 17, 2021. |
| 59 |
DESCRIPTION OF OUR SECURITIES
General
The following descriptions of our capital stock and certain provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to the amended and restated certificate of incorporation and amended and restated bylaws that will be in effect upon completion of this offering. Copies of these documents will be filed with the Securities and Exchange Commission as exhibits to our registration statement, of which this prospectus forms a part. The descriptions of the common stock and preferred stock reflect changes to our capital structure that will occur upon the completion of this offering and Delaware law.
Upon the completion of this offering, and after giving effect to the reverse stock split, our amended and restated certificate of incorporation will provide for common stock and will authorize shares of undesignated preferred stock, the rights, preferences and privileges of which may be designated from time to time by our board of directors. In addition, our certificate of designation filed in connection with Series A preferred stock will continue to remain valid unless terminated.
Our authorized capital stock consists of 305,000,000 shares, all with a par value of $0.0001 per share, of which 300,000,000 shares are authorized as common stock and 5,000,000 shares are designated as preferred stock.
As of December 17, 2021, we had outstanding 66,169,164 shares of common stock held by approximately 450 stockholders of record, 420 shares of Series A preferred stock and 1,509 shares of Series B preferred stock.
Common Stock
Dividend Rights
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of legally available funds. See “Dividend Policy.”
Voting Rights
Except as required by law or matters relating solely to the terms of preferred stock, each outstanding share of common stock is entitled to one vote on all matters submitted to a vote of stockholders. Holders of shares of our common stock shall have no cumulative voting rights. Except in respect of matters relating to the election and removal of directors on our board of directors and as otherwise provided in our amended and restated certificate of incorporation or required by law, all matters to be voted on by our stockholders must be approved by a majority of the shares present in person or by proxy at the meeting and entitled to vote on the subject matter. In the case of election of directors, all matters to be voted on by our stockholders must be approved by a plurality of the votes of the shares present or represented by proxy at the meeting or via written consent in lieu of the meeting.
Liquidation
In the event of liquidation, dissolution or winding up of our Company, holders of our common stock are entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences and privileges of the holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock.
Preferred Stock
As of December 17, 2021, there were 1,509 shares of Series B convertible preferred stock outstanding, which will automatically convert upon the closing of this offering into approximately 9.43 million shares of common stock, not including accrued dividends also payable in common stock.
| 60 |
The Company has designated 1,500 shares as Series A preferred stock. As of December 17, 2021, there were 420 shares of Series A preferred stock outstanding. The Series A preferred stock (“Series A Stock”) currently converts into common stock at $0.16 per share, subject to price protection provisions in the instance certain shares are issued at a lower price. The Series A Stock bears a 6% dividend per annum, calculable and payable per quarter in cash or additional shares of common stock as determined in the Certificate of Designation. The Series A Stock has no voting rights until converted to common stock and has a liquidation preference equal to the aggregate purchase price of $600,000 plus accrued dividends. The Series A Stock is currently in default for failure to redeem all the shares by July 1, 2019, and the Company is negotiating a modification with the holders, including the possible conversion of these shares into common stock.
On December 6, 2021 all holders of the Company’s Series E-1 preferred stock agreed to exchange their preferred shares into 28,839,428 shares of common stock, and all Series E-1 stock was retired. All shares of common stock issued to these shareholders remain subject to the same vesting schedules as was originally provided in each shareholder’s E-1 shares issuance agreement and as further mentioned in the exchange agreement and plan of reorganization, and therefore, are forfeitable if certain employment conditions are not met prior to vesting as described in their respective employment agreements.
Convertible Notes
Beginning October 31, 2021, we entered into convertible note purchase agreements with eight accredited investors, pursuant to which we issued an aggregate of $605,000 of convertible notes (the “Notes”). The Notes mature on December 31, 2023 and are convertible into shares of common stock of the Company in the event of future equity financing of $5 million or greater, NASDAQ uplisting, or at the discretion of the noteholders, at a conversion price of $0.20 per share. The obligations under the Notes are unsecured. The Company has agreed to pay simple interest at the rate of 6% per annum on the outstanding amount of the Notes until fully repaid or converted. In connection with the Notes offering, the Company issued 1,008,334 warrants to the noteholders, with each warrant convertible into one share of common stock at an exercise price of $0.60 per share beginning from the date of the warrant until October 31, 2022. The outstanding balance of the Notes as of December 17, 2021 was $605,000, exclusive of accrued interest.
Options
As of December 17, 2021, there were options outstanding to purchase 1,112,619 shares of our common stock pursuant to the Company’s Equity Incentive Plans.
Warrants
As of December 17, 2021, there were warrants outstanding to purchase 1,483,322 shares of common stock, not including an additional 1,237,664 warrants that the Company is obligated to issue under a service contract that has not been issued as of December 17, 2021.
Authorized but Unissued Capital Stock
We have authorized but unissued shares of preferred stock and common stock, and our board of directors may authorize the issuance of one or more series of preferred stock without stockholder approval. These shares could be used by our board of directors to make it more difficult or to discourage an attempt to obtain control of us through a merger, tender offer, proxy contest or otherwise.
Limitation on Liability and Indemnification Matters
Our certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| ● | any breach of the director’s duty of loyalty to us or our stockholders; | |
| ● | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or | |
| ● | any transaction from which the director derived an improper personal benefit. |
Our certificate of incorporation provides that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. We also maintain directors’ and officers’ liability insurance.
The limitation of liability and indemnification provisions in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against our directors and officers for breach of their fiduciary duty. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder’s investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers as required by these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
Transfer Agent and Registrar
The stock transfer agent for our securities is Transfer Online Inc., 512 SE Salmon Street, Portland, OR 97214, and its telephone number is (503) 227-2950.
Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “QSAM”. No assurance can be given that our application will be approved or that a trading market will develop. Our common stock is currently traded on OTCQB under the symbol “QSAM.” On December 15, 2021, the last reported sale price of our common stock was $0.28 per share.
| 61 |
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering our common stock was traded on the OTCQB. In connection with this offering, we have applied to list our common stock on the Nasdaq Capital Market. No assurance can be given that our application will be approved. Sales of substantial amounts of our common stock in the public market, or the perception that such sales could occur, could adversely affect prevailing market prices of our common stock. Upon completion of this offering, we will have an aggregate of shares of common stock issued and outstanding, assuming the underwriter does not exercise its over-allotment option. Of these shares, all of the common stock sold in this offering will be freely transferable without restriction or further registration under the Securities Act, except that any shares purchased by one of our “affiliates,” as that term is defined in Rule 144 under the Securities Act, or Rule 144, may be sold only in compliance with the limitations described below. The remaining shares of common stock held by our existing stockholders are “restricted securities” as defined in Rule 144. Restricted shares may be sold in the public market only if registered under the Securities Act or if they qualify for an exemption from registration, including, among others, the exemptions provided by Rule 144. As a result of the contractual 180-day lock-up period described in “Underwriting” and the provisions of Rules 144, these shares will be available for sale in the public market as follows:
| ● | beginning on the date of this prospectus, the shares of common stock sold in this offering will be immediately available for sale in the public market; | |
| ● | beginning 91 and 181 days after the date of this prospectus respectively, and additional shares of common stock may become eligible for sale in the public market upon the satisfaction of certain conditions as set forth in “Lock-Up Agreements,” all of which shares would be held by our affiliates and subject to the volume and other restrictions of Rule 144, as described below; | |
| ● | the remainder of the shares of common stock will be eligible for sale in the public market from time to time thereafter, subject in some cases to the volume and other restrictions of Rule 144, as described below. |
Lock-up Agreements
All of our directors and executive officers and certain holders of our outstanding common stock signed lock-up agreements in connection with this offering pursuant to which, subject to certain exceptions, they agreed not to sell or otherwise dispose of their shares of common stock or any securities convertible into or exchangeable for common stock for a period of at least 180 days (in the case of directors and officers) or 90 days in the case of other stockholders after the date of this prospectus without the prior written consent of the underwriter. Approximately % of our issued and outstanding common stock prior to the offering will be subject to such lock-up agreements.
Rule 144
Affiliate Resales of Restricted Securities
In general, a person who is an affiliate of ours or was an affiliate at any time during the 90 days before a sale and has completed the lock-up period mentioned in its lock-up agreement, provided they have beneficially owned shares of our common stock for at least 180 days, would be entitled to sell in “broker’s transactions” or certain “riskless principal transactions” or to market makers, a number of shares within any three-month period that does not exceed the greater of:
| ● | 1% of the number of shares of our common stock then outstanding; and | |
| ● | the average weekly trading volume in our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Affiliate resales under Rule 144 are also subject to the availability of current public information about us. In addition, if the number of shares being sold under Rule 144 by an affiliate during any three-month period exceeds 5,000 shares or has an aggregate sale price in excess of $50,000, the seller must file a notice on Form 144 with the SEC and NASDAQ concurrently with either the placing of a sale order with the broker or the execution directly with a market maker.
Non-Affiliate Resales of Restricted Securities
Under Rule 144, a person who is not an affiliate of ours at the time of sale, and has not been an affiliate at any time during the 90 days preceding a sale, and who has beneficially owned shares of our common stock for at least six months, is entitled to sell such shares subject only to the availability of current public information about us. If such person has held our shares for at least one year, such person can resell without regard to any Rule 144 restrictions, including the 90-day public company requirement and the current public information requirement.
Non-affiliate resales are not subject to the manner of sale, volume limitation or notice filing provisions of Rule 144.
Form S-8 Registration Statement
Following the completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register the common stock issued or reserved for issuance under our 2016 Equity Compensation Plan and other stock options granted by the Company, or collectively Equity Incentive Plans. The registration statement on Form S-8 will become effective automatically upon filing. Common stock issued upon exercise of a share option and registered under the Form S-8 registration statement will, subject to vesting and lock-up provisions and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY. IT DOES NOT COVER ALL SHARE TRANSFER RESTRICTION MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN LEGAL ADVISOR REGARDING THE PARTICULAR SECURITIES LAWS AND TRANSFER RESTRICTION CONSEQUENCES OF PURCHASING, HOLDING, AND DISPOSING OF THE COMMON STOCK INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGE IN APPLICABLE LAWS.
| 62 |
UNDERWRITING
ThinkEquity LLC is acting as representative of the underwriters. Subject to the terms and conditions of an underwriting agreement between us and the representative, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of shares of common stock listed next to its name in the following table:
| Underwriter | Number Shares of Common Stock | |||
| ThinkEquity LLC | ||||
| Total | ||||
The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to various conditions and representations and warranties, including the approval of certain legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are committed to take and pay for all of the shares being offered, if any are taken, other than the shares covered by the option described below unless and until this option is exercised.
Over-Allotment Option
We have granted a 45-day option to the representative of the underwriters to purchase up to additional shares of our common stock at a public offering price of $ per share, solely to cover over-allotments, if any. The underwriters may exercise this option for 45 days from the date of this prospectus solely to cover sales of shares of common stock by the underwriters in excess of the total number of shares of common stock set forth in the table above. If any of these additional shares are purchased, the underwriters will offer the additional shares on the same terms as those on which the shares are being offered.
Discounts and Commissions; Expenses
The underwriters propose initially to offer the shares of common stock to the public at the public offering price set forth on the cover page of this prospectus and to dealers at those prices less a commission not in excess of $ per share of common stock.
The following table shows the public offering price, underwriting discounts and commissions and proceeds before expenses to us. The information assumes either no exercise or full exercise of the over-allotment option we granted to the representative of the underwriters.
| Per Share | Total Without Over-allotment Option | Total With Over-allotment Option | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discount (7.5%) | $ | $ | $ | |||||||||
| Proceeds to us, before expenses | $ | $ | $ | |||||||||
We have agreed to pay a non-accountable expense allowance to the representative of the underwriters equal to 1% of the gross proceeds received at the closing of the offering. We have paid an advance of $35,000 to the representative, which will be applied against the out-of-pocket accountable expenses that will be paid by us to the underwriters in connection with this offering, and will be reimbursed to us to the extent not actually incurred in compliance with applicable FINRA rules.
| 63 |
We have also agreed to pay certain of the representative’s expenses relating to the offering, including (a) filing fees and expenses associated with the registration of shares by the Commission and review of the offering by FINRA; (b) all fees and expenses relating to the listing of the common stock on the Nasdaq Capital Market, including any fees charges by The Depository Trust for new securities; (c) all fees, expenses and disbursements relating to background checks of our officers and directors in an amount not to exceed $10,000 in the aggregate; (d) all fees, expenses and disbursements relating to the registration or qualification of the shares of our common stock under the “blue sky” securities laws of such states and other jurisdictions as the representative may reasonably designate; (e) all fees, expenses and disbursements relating to the registration, qualification or exemption of the shares of our common stock under the securities laws of such foreign jurisdictions as the representative may reasonably designate; (f) the costs associated with post-closing advertising of the offering in the national editions of the Wall Street Journal and New York Times; (g) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and Lucite tombstones, each of which the Company or its designee shall provide within a reasonable time after the closing of this offering in such quantities as the representative may reasonably request in an amount not to exceed $3,000 in the aggregate; (h) the fees and expenses of the Company’s accountants, transfer agents and public relations firm; (i) fees and expenses of the underwriters’ legal counsel not to exceed $125,000; (j) a $29,500 cost associated with the underwriters use of Ipreo’s book-building, prospectus tracking and compliance software for the offering; (k) up to $10,000 of ThinkEquity’s actual accountable “road show” expenses; (l) up to $30,000 of the representative’s market making and trading, and clearing firm settlement expenses for the offering; (m) the costs of all mailing and printing of the underwriting documents, Registration Statements, Prospectuses and all amendments, supplements and exhibits thereto and as many preliminary and final Prospectuses as ThinkEquity may reasonably deem necessary; (n) the costs of preparing, printing and delivering certificates representing the shares; and (o) $10,000 for data services and communications expenses; and (p) stock transfer and/or stamp taxes, if any, payable upon the transfer of securities from the Company to ThinkEquity.
Our total estimated expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, are approximately $ .
Representative’s Warrants
Upon closing of this offering, we have agreed to issue to the representative as compensation warrants to purchase up to shares of common stock (5% of the aggregate number of shares of common stock sold in this offering exclusive of the over-allotment option, or the representative’s warrants). The representative’s warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per share in this offering (excluding the over-allotment option). The representative’s warrants are exercisable at any time and from time to time, in whole or in part, during the four year period commencing six months from the effective date of the registration statement of which this prospectus is a part.
The representative’s warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(e)(1)(A) of FINRA. The representative (or permitted assignees under Rule 5110(e)(1)(A)) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants, nor will they engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from the effective date of the registration statement of which this prospectus is a part. In addition, the warrants provide for registration rights upon request, in certain cases. The sole demand registration right provided will not be greater than five years from the effective date of the registration statement in compliance with Rule 5110(g) of FINRA. The piggyback registration rights provided will not be greater than seven years from the effective date of the registration statement in compliance with Rule 5110(g) of FINRA. We will bear all fees and expenses attendant to registering the shares of common stock issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend or our recapitalization, reorganization, merger or consolidation. However, the warrant exercise price or underlying shares will not be adjusted for issuances of shares of common stock at a price below the warrant exercise price.
Lock-Up Agreements
Pursuant to “lock-up” agreements, we, our executive officers and directors, and holders of 5% or more of the outstanding shares of common stock, have agreed, without the prior written consent of the representative, not to directly or indirectly, offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of our common stock (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of our common stock), enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any other securities of ours or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of 180 days after the date of this prospectus in the case of our directors, executive officers, the Company and any successor of the Company and 90 days after the date of this prospectus in the case of 5% or more stockholders.
Pricing of this Offering
The public offering price for our common stock will be determined through negotiations between us and the underwriters. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
| 64 |
We offer no assurances that the public offering price of our common stock will correspond to the price at which our common stock will trade in the public market subsequent to this offering or that an active trading market for our common stock and warrants will develop and continue after this offering.
Right of First Refusal
Until 12 months from the closing date of this offering, the representative will have an irrevocable right of first refusal, in its sole discretion, to act as sole investment banker, book-runner, and/or sole exclusive placement agent, at the representative’s sole discretion, for each and every future public and private equity and debt offering, including all equity linked financings, during such 12 month period, on terms customary to the representative.
Discretionary Accounts
The underwriters do not intend to confirm sales of the shares of common stock offered hereby to any accounts over which they have discretionary authority.
Trading; Nasdaq Capital Market Listing
We have applied to list our common stock on the Nasdaq Capital Market under the symbol “QSAM.” No assurance can be given that our application will be approved or that a trading market will develop. Our common stock is currently traded on OTCQB, under the symbol “QSAM.” On December 15, 2021, the last reported sale price of our common stock was $0.28 per share.
Other
From time to time, certain of the underwriters and/or their affiliates may in the future provide, various investment banking and other financial services for us for which they may receive customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock in accordance with Regulation M under the Exchange Act. Specifically, the underwriters may over-allot in connection with this offering by selling more shares than are set forth on the cover page of this prospectus. This creates a short position in our common stock for its own account. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of common stock over-allotted by the underwriters is not greater than the number of shares of common stock that they may purchase in the over-allotment option. In a naked short position, the number of shares of common stock involved is greater than the number of shares common stock in the over-allotment option. To close out a covered short position, the underwriters may elect to exercise all or part of the over-allotment option. The underwriters must cover any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of common stock made by the underwriters in the open market prior to the completion of the offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter or dealer repays selling concessions allowed to it for distributing shares of common stock in this offering because the underwriter repurchases the shares of common stock in stabilizing or short covering transactions.
Finally, the underwriters may bid for, and purchase, shares of our common stock in market making transactions, including “passive” market making transactions as described below.
These activities may stabilize or maintain the market price of our common stock at a price that is higher than the price that might otherwise exist in the absence of these activities. The underwriters are not required to engage in these activities, and may discontinue any of these activities at any time without notice. These transactions may be effected on the national securities exchange on which our shares of common stock are traded, in the over-the-counter market, or otherwise.
Electronic Distribution
This prospectus in electronic format may be made available on websites or through other online services maintained by one or more of the underwriters, or by their affiliates. Other than this prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
| 65 |
Selling Restrictions
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of our common stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or our common stock in any jurisdiction where action for that purpose is required. Accordingly, our common stock may not be offered or sold, directly or indirectly, and this prospectus or any other offering material or advertisements in connection with our common stock may be distributed or published, in or from any country or jurisdiction, except in compliance with any applicable rules and regulations of any such country or jurisdiction.
European Economic Area Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| ● | to any legal entity which is a qualified investor as defined under the Prospectus Directive; | |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); | |
| ● | to fewer than 150 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
| 66 |
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts, or (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code Monétaire et Financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 ;and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1; and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
| 67 |
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ — $$ — Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and | |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and | |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
| 68 |
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
LEGAL MATTERS
Dickinson Wright PLLC, Ft. Lauderdale, Florida will pass upon the validity of the securities offered hereby. Certain legal matters in connection with this offering will be passed upon for the underwriter by Sichenzia Ross Ference LLP, New York, New York.
EXPERTS
The financial statements of QSAM Biosciences, Inc. as of December 31, 2020 and 2019 and for the interim period ended September 30, 2021 included in this Registration Statement, of which this Prospectus forms a part, have been so included in reliance on the report of D. Brooks and Associates CPAs, P.A., an independent registered public accounting firm (the report on the financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern) appearing elsewhere herein, given on the authority of said firm as experts in auditing and accounting.
| 69 |
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed with the registration statement. For further information about us and the securities offered hereby, we refer you to the registration statement and the exhibits filed with the registration statement. Statements contained in this prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. The SEC also maintains an internet website that contains reports, proxy statements and other information about registrants, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
In addition, we file periodic reports, proxy statements, and other information with the SEC pursuant to the Exchange Act. These reports, proxy statements, and other information will be available on the website of the SEC referred to above.
We also maintain a website at www.qsambio.com., through which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. However, the information contained in or accessible through our website is not part of this prospectus or the registration statement of which this prospectus forms a part, and investors should not rely on such information in making a decision to purchase our common stock in this offering.
| 70 |
QSAM Biosciences Inc.
CONSOLIDATED FINANCIAL STATEMENTS
TABLE OF CONTENTS
| F-1 |
QSAM BIOSCIENCES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
| September 30, | December 31, | |||||||
2021 | 2020 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses and other current assets | ||||||||
| Deferred offering costs | - | |||||||
| TOTAL CURRENT ASSETS | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Accrued payroll and related expenses | ||||||||
| Accrued interest - related parties | - | |||||||
| Notes payable - related parties | ||||||||
| Paycheck Protection Program Loan - current portion | - | |||||||
| Debentures | ||||||||
| Convertible bridge notes, at fair value | - | |||||||
| TOTAL CURRENT LIABILITIES | ||||||||
| Paycheck Protection Program Loan - net of current portion | - | |||||||
| TOTAL LIABILITIES | ||||||||
| Redeemable convertible preferred stock - Series A; $par value, designated
Series A, and and shares issued and outstanding (liquidation preference of $ | ||||||||
| STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Preferred stock, Series B, $par value; shares authorized, and shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | - | |||||||
| Preferred stock, Series E-1, $par value; shares authorized, and shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | - | |||||||
| Common stock, $par value, shares authorized, and shares issued and outstanding as of September 30, 2021 and December 31, 2020, respectively | ||||||||
| Unearned deferred compensation | ( | ) | ( | ) | ||||
| Subscription receivable | - | ( | ) | |||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) | ( | ) | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | $ | ||||||
See notes to the unaudited condensed consolidated financial statements.
| F-2 |
QSAM BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited)
| For the three months ended | For the nine months ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| REVENUES | $ | $ | $ | $ | ||||||||||||
| OPERATING EXPENSES FROM CONTINUING OPERATIONS | ||||||||||||||||
| Compensation and related expenses | ||||||||||||||||
| Professional fees | ||||||||||||||||
| General and administrative | ||||||||||||||||
| Research and development expenses | ||||||||||||||||
| Total Operating Expenses | ||||||||||||||||
| LOSS FROM CONTINUING OPERATIONS | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| OTHER INCOME (EXPENSE) FROM CONTINUING OPERATIONS | ||||||||||||||||
| Financing costs including interest | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Change in fair value of convertible bridge notes | - | ( | ) | - | ( | ) | ||||||||||
| Other miscellaneous income | - | - | - | |||||||||||||
| Gain on sale of equity method investment | - | - | ||||||||||||||
| Loss on debentures and accrued expenses converted to common stock | - | - | ( | ) | - | |||||||||||
| Gain on forgiveness of debt from Paycheck Protection Program | - | - | ||||||||||||||
| Loss on conversion of bridge notes including accrued interest and debt forgiveness | - | ( | ) | ( | ) | ( | ) | |||||||||
| Total Other Income (Expense) | ( | ) | ( | ) | ( | ) | ||||||||||
| Loss from continuing operations before income taxes | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| INCOME TAXES | - | - | - | - | ||||||||||||
| Loss from continuing operations | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| DISCONTINUED OPERATIONS: | ||||||||||||||||
| Income from discontinued operations before income taxes | - | - | ||||||||||||||
| INCOME TAXES | - | - | - | - | ||||||||||||
| Income from discontinued operations | - | - | ||||||||||||||
| NET LOSS | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| PREFERRED STOCK | ||||||||||||||||
| Series A and Series B preferred contractual dividends and deemed dividends | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS: BASIC AND DILUTED: | ||||||||||||||||
| CONTINUING OPERATIONS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| DISCONTINUED OPERATIONS | - | - | ||||||||||||||
| $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||
| WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: | ||||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| F-3 |
QSAM BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE THREE AND NINE MONTHS ENDED SEPTEMBER 30, 2021 AND 2020
(UNAUDITED)
| Series B Preferred Stock | Series E-1 Preferred Stock | Common Stock | Deferred | Additional | Total Stockholders’ | |||||||||||||||||||||||||||||||||||||||
| Shares | Par Value |
Shares | Par Value |
Shares | Par Value |
Stock-based Compensation |
Paid-In Capital |
Stock Subscription |
Accumulated Deficit |
Equity (Deficit) |
||||||||||||||||||||||||||||||||||
| Balance, January 1, 2021 | - | $ | $ | ( |
) | $ | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||||||||||||||||||||||||||||
| Stock-based compensation for services | - | - | - | - | ||||||||||||||||||||||||||||||||||||||||
| Conversion of debentures and accrued expenses | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||||
| Conversion of bridge notes and accrued interest to common stock | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||||
| Conversion of Series A preferred stock to common stock | - | - | - | - | - | - | ( |
) | ||||||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | |||||||||||||||||||||||||||||||
| Issuance of Series B, preferred stock for cash | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||||
| Issuance of Series B, conversion of notes payable to preferred stock | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Stock-based compensation to employees and directors | - | - | - | - | ( |
) | - | - | ||||||||||||||||||||||||||||||||||||
| Net loss for the three months ended March 31, 2021 | - | - | - | - | - | - | - | - | ( |
) | ( |
) | ||||||||||||||||||||||||||||||||
| Balance, March 31, 2021 | ( |
) | - | ( |
) | |||||||||||||||||||||||||||||||||||||||
| Stock-based compensation for services and warrant modification | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Deemed dividend from warrant modification | - | - | - | - | - | - | - | - | ( |
) | - | |||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | |||||||||||||||||||||||||||||||
| Stock-based compensation to employees and directors | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||
| Net loss period for the three months ended June 30, 2021 | - | - | - | - | - | - | - | - | - | ( |
) | ( |
) | |||||||||||||||||||||||||||||||
| Balance, June 30, 2021 | ( |
) | - | ( |
) | |||||||||||||||||||||||||||||||||||||||
| Conversion of Series B preferred stock to common stock | ( |
) | - | - | - | - | ( |
) | - | - | - | |||||||||||||||||||||||||||||||||
| Issuance of stock for services | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||||
| Incremental value from warrant modifications | - | - | - | - | - | - | - | - | ( |
) | - | |||||||||||||||||||||||||||||||||
| Cumulative contractual dividends of Series B preferred stock | - | - | - | - | - | - | - | - | ( |
) | - | |||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | |||||||||||||||||||||||||||||||
| Cumulative contractual dividends of Series B preferred stock converted to common stock | - | - | - | - | - | - | - | - | ( |
) | - | |||||||||||||||||||||||||||||||||
| Compensation expense due to warrant modification | - | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||
| Stock-based compensation to employees and directors | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Net loss for the three months ended September 30, 2021 | - | - | - | - | - | - | - | - | - | ( |
) | ( |
) | |||||||||||||||||||||||||||||||
| Balance, September 30, 2021 | $ | $ | $ | $ | ( |
) | $ | $ | $ | ( |
) | $ | ||||||||||||||||||||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| F-4 |
| Series B Preferred Stock | Series E-1 Preferred Stock | Common Stock | Deferred Stock-based | Additional Paid In |
Stock |
Accumulated | Total Stockholders’ |
||||||||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Shares | Value | Compensation | Capital | Receivable | Deficit | Deficit | |||||||||||||||||||||||||||||||||
| Balance, December 31, 2019 | $ | $ | - | $ | $ | $ | $ | - | $ | ( |
) | $ | ( |
) | |||||||||||||||||||||||||||||
| Stock-based compensation for services | - | - | - | - | ( |
) | - | - | |||||||||||||||||||||||||||||||||||
| Stock-based compensation expense and stock option modification | - | - | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | ||||||||||||||||||||||||||||||
| Net loss for the three months ended March 31, 2020 | - | - | - | - | - | - | - | - | ( |
) | ( |
) | |||||||||||||||||||||||||||||||
| Balance, March 31, 2020 | $ | $ | - | $ | $ | ( |
) | $ | $ | - | $ | ( |
) | $ | ( |
) | |||||||||||||||||||||||||||
| Stock-based compensation for services | - | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | ||||||||||||||||||||||||||||||
| Net loss period for the three months ended June 30, 2020 | - | - | - | - | - | - | - | - | ( |
) | ( |
) | |||||||||||||||||||||||||||||||
| Balance, June 30, 2020 | $ | $ | - | $ | $ | ( |
) | $ | $ | - | $ | ( |
) | $ | ( |
) | |||||||||||||||||||||||||||
| Stock-based compensation for services | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||||
| Conversion of bridge notes and accrued interest to common stock | - | - | - | - | - | - | - | ||||||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | - | - | - | ( |
) | - | - | ( |
) | ||||||||||||||||||||||||||||||
| Net loss for the three months ended September 30, 2020 | - | - | - | - | - | - | - | - | ( |
) | ( |
) | |||||||||||||||||||||||||||||||
| Balance, September 30, 2020 | $ | $ | - | $ | $ | $ | $ | - | $ | ( |
) | $ | ( |
) | |||||||||||||||||||||||||||||
See notes to the unaudited condensed consolidated financial statements.
| F-5 |
QSAM BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS
(UNAUDITED)
For the nine months ended | For the nine months ended | |||||||
| September 30, | September 30, | |||||||
| 2021 | 2020 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Stock-based compensation for services and warrant modification | ||||||||
| Stock-based compensation to employees and directors | ||||||||
| Stock-based compensation and stock option modification | - | |||||||
| Loss on conversion bridge notes and accrued interest | - | |||||||
| Loss on conversion of debentures and accrued expense to common stock | - | |||||||
| Change in fair value of convertible bridge notes | - | |||||||
| Amortization of debt issuance costs | - | |||||||
| Paid-in-kind interest - convertible bridge notes | ||||||||
| Loss on extinguishment of debt | - | |||||||
| Gain on forgiveness of debt | ( | - | ||||||
| Changes in operating assets and liabilities | ||||||||
| Increase in prepaid expenses and other current assets | ( | ) | ( | ) | ||||
| Deferred offering costs | ( | ) | - | |||||
| (Decrease) increase in accounts payable and accrued expenses | ( | ) | ||||||
| Increase accrued payroll and related expenses | - | |||||||
| Increase in accrued interest– related parties | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from convertible notes payable and notes payable - related parties | - | |||||||
| Repayment on promissory notes – related party | ( | ) | ||||||
| Proceeds from convertible notes payable | - | |||||||
| Proceeds for the issuance of preferred stock – Series B | ||||||||
| Proceeds from Paycheck Protection Program | - | |||||||
| Net cash provided by financing activities | ||||||||
| NET INCREASE IN CASH | ||||||||
| CASH - Beginning of period | ||||||||
| CASH - End of period | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOW DISCLOSURES: | ||||||||
| Payment of interest in cash | $ | $ | ||||||
| Payment of income taxes | $ | $ | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Accrual of contractual dividends on Series A convertible preferred stock | $ | $ | ||||||
| Accrual of contractual dividends on Series B convertible preferred stock | $ | $ | - | |||||
| Deemed dividend on conversion of Series A preferred stock to common stock | $ | $ | ||||||
| Deemed Dividend on warrant modifications | $ | $ | ||||||
| Conversion of convertible bridge notes and accrued interest to 6,627,692 and 11,418,069 shares of common stock, respectively | $ | $ | ||||||
| Conversion of debentures and accrued expenses to common stock | $ | $ | ||||||
| Conversion of Series A preferred stock to common stock | $ | $ | ||||||
| Conversion of notes payable with related parties to Series B preferred stock and warrants | $ | $ | ||||||
See notes to the condensed consolidated financial statements.
| F-6 |
QSAM BIOSCIENCES INC.
NOTES TO THE UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
QSAM
Biosciences Inc. (hereinafter the “Company”, “we”, “our”, “us”), incorporated in Delaware
on August 26, 2004, is currently engaged in the business of developing a novel radiopharmaceutical drug candidate for the treatment of
bone cancer. This business line commenced in earnest in the fourth fiscal quarter of 2020 as a result of the separation and transfer
pursuant to an Omnibus Separation Agreement dated November 6, 2020 (the “Separation Agreement”) of the Company’s prior
business of managing compost and soil manufacturing facilities (the “Legacy Business”) through an unconsolidated investee
entity called Earth Property Holdings LLC, a Delaware limited liability company (“EPH”). Pursuant to the Separation Agreement,
the Company transferred to EPH all assets and related liabilities in connection with the Legacy Business in return for a forgiveness
of debt. The financial statements presented herein have been adjusted to account for the Legacy Business as discontinued operations (see
Note 4 – Separation Agreement and Note 9 – Discontinued Operations). The Company sold its entire equity interest in EPH to
a third party in the first quarter of 2021 for $
In April 2020, the Company established QSAM Therapeutics Inc. (“QSAM”) as a wholly-owned subsidiary incorporated in the state of Texas, and through QSAM, executed a Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) with IGL Pharma, Inc. (“IGL”). The License Agreement provides QSAM with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and knowhow with respect to Samaium-153 DOTMP aka CycloSam® (the “Technology”), a clinical stage novel radiopharmaceutical meant to treat different types of bone cancer and related diseases.
In connection with the transition to the biosciences sector, the Company changed its name to QSAM Biosciences Inc. on September 4, 2020, and subsequently changed its stock symbol to QSAM, to better reflect its business moving forward.
Prior to 2017, the Company owned and licensed technology that converts waste fuels and heat to power, which it sold to a licensee in August of that year. Much of these operations were conducted through a wholly-owned subsidiary of the Company called Q2Power Corp. (“Q2P”), which still exists but has no current operations. Q2P and QSAM are sometimes referred to herein as the “Subsidiary”. Formerly, the Company’s name was Q2Power Technologies, Inc., and before that, Anpath Group, Inc.
The recent outbreak of the novel coronavirus (COVID-19) is impacting worldwide economic activity. COVID-19 poses the risk that we or our employees and our other partners may be prevented from conducting business activities for an indefinite period of time, including due to the spread of the disease or shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the full impact that COVID-19 could have on our business, the continued spread of COVID-19 could disrupt our research and development of CycloSam and other related activities, which could have a material adverse effect on our business, financial condition and results of operations. In addition, a severe or prolonged economic downturn could result in a variety of risks to the business. While we have not yet experienced any material disruptions in our business or other negative consequences relating to COVID-19, the extent to which the COVID-19 pandemic impacts our results will depend on future developments that are highly uncertain and cannot be predicted.
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN
The accompanying unaudited condensed financial statements are prepared in accordance with Rule 8-01 of Regulation S-X of the Securities Exchange Commission (“SEC”). Accordingly, certain information and note disclosures normally included in financial statements prepared in accordance with generally accepted accounting principles in the United States of America (“US GAAP”) have been condensed or omitted pursuant to such rules and regulations. However, the Company believes that the disclosures included in these unaudited condensed financial statements are adequate to make the information presented not misleading. The unaudited condensed financial statements included in this document have been prepared on the same basis as the annual financial statements, and in our opinion reflect all adjustments, which include normal recurring adjustments necessary for a fair presentation in accordance with US GAAP and SEC regulations for interim financial statements. The results for the three and nine months ended September 30, 2021 are not necessarily indicative of the results that the Company will have for any subsequent period or for the calendar year ended December 31, 2021. These unaudited condensed financial statements should be read in conjunction with the audited financial statements and the notes to those statements for the year ended December 31, 2020 which was filed with the SEC on April 15, 2021.
| F-7 |
The
Company raised a total of $
The
Company’s convertible debentures of $
For
the nine months ended September 30, 2021, the Company used net cash in operating activities for its continuing operations of $
The Company has supported operations through the issuance of common stock, preferred stock and debt over the last 12 months. This includes the Series B preferred stock offering in the first quarter of 2021, the recent exercise of warrants issued in connection with the Series B offering, and also a recent convertible debt offering conducted after the end of the third quarter of 2021. Management expects expenses to increase in 2022 as our drug technology enters into clinical trials, and as a result, we will need to raise additional capital to support these operations. Management believes that it can do so through equity raises in 2022; however, there is no guarantee that such plan will be successful. If we are not successful in raising additional capital, we may need to delay clinical trials, reduce overhead, or in the most extreme scenario, shut down operations.
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. There is no guarantee whether the Company will be able to generate revenue and/or raise capital sufficient to support its continuing operations. The ability of the Company to continue as a going concern is dependent on management’s plans which include implementation of its business model to develop and commercialize its drug candidate, seek strategic partnerships to advance clinical trials and other research endeavors which could provide additional capital to the Company, and continue to raise funds for the Company through equity or debt offerings. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. The unaudited condensed financial statements do not include any adjustments that might result from the outcome of these uncertainties.
In
January 2021, the Company closed a $
Subsequent to
September 30, 2021, eight non-affiliated investors in the Company’s Series B Preferred stock exercised their warrants at
$
Also, subsequent to
September 30, 2021, the Company issued six convertible promissory notes in a private placement offering among six non-affiliated
accredited investors in the total amount of $
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The unaudited condensed financial statements include the accounts of the Company and its Subsidiaries. All significant inter-company transactions and balances have been eliminated in consolidation. References herein to the Company include the Company and its Subsidiary unless the context otherwise requires.
| F-8 |
Cash and Cash Equivalents
The
Company considers cash, short-term deposits, and other investments with original maturities of no more than ninety days when acquired
to be cash and cash equivalents for the purposes of the statement of cash flows. The Company maintains cash balances at one financial
institution and has experienced no losses with respect to amounts on deposit. The Company held
Revenue Recognition
The Company recognizes revenue in accordance with ASC Topic 606, “Revenue from Contracts with Customers (“ASC 606”) and all the related amendments.
The core principle of ASC 606 requires that an entity recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. ASC 606 defines a five-step process to achieve this core principle and, in doing so, it is possible more judgment and estimates may be required within the revenue recognition process than previously required under U.S. GAAP, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation.
The
Company had
The Company applies the fair value method of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718, “Share Based Payment”, in accounting for its stock-based compensation with employees and non-employees. This standard states that compensation cost is measured at the grant date based on the fair value of the award and is recognized over the service period, which is usually the vesting period. The Company values stock-based compensation at the market price for the Company’s common stock and other pertinent factors at the grant date.
The Black-Scholes option pricing valuation method is used to determine fair value of stock options consistent with ASC 718, “Share Based Payment”. Use of this method requires that the Company make assumptions regarding stock volatility, dividend yields, expected term of the awards and risk-free interest rates.
Research and Development
Research
and development costs are expensed as incurred. Research and development costs were $
Fair Value Measurement
The Company measures fair value in accordance with a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The Company’s convertible Bridge Notes are valued by using Monte Carlo Simulation methods and discounted future cash flow models. Where possible, the Company verifies the values produced by its pricing models to market prices. Valuation models require a variety of inputs, including contractual terms, market prices, yield curves, credit spreads, measures of volatility and correlations of such inputs. These convertible Bridge Notes do not trade in liquid markets, and as such, model inputs cannot generally be verified and do involve significant management judgment. Such instruments are typically classified within Level 3 of the fair value hierarchy.
| F-9 |
Equity Method Investment
Investments in partnerships, joint ventures and less-than majority-owned subsidiaries in which we have significant influence are accounted for under the equity method. The Company’s consolidated net income includes the Company’s proportionate share of the net income or loss of our equity method investee. When we record our proportionate share of net income, it increases income (loss) — net in our consolidated statements of operations and our carrying value in that investment. Conversely, when we record our proportionate share of a net loss, it decreases income (loss) — net in our consolidated statements of income and our carrying value in that investment. The Company’s proportionate share of the net income or loss of our equity method investees includes significant operating and nonoperating items recorded by our equity method investee. These items can have a significant impact on the amount of income (loss) — net in our consolidated statements of operations and our carrying value in those investments. The Company divested its investment in its equity method investee in March 2021.
Discontinued Operations
In accordance with ASC 205-20 Presentation of Financial Statements: Discontinued Operations, a disposal of a component of an entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results when the components of an entity meets the criteria in paragraph 205-20-45-10. In the period in which the component meets held-for-sale or discontinued operations criteria the major current assets, other assets, current liabilities, and noncurrent liabilities shall be reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations, less applicable income taxes (benefit), shall be reported as components of net income (loss) separate from the net income (loss) of continuing operations.
The Company disposed of a component of its business pursuant to a Separation Agreement in November 2020, which met the definition of a discontinued operation. Accordingly, the operating results of the business disposed are reported as income (loss) from discontinued operations in the accompanying unaudited condensed statements of operations for the three months ended September 30, 2021 and 2020. For additional information, see Note 4 – Separation Agreement and Note 9 - Discontinued Operations.
Income Taxes
Income
taxes are accounted for under the asset and liability method as stipulated by FASB ASC 740, “Income Taxes” (“ASC
740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the
financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit
carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years
in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities
or a change in tax rate is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced to estimated
amounts to be realized by the use of a valuation allowance.
In the event that an uncertain tax position exists in which the Company could incur income taxes, the Company would evaluate whether there is a probability that the uncertain tax position taken would be sustained upon examination by the taxing authorities. Reserves for uncertain tax positions would be recorded if the Company determined it is probable that a position would not be sustained upon examination or if payment would have to be made to a taxing authority and the amount is reasonably estimated. As of September 30, 2021 and December 31, 2020, the Company does not believe it has any uncertain tax positions that would result in the Company having a liability to the taxing authorities; however, federal returns have not been filed since the Company’s inception in 2014. Such delinquencies are being resolved by management and a retained tax expert. Interest and penalties related to any unrecognized tax benefits is recognized in the unaudited condensed consolidated financial statements as a component of income taxes. The Company will need to be in compliance with the tax authorities by filing past federal and state income tax returns.
| F-10 |
Net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period plus any potentially dilutive shares related to the issuance of stock options, shares from the issuance of stock warrants, shares issued from the conversion of redeemable convertible preferred stock and shares issued for the conversion of convertible debt.
| Shares from the conversion of Series B Preferred Stock not inclusive of dividends | ||||
| Shares from the conversion of Series E-1 Preferred Stock (subject to and potential forfeiture) | ||||
| Shares from common stock options | ||||
| Shares from common stock warrants | ||||
| Shares from the conversion of debentures | ||||
| Shares from the conversion of redeemable convertible preferred stock (based upon an assumed conversion price at September 30, 2021 of $ |
As of September 30, 2020, there were the following potentially dilutive securities that were excluded from diluted net loss per share because their effect would be anti-dilutive:
| Shares from common stock options | ||||
| Shares from common stock warrants | ||||
| Shares from the conversion of debentures | ||||
| Shares that may be converted from Bridge Notes (based upon an assumed conversion price at September 30, 2020 of $ per share) | ||||
| Shares from the conversion of redeemable convertible preferred stock (inclusive of cumulative dividends which may be converted to shares of common stock under certain conditions). |
Significant Estimates
U.S. Generally Accepted Accounting Principles (“GAAP”) requires the Company to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the unaudited financial statements, the reported amounts of revenues and expenses, cash flows and the related footnote disclosures during the period. On an on-going basis, the Company reviews and evaluates its estimates and assumptions, including, but not limited to, those that relate to the fair value of stock-based compensation fair value of convertible bridge notes, and a valuation allowance on deferred tax assets and contingencies. Actual results could differ from these estimates.
Recent Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity. ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible instruments. ASU 2020-06 is effective January 1, 2022 and should be applied on a full or modified retrospective basis, with early adoption permitted beginning on January 1, 2021. The Company adopted ASU 2020-06 effective January 1, 2021. The adoption of ASU 2020-06 did not have an impact on the Company’s financial statements.
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on its unaudited financial statements.
Reclassifications
Certain reclassifications of prior year amounts have been made to conform to the 2021 presentation. These reclassifications had no effect on net loss or loss per share as previously reported.
| F-11 |
Concentration of Risk
The
Company expects cash to be the asset most likely to subject the Company to concentrations of credit risk. The Company’s bank deposits
may at times exceed federally insured limits. The Company’s policy is to maintain its cash with high credit quality financial institutions
to limit its risk of loss exposure. The Company’s cash balance as of September 30, 2021, is in excess of FDIC limits in the amount
of approximately $
The Company is subject to a number of risks similar to those of other companies at a clinical-stage for radiopharmaceutical drug candidates, including dependence on key individuals; the need to develop commercially viable therapeutics; competition from other companies, many of which are larger and better capitalized; and the need to obtain adequate additional financing to fund the development of its products. The Company currently depends on third-party, suppliers for key materials and services used in its research and development manufacturing process, and is subject to certain risks related to the loss of these third-party suppliers or their inability to supply the Company with adequate materials and services.
The Company had no revenue from its continuing operations for the three and nine months ended September 30, 2021 and 2020. Revenue included in discontinued operations was generated from one related customer for the three and nine months ended September 30, 2020.
Fair Value of Financial Instruments
In accordance with Accounting Standards Codification (“ASC”) 825, Financial Instruments, disclosures of fair value information about financial instruments are required, whether or not recognized in the balance sheet, for which it is practicable to estimate that value. Cash is carried fair value.
Other financial instruments, including accounts payable, accrued liabilities and short-term debt, are carried at cost, which approximates fair value given their short-term nature.
Deferred Offering Cost
Costs incurred prior to an equity offering are capitalized until the offering occurs. Upon the equity offering, all accumulated costs are charged against proceeds. If the Company determines that the equity offering will not occur, the accumulated costs are charged to operations.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. To date, the Company views its operations and manages its business as one segment.
NOTE 4 – SEPARATION AGREEMENT
On November 6, 2020, the Company entered into the Separation Agreement with its unconsolidated investee, EPH. The Company’s board of directors approved the Separation Agreement in support of the Company’s previously disclosed plan to secure new technologies and business opportunities in the broader biosciences sector, and to significantly reduce debt and liabilities of the Company and eliminate under-performing assets and agreements. The Separation Agreement resulted in the discontinuance of the Company’s management of businesses and assets focused on compost and soil manufacturing to focus solely on the development of its exclusively licensed pharmaceutical Technology, as well as other drug candidates that it may license or otherwise secure in the future. Pursuant to the Separation Agreement:
| ● | The Management Agreement, dated January 18, 2019, as amended, between EPH and the Company was terminated by mutual agreement of the parties. Fees from this agreement constituted most of the Company’s revenue over the prior two years. |
| ● | In
lieu of any severance or other termination payments due under the Management Agreement, EPH released the Company from a total of
$ | |
| ● | The
Company agreed to transfer to EPH its license agreement with Agrarian Technologies LLC and Mulch Masters Inc. for the ABS soil enhancement
product and all associated knowhow, trade secrets and trademark/service marks. Accrued license fees in connection with this license
agreement were also assumed by EPH in the amount of $ | |
| ● | The prior officers and employees of the Company engaged in the Legacy Business were released from any non-competition, non-solicitation or other restricted covenant pursuant to their respective employment agreements. Effective October 1, 2020, several of these employees had already separated from the Company. | |
| ● | EPH received the right in its sole discretion to use the name “Q2Earth” in all jurisdictions of the United States and worldwide. |
| F-12 |
Pursuant to ASC 205-20 Presentation of Financial Statements: Discontinued Operations and amended by ASU No. 2014-08, management has determined that the Separation Agreement results in the disposal of a component that represents a strategic shift in the Company’s business operations that will have a major effect on the Company’s operations and financial results. Therefore, the net income (loss) generated from this disposed component have been presented as discontinued operations for the period ended September 30, 2020 on the statement of operations.
NOTE 5 – EQUITY METHOD INVESTMENT
During
November 2018, the Company invested $
Our prior Chairman and CEO of the Company, also serves as President of EPH; and Christopher Nelson, General Counsel and Director of the Company, also serves as General Counsel and Secretary of EPH. See Note 6 – Related Party Transactions for transactions with our equity method investment during the three and nine months ended September 30, 2021 and 2020.
NOTE 6 – RELATED PARTY TRANSACTIONS
The
Company currently has a License Agreement with IGL Pharma, Inc., an entity in which the Company’s Executive Chairman serves as
President, holds options to purchase less than a
The Company currently maintains an executive office in Florida, which is leased by an investment firm in which the Company’s General Counsel serves as an officer but does not hold any equity or voting rights. The Company has no formal agreement for this space and pays no rent.
During
the three and months ended September 30, 2020, the Company received $
In
the nine-month period ended September 30, 2021, the Company paid to EPH $
During
the year ended December 31, 2020, the Company received $
| F-13 |
During
the three and nine months ended September 30, 2021, the Company incurred $
NOTE 7 – DEBENTURES, CONVERTIBLE BRIDGE NOTES, AND NOTES PAYABLE
Debentures
The
Company has Original Issue Discount Senior Secured Convertible Debentures (the “Debentures”) in the aggregate amount of $
Convertible Bridge Notes
In
2017 and 2018, the Company issued a total of $
As
of March 31, 2021, all Bridge Notes remaining at the end of 2020, inclusive of principal and accrued and capitalized interest, were settled
with the holders of these notes converting their debt into a total of shares of common stock of the Company with a
fair value of $
Pursuant
to ASC 825-10-25-1, Fair Value Option, the Company made an irrevocable election at the time of issuance to report the Bridge Notes at
fair value, with changes in fair value recorded through the Company’s condensed consolidated statements of operations as other
income (expense) in each reporting period. The estimated fair value of the remaining outstanding Bridge Notes as of September 30, 2021
and December 31, 2020 was $
Paycheck Protection Program
On
April 14, 2020, the Company received $
| F-14 |
NOTE 8 – FAIR VALUE MEASUREMENT
The Company measures fair value in accordance with a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described below:
| Level 1 | Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities; | |
| Level 2 | Quoted prices in markets that are not active, or inputs that are observable, either directly or indirectly, for substantially the full term of the asset or liability; and | |
| Level 3 | Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (supported by little or no market activity). |
As disclosed in Note 7, the Bridge Notes are reported at fair value, with changes in fair value recorded through the Company’s condensed consolidated statements of operations as a component of other income (expense) in each reporting period.
The following tables set forth the Company’s unaudited financial assets and liabilities measured at fair value by level within the fair value hierarchy as of September 30, 2021 and December 31, 2020. Assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible Bridge Notes | $ | $ | $ | $ | ||||||||||||
| Fair value as of September 30, 2021 | $ | $ | $ | $ | ||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible Bridge Notes | $ | $ | $ | - | $ | |||||||||||
| Fair value as of December 31, 2020 | $ | $ | $ | $ | ||||||||||||
The following tables present a reconciliation of the beginning and ending balances of items measured at fair value on a recurring basis that use significant unobservable inputs (Level 3) that has been recorded in the condensed consolidated balance sheets which is as follows:
| Fair value, December 31, 2020 | $ | |||
| Accrued interest | ||||
| Conversion to shares of common stock | ( | ) | ||
| Fair value, September 30, 2021 | $ |
NOTE 9 – DISCONTINUED OPERATIONS
On November 6, 2020, the Company executed a Separation Agreement (see Note 4 – Separation Agreement), whereby the Company transferred its Legacy Business and the related assets and liabilities to EPH, a related party and equity method investee.
ASC 205-20 “Discontinued Operations” establishes that the disposal or abandonment of a component of an entity or a group of components of an entity should be reported in discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s unaudited operations and financial results. As a result, the component’s results of operations have been reclassified as discontinued operations on a retrospective basis for the period ended September 30, 2020. There were no results of operations from the component in the current period. As of September 30, 2021, there were no assets or liabilities held associated with this business. The results of operations of this component, for all periods, are separately reported as “discontinued operations” on the unaudited condensed statements of operations.
As disclosed in Note 4 – Separation Agreement, the Company sold its equity interest in EPH as of March 31, 2021. There have been no transactions between the Company and EPH since the Separation Agreement.
A reconciliation of the major classes of line items constituting the income (loss) from discontinued operations, net of income taxes as is presented in the consolidated statements of operations for the three and nine month ended September 30, 2021 and 2020, are summarized below:
| F-15 |
Reconciliation of revenue and expense items in discontinued operations in the unaudited condensed statements of operations:
| For the Three Months Ended | For the Nine Months Ended | |||||||
| September 30, | September 30, | |||||||
| 2020 | 2020 | |||||||
| REVENUES | $ | $ | ||||||
| OPERATING EXPENSES | ||||||||
| Payroll and related expenses | 106,310 | 320,463 | ||||||
| General and administrative | ||||||||
| Total operating expenses | 119,872 | 371,838 | ||||||
| Operating Income | ||||||||
| Financing costs including interest | ( | ) | ( | ) | ||||
| OTHER EXPENSE | ( | ) | ( | ) | ||||
| INCOME FROM DISCONTINUED OPERATIONS | $ | $ | ||||||
Reconciliation of cash flows from operating activities and financing activities on the unaudited condensed statements of cash flows:
| Nine Months ended | ||||
| September 30, | ||||
| 2020 | ||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||
| Net income from discontinued operations | $ | |||
| Adjustments to reconcile net income to net cash provided by discontinued operations: | ||||
| Changes in operating assets and liabilities | ||||
| Increase in accounts payable and accrued expenses | ||||
| Increase in accrued interest - related party | ||||
| Net cash provided by operating activities | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||
| Proceeds from promissory notes - related parties | ||||
| Net cash provided by financing activities | ||||
| Net cash provided by discontinued operations | $ | |||
NOTE 10 – COMMON STOCK, PREFERRED STOCK AND WARRANTS
Common Stock
During
the three months ended September 30, 2021, the Company issued shares of common stock in connection with
the conversion of Series B Preferred stock with an original investment amount of $
As
of March 31, 2021, $
In September 2020, the Company
issued shares of common stock to three consultants in connection with services provided. All services were provided in the third
quarter of 2020, and the Company incurred a professional fee expense of $
The Company issued
shares of common stock in the first quarter of 2020 in connection with a services contract valued at $
| F-16 |
Series A Redeemable Convertible Preferred Stock
The
Company has 480 shares of Preferred Stock issued and outstanding as of September 30, 2021, which currently are convertible at $
The
Preferred Stock has price protection provisions in the case that the Company issues any shares of stock not pursuant to an “Exempt
Issuance” at a price below the Conversion Price. Exempt Issuances include: (i) shares of Common Stock or common stock equivalents
issued pursuant to the original merger of the company or any funding contemplated by that transaction; (ii) any common stock or convertible
securities outstanding as of the date of closing; (iii) common stock or common stock equivalents issued in connection with strategic
acquisitions; (iv) shares of common stock or equivalents issued to employees, directors or consultants pursuant to a plan, subject to
limitations in amount and price; and (v) other similar transactions. The Certificate of Designation contains restrictive covenants not
to incur certain debt, repurchase shares of common stock, pay dividends or enter into certain transactions with affiliates without consent
of holders of
Management
has determined that the Preferred Stock is more akin to a debt security than equity primarily because it contains a mandatory
The
Preferred Stock carries a
Series B Preferred Stock
In
December 2020, the Company filed an amendment to its Articles of Incorporation to authorize the issuance of up to shares of Series B Convertible Preferred Stock
(the “Series B Stock”), par value $per share, pursuant to a Certificate of Designation.
The Series B Preferred Shares provide the holders a
| F-17 |
In
January 2021, the Company closed a private offering of its Series B Stock for $per share, raising a total of $
Series E-1 Preferred Stock
On
December 3, 2020, the Company filed an amendment to its Articles of Incorporation to authorize the issuance of up to shares of
Series E-1 Preferred Stock (the “Series E-1 Stock”) pursuant to a Certificate of Designation. The shares of Series E-1 Stock
are incentive-based, vesting and forfeitable securities that provide the holders the right in the aggregate to receive an “earnout”
equal to 20% of the total consideration received by the Company in the instance of a sale or sub-license of its core licensed radiopharmaceutical
Technology, or sale or merger of the Company, which is paid on a priority, senior basis. In addition, the holders of the Series E-1 Stock
can convert their vested preferred stock at anytime or after an event resulting in an earnout payment, such as an acquisition of the
Company, into an aggregate of
On
December 30, 2020, shares of Series E-1 Stock were issued to five
individuals, including the Company’s Executive Chairman, CEO, and General Counsel, which vest starting in July 2021
through January 2023 and are forfeitable by the holders prior to vesting. In February 2021, the remaining shares of Series E-1 Stock were issued to one
newly-appointed director, vesting half in February 2022 and the balance in February 2023. Upon these shares of Series E-1 preferred stock
becoming fully vested, they are convertible in the aggregate into shares of common stock. During the three and
nine months ended September 30, 2021, employee and director stock option expense for vested Series E-1 amounted to $and $,
respectively, which is included in compensation and related expenses on the condensed statements of operations. As of September 30, 2021,
$
The Company computed the total grant date fair value of the Series E-1 Stock to be approximately $ using an option pricing model and the following assumptions: (1) with respect to the shares granted in 2020: expected term of , dividend yield of --%, volatility of %, and a risk-free rate of %; and (2) with respect to the shares granted in 2021: expected term of , dividend yield of %, volatility of %, and a risk-free rate of %. The value of these shares will be recognized as stock-based compensation expense over the vesting period through February 2023.
Warrants
During
the nine months ended September 30, 2021, the Company issued
| F-18 |
The following is a summary of the outstanding common stock warrants as of September 30, 2021:
| Number of Warrants | Exercise price per share | Expiration Date | ||||||||||
| Warrants issued in connection with issuance of Series B Preferred Stock (1) | $ | |||||||||||
| Warrants issued in connection with issuance of Series B Preferred Stock to lead investor | $ | |||||||||||
| Warrants issued in connection with the issuance of Series B Preferred Stock to directors | $ | |||||||||||
| Warrants issued to a service provider (2) | $ | |||||||||||
| (1) |
| (2) | On September 22, 2021, the Company’s board of directors approved an extension of the expiration date of these warrants issued to a service provider from September 30, 2021 to October 15, 2021. |
During the period ended March 31, 2021, the Company issued a service provider fully vested warrants in connection with the execution of a service agreement to purchase shares of common stock for an exercise price of $and exercise period of six months. The Company computed the total grant date fair value of the warrants to be approximately $using a Black-Scholes option pricing model and the following assumptions: expected term of 0years, dividend yield of -%-, volatility of %, and a risk-free rate of %. The value of these warrants was recognized as stock-based compensation expense on the date of grant and is included in professional fees on the condensed consolidated statement of operations for the nine months ended September 30, 2021, as the warrants were fully earned upon issuance. On June 17, 2021, the term of these warrants was extended until September 30, 2021, resulting in incremental compensation expense of $ which has been included in professional fees in the condensed statement of operations for the nine months ended September 30, 2021.
On September 22,
2021, the term of the
On June 22, 2021, the
maturity of the
On September 22,
2021, the terms of all
In 2016 to compensate officers, directors and other key service providers with equity grants, the Board approved the 2016 Omnibus Equity Incentive Plan (“2016 Plan”), which initially allowed for shares of common stock, stock options, stock rights (restricted stock units), or stock appreciation rights to be granted by the Board in its discretion. This authorized amount was increased to shares by Board resolution and amendment in 2017, and further increased to million shares by Board resolution in 2021. There are currently no shares available under the 2016 Plan for future issuance; however, the Board may increase the authorized shares under the 2016 Plan each year.
| F-19 |
The Company issued stock options to purchase common stock to officers and directors of the Company during the three and nine months ended September 30, 2021 to officers and directors of the Company. These options have a year term, a vesting period of , and an exercise price of $per share. The Company recorded $of stock-based compensation expense to compensation and related expenses for the three and nine month ended September 30, 2021 for these stock options.
| Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||||
| Outstanding as of December 31, 2020 | $ | $ | - | |||||||||||||
| Granted | $ | $ | ||||||||||||||
| Outstanding as of September 30, 2021 | $ | $ | ||||||||||||||
| Exercisable as of September 30, 2021 | $ | 1.31 | $ | |||||||||||||
The aggregate intrinsic value of options exercised is the difference between the fair market value of the Company’s closing price of our common stock at each reporting date, less the exercise price multiplied by the number of options granted which was nil at September 30, 2021.
As of September 30, 2021, the unrecognized stock-based compensation of approximately $ is expected to be expensed over the next nine months based on the option vesting requirements. The weighted average fair value of options granted was $per share for the nine months ended September 30, 2021.
We estimate the fair value of stock-based awards on the date of grant using the Black-Scholes option pricing model using the fair market value of our common stock on the date of grant and a number of other assumptions. These assumptions include estimates regarding the expected term of the awards, estimates of the stock volatility over a duration that approximates the expected term of the awards, estimates of the risk-free rate, and estimates of expected dividend rates.
| Expected term (years) | ||||
| Expected volatility | % | |||
| Risk-free interest rate | % | |||
| Expected dividend yield | % | |||
Option Repricing
On January 6, 2020, the compensation committee of the Company’s Board of Directors, approved a one-time stock option repricing program (the “Option Repricing”) to permit the Company to reprice certain options to purchase the Company’s Common Stock held by its current directors, officers and employees (the “Eligible Options”), which actions became effective on January 6, 2020. Under the Option Repricing, Eligible Options with an exercise price at or above $per share (representing an aggregate of options, or of the total outstanding) were amended to reduce such exercise price to $per share.
The
impact of the Option Repricing was a one-time incremental non-cash charge of $
NOTE 12 – COMMITMENTS AND CONTINGENCIES
The employment agreements for the Company’s Executive Chairman and CEO each contain termination provisions whereby if they are terminated without cause or following a material change, as defined therein, they will receive salary through the date of termination plus an additional 12 months, bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest and healthcare benefits will continue for 12 months. The Company’s General Counsel’s employment agreement contains a twelve month severance payment in the instance of a termination without cause, as defined therein.
The
QSAM License Agreement requires multiple milestone-based payments including: $
Pursuant
to a services agreement signed in 2018, an additional
NOTE 13 – SUBSEQUENT EVENTS
On October 15, 2021,
eight non-affiliated accredited investors in the Company’s Series B Preferred stock offering exercised their warrants at
$
Commencing on October
31 and as of November 15, 2021, the Company issued six convertible promissory notes in a private placement offering among six
non-affiliated investors in the total amount of $
| F-20 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of QSAM Biosciences, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of QSAM Biosciences, Inc (f/k/a Q2Earth, Inc., and referred to as the “Company”) as of December 31, 2020 and 2019, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for the years then ended and the related notes to the consolidated financial statements (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred operating losses, has incurred negative cash flows from operations and has a working capital deficit. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plan regarding these matters is also described in Note 2 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit includes performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2019.
|
| Palm Beach Gardens, FL |
| April 15, 2021 |
| F-21 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2020 | 2019 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses and other assets | ||||||||
| TOTAL CURRENT ASSETS | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Accrued payroll and related expenses | ||||||||
| Accrued bonus | - | |||||||
| Notes payable - related parties | - | |||||||
| Debentures | ||||||||
Paycheck protection program loan - current portion | - | |||||||
| Convertible bridge notes, at fair value | ||||||||
| Current liabilities held for disposal | - | |||||||
| TOTAL CURRENT LIABILITIES | ||||||||
Paycheck protection program loan - net of current portion | - | |||||||
| Convertible bridge notes, at fair value | - | |||||||
| TOTAL LIABILITIES | ||||||||
| Redeemable convertible preferred stock - Series A; $ par value, designated Series A,
shares issued and outstanding (liquidation preference of $ | ||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Preferred stock, Series B, $par value; shares authorized, and shares issued and outstanding at December 31, 2020 and December 31, 2019, respectively | - | - | ||||||
| Preferred stock, Series E-1, $ par value; shares authorized, and shares issued and outstanding at December 31, 2020, and December 31, 2019, respectively | - | - | ||||||
| Common stock, $par value, shares authorized, and shares issued and outstanding at December 31, 2020 and December 31, 2019, respectively | ||||||||
| Unearned deferred compensation | ( | ) | - | |||||
| Subscription receivable | ( | ) | - | |||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | ( | ) | ( | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | $ | ||||||
See notes to the consolidated financial statements.
| F-22 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the years ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| REVENUES | $ | $ | ||||||
| OPERATING EXPENSES FROM CONTINUED OPERATIONS | ||||||||
| Payroll and related expenses | ||||||||
| Professional fees | ||||||||
| General and administrative | ||||||||
| Research and development expenses | - | |||||||
| Total Operating Expenses | ||||||||
| LOSS FROM CONTINUING OPERATIONS | ( | ) | ( | ) | ||||
| OTHER INCOME (EXPENSE) FROM CONTINUING OPERATIONS | ||||||||
| Financing costs including interest | ( | ) | ( | ) | ||||
| Change in fair value of convertible bridge notes | ( | ) | ||||||
| Loss on equity method investment | - | ( | ) | |||||
| Loss on convertible debt and other liabilities converted to equity | ( | ) | - | |||||
Total Other (Expense) Income | ( | ) | ||||||
| Loss from continuing operations before income taxes | ( | ) | ( | ) | ||||
| INCOME TAXES | - | - | ||||||
| Loss from continuing operations | ( | ) | ( | ) | ||||
| DISCONTINUED OPERATIONS: | ||||||||
| Income (Loss) from discontinued operations before income taxes | ( | ) | ||||||
| INCOME TAXES | - | - | ||||||
| Income (Loss) from discontinued operations | ( | ) | ||||||
| NET LOSS | ( | ) | ( | ) | ||||
| PREFERRED STOCK | ||||||||
| Series A convertible contractual dividends | ( | ) | ( | ) | ||||
| NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | ( | ) | $ | ( | ) | ||
| NET LOSS ATTRIBUTABLE TO COMMON STOCKHOLDERS: BASIC AND DILUTED: | ||||||||
| CONTINUING OPERATIONS | $ | ( | ) | $ | ( | ) | ||
| DISCONTINUED OPERATIONS | ( | ) | ||||||
| $ | ( | ) | $ | ( | ) | |||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: BASIC AND DILUTED | ||||||||
See notes to the consolidated financial statements
| F-23 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ DEFICIT
| Preferred Stock | Common Stock | Additional Paid In | Deferred Stock-based | Subscription | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Capital | Compensation | Receivable | Deficit | Deficit | ||||||||||||||||||||||||||||
| Balance, December 31, 2018 | - | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||
| Issuance of stock to consultants for services | - | - | - | - | ||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | ( | ) | - | - | ( | ) | ||||||||||||||||||||||||||
| Net loss period ended December 31, 2019 | - | - | - | - | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Balance, December 31, 2019 | - | $ | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||||||
| Stock-based compensation for services | - | ( | ) | - | - | |||||||||||||||||||||||||||||||
| Stock-based compensation expense and stock option modification | - | - | - | - | - | - | - | |||||||||||||||||||||||||||||
| Conversion of debenture and promissory note with unrelated parties | - | - | - | - | ||||||||||||||||||||||||||||||||
| Conversion of bridge notes and accrued interest to common stock | - | - | - | |||||||||||||||||||||||||||||||||
| Conversion of accrued salary and bonus, director fees, and promissory notes with related parties | - | - | - | |||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | - | - | ( | ) | - | - | - | ( | ) | |||||||||||||||||||||||||
| Conversion of debt to Series B preferred stock | - | - | - | - | - | - | ||||||||||||||||||||||||||||||
| Sale of Series B preferred stock | - | - | - | - | ( | ) | - | |||||||||||||||||||||||||||||
| Net loss period ended December 31, 2020 | - | - | - | - | - | - | - | ( | ) | ( | ) | |||||||||||||||||||||||||
| Balance, December 31, 2020 | - | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||||||||||||||||||
See notes to the consolidated financial statements.
| F-24 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the years ended | ||||||||
| December 31, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash provided by operations: | ||||||||
| Depreciation | - | |||||||
| Loss on equity investment | - | |||||||
| Stock-based compensation for services | ||||||||
| Stock-based compensation and stock option modification | - | |||||||
| Loss on conversion of bridge notes and accrued interest | - | |||||||
| Loss on conversion of debentures and notes payable with unrelated parties | - | |||||||
| Loss on conversion of accrued salary and bonus, director fees, and notes payable with related parties | - | |||||||
| Change in fair value of convertible bridge notes | ( | ) | ||||||
| Amortization of debt issuance costs | ||||||||
| Paid-in-kind interest - convertible bridge notes | ||||||||
| Gain on forgiveness or assumption of notes payable and accrued expenses | ( | ) | - | |||||
| Changes in operating assets and liabilities | ||||||||
| Increase in prepaid expenses and other current assets | ( | ) | ( | ) | ||||
| Increase in accounts payable and accrued expenses | ||||||||
| Increase accrued payroll and related expenses | ||||||||
| Increase in contract liabilities - related party | - | ( | ) | |||||
| Increase in accrued interest – related party | ||||||||
| Increase in accrued interest | - | |||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from notes payable - related parties | ||||||||
| Repayments on notes payable - related parties | ( | ) | ||||||
| Proceeds from notes payable - unrelated parties | - | |||||||
| Proceeds from convertible notes payable | - | |||||||
| Proceeds from issuance of Series B Preferred Stock | - | |||||||
| Proceeds from Paycheck Protection Program | - | |||||||
| Net cash provided by financing activities | ||||||||
| NET INCREASE (DECREASE) IN CASH | ( | ) | ||||||
| CASH - Beginning of year | ||||||||
| CASH - End of year | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOW DISCLOSURES: | ||||||||
| Payment of interest in cash | $ | $ | ||||||
| Payment of income taxes | $ | $ | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Accrual of contractual dividends on Series A convertible preferred stock | $ | $ | ||||||
| Investment purchased with a subscription payable | $ | $ | ||||||
| Conversion of convertible bridge notes and accrued interest of common stock, fair value | $ | $ | ||||||
| Conversion of debentures and notes payable with unrelated parties to common stock | $ | $ | ||||||
| Conversion of accrued salary and bonus, director fees, and notes payable with related parties to common stock | $ | $ | ||||||
| Conversion of notes payable to Series B Preferred Stock | $ | $ | ||||||
| Series B Preferred Stock purchased with a stock subscription receivable | $ | $ | ||||||
See notes to the consolidated financial statements.
| F-25 |
QSAM BIOSCIENCES INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
QSAM
Biosciences Inc. (f/k/a Q2Earth, Inc.) (hereinafter the “Company”, “we”, “our”, “us”),
incorporated in Delaware on August 26, 2004, is currently engaged in the business of developing a novel radiopharmaceutical
drug candidate for the treatment of bone cancer. This business line commenced in earnest in the fourth fiscal quarter of 2020
as a result of the separation and transfer pursuant to an Omnibus Separation Agreement dated November 6, 2020 (the “Separation
Agreement”) of the Company’s prior business of managing compost and soil manufacturing facilities (the “Legacy
Business”) through an unconsolidated investee entity called Earth Property Holdings LLC, a Delaware limited liability company
(“EPH”). Pursuant to the Separation Agreement, the Company transferred to EPH all assets and related liabilities in
connection with the Legacy Business in return for a forgiveness of debt. The financial statements presented herein have been adjusted
to account for the Legacy Business as discontinued operations (see Notes 4 – Separation Agreement and 9 –
Discontinued Operations). The Company owns approximately an
In April 2020, the Company established QSAM Therapeutics Inc. (“QSAM”) as a wholly-owned subsidiary incorporated in the state of Texas, and through QSAM, executed a Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) with IGL Pharma, Inc. (“IGL”). The License Agreement provides QSAM with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and knowhow with respect to Samaium-153 DOTMP aka CycloSam® (the “Technology”), a clinical stage novel radiopharmaceutical meant to treat different types of bone cancer and related diseases. The establishment of QSAM and execution of the License Agreement and the Separation Agreement are part of the Company’s strategic plan to transition its business into the broader biosciences sector which currently is the Company’s focus.
In connection with the transition to the biosciences sector, the Company changed its name to QSAM Biosciences Inc. on September 4, 2020, and subsequently changed its stock symbol to QSAM, to better reflect its business moving forward.
On
September 4, 2020,
Prior to 2017, the Company owned and licensed technology that converts waste fuels and heat to power, which it sold to a licensee in August of that year. Much of these operations were conducted through a wholly-owned subsidiary of the Company called Q2Power Corp. (“Q2P”), which still exists but has no current operations. Q2P and QSAM are sometimes referred to herein as the “Subsidiaries”. Formerly, the Company’s name was Q2Power Technologies, Inc., and before that, Anpath Group, Inc.
The recent outbreak of the novel coronavirus (COVID-19) is impacting worldwide economic activity. COVID-19 poses the risk that we or our employees and other partners may be prevented from conducting business activities for an indefinite period of time, including due to the spread of the disease or shutdowns that may be requested or mandated by governmental authorities. While it is not possible at this time to estimate the full impact that COVID-19 could have on our business, the continued spread of COVID-19 could disrupt our research and development of CycloSam and other related activities, which could have a material adverse effect on our business, financial condition and results of operations. In addition, a severe or prolonged economic downturn could result in a variety of risks to the business. While we have not yet experienced any material disruptions in our business or other negative consequences relating to COVID-19, the extent to which the COVID-19 pandemic impacts our results will depend on future developments that are highly uncertain and cannot be predicted.
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN
For
the year ended December 31, 2020, the Company used cash in operating activities for its continuing operations of $
| F-26 |
The
Company raised a total of $
The
Company’s convertible debentures totaling $
As
of December 31, 2020, the Company had a working capital deficit of $
These conditions raise substantial doubt about the Company’s ability to continue as a going concern. There is no guarantee whether the Company will be able to generate revenue and/or raise capital sufficient to support its continuing operations. The ability of the Company to continue as a going concern is dependent on management’s plans which include implementation of its business model to develop and commercialize its drug candidate, seek strategic partnerships to advance clinical trials and other research endeavors which could provide additional capital to the Company, and continue to raise funds for the Company through equity or debt offerings. The consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
In
2018, the Company signed an eight-year Management Agreement with EPH to oversee all of the operations of EPH and its acquired
subsidiaries for an initial annual fee of $
Our net loss from continuing operations in 2020 and 2019 resulted largely from activities related to the public company and in 2020 also from certain license and research expenses in connection with the continuing operations of the Company’s drug development business. All income and losses related to expenses from the Legacy Business are included in discontinued operations (see Note 9 - Discontinued Operations).
Management
is taking steps to improve its balance sheet and negative cashflow. Commencing in December 2020 and closing in February
2021, management raised $
| F-27 |
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its Subsidiaries. All significant inter-company transactions and balances have been eliminated in consolidation. References herein to the Company include the Company and its Subsidiaries unless the context otherwise requires.
Cash
The
Company considers cash, short-term deposits, and other investments with original maturities of no more than ninety days when acquired
to be cash and cash equivalents for the purposes of the statement of cash flows. The Company maintains cash balances at two financial
institutions and has experienced no losses with respect to amounts on deposit. The Company held
Revenue Recognition
On January 1, 2018, the Company adopted ASC Topic 606, “Revenue from Contracts with Customers (“ASC 606”) and all the related amendments. The Company elected to adopt this guidance using the modified retrospective method. The adoption of this guidance did not have a material effect on the Company’s financial position, results of operations, or cash flows.
The core principle of ASC 606 requires that an entity recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. ASC 606 defines a five-step process to achieve this core principle and, in doing so, it is possible more judgment and estimates may be required within the revenue recognition process than previously required under U.S. GAAP, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation.
The Company had revenue in 2020 and 2019 from continuing operations.
| F-28 |
The Company applies the fair value method of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718, “Share Based Payment”, in accounting for its stock-based compensation with employees and non-employees. This standard states that compensation cost is measured at the grant date based on the fair value of the award and is recognized over the service period, which is usually the vesting period. The Company values stock-based compensation at the market price for the Company’s common stock and other pertinent factors at the grant date.
The Black-Scholes option pricing valuation method is used to determine fair value of stock options consistent with ASC 718, “Share Based Payment”. Use of this method requires that the Company make assumptions regarding stock volatility, dividend yields, expected term of the awards and risk-free interest rates.
Research and Development
Research
and development costs are expensed as incurred. Research and development costs were $
Fair Value Measurement
The Company measures fair value in accordance with a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The Company’s convertible Bridge Notes are valued by using Monte Carlo Simulation methods and discounted future cash flow models. Where possible, the Company verifies the values produced by its pricing models to market prices. Valuation models require a variety of inputs, including contractual terms, market prices, yield curves, credit spreads, measures of volatility and correlations of such inputs. These convertible Bridge Notes do not trade in liquid markets, and as such, model inputs cannot generally be verified and do involve significant management judgment. Such instruments are typically classified within Level 3 of the fair value hierarchy.
Equity Method Investment
Investments in partnerships, joint ventures and less-than majority-owned subsidiaries in which we have significant influence are accounted for under the equity method. The Company’s consolidated net income includes the Company’s proportionate share of the net income or loss of our equity method investee. When we record our proportionate share of net income, it increases income (loss) — net in our consolidated statements of operations and our carrying value in that investment. Conversely, when we record our proportionate share of a net loss, it decreases income (loss) — net in our consolidated statements of income and our carrying value in that investment. The Company’s proportionate share of the net income or loss of our equity method investees includes significant operating and nonoperating items recorded by our equity method investee. These items can have a significant impact on the amount of income (loss) — net in our consolidated statements of operations and our carrying value in those investments.
| F-29 |
Discontinued Operations
In accordance with ASC 205-20 Presentation of Financial Statements: Discontinued Operations, a disposal of a component of an entity or a group of components of an entity is required to be reported as discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results when the components of an entity meets the criteria in paragraph 205-20-45-10. In the period in which the component meets held-for-sale or discontinued operations criteria the major current assets, other assets, current liabilities, and noncurrent liabilities shall be reported as components of total assets and liabilities separate from those balances of the continuing operations. At the same time, the results of all discontinued operations, less applicable income taxes (benefit), shall be reported as components of net income (loss) separate from the net income (loss) of continuing operations.
The Company disposed of a component of its business pursuant to a Separation Agreement in November 2020, which met the definition of a discontinued operation. Accordingly, the operating results of the business disposed are reported as income (loss) from discontinued operations in the accompanying consolidated statements of operations for the years ended December 31, 2020, and 2019. For additional information, see Note 4 – Separation Agreement and Note 14 - Discontinued Operations.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed on the straight-line method, based on the estimated useful lives of the assets as follows:
| Years | ||||
| Furniture and equipment | ||||
| Computers | ||||
Expenditures for maintenance and repairs are charged to operations as incurred.
Income Taxes
Income
taxes are accounted for under the asset and liability method as stipulated by FASB ASC 740, “Income Taxes”
(“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences
between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating
loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply
to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the
effect on deferred tax assets and liabilities or a change in tax rate is recognized in income in the period that includes the
enactment date. Deferred tax assets are reduced to estimated amounts to be realized by the use of a valuation allowance.
In the event that an uncertain tax position exists in which the Company could incur income taxes, the Company would evaluate whether there is a probability that the uncertain tax position taken would be sustained upon examination by the taxing authorities. Reserves for uncertain tax positions would be recorded if the Company determined it is probable that a position would not be sustained upon examination or if payment would have to be made to a taxing authority and the amount is reasonably estimated. As of December 31, 2020, the Company does not believe it has any uncertain tax positions that would result in the Company having a liability to the taxing authorities; however, federal returns have not been filed since the Company’s inception in 2014. Such delinquencies are being resolved by management and a retained tax expert. Interest and penalties related to any unrecognized tax benefits is recognized in the consolidated financial statements as a component of income taxes.
Net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period plus any potentially dilutive shares related to the issuance of stock options, shares from the issuance of stock warrants, shares issued from the conversion of redeemable convertible preferred stock and shares issued for the conversion of convertible debt.
| F-30 |
At December 31, 2020, there were the following potentially dilutive securities that were excluded from diluted net loss per share because their effect would be anti-dilutive (all shares adjusted to reflect a 25:1 reverse stock split effected on September 4, 2020):
| Shares from the conversion of Series B Preferred Stock | ||||
| Shares from the conversion of Series E-1 Preferred Stock (subject to ) | ||||
| Shares from common stock options | ||||
| Shares from common stock warrants | ||||
| Shares from the conversion of debentures | ||||
| Shares that may be converted from Bridge Notes (based upon an assumed conversion price at December 31, 2020 of $ per share) | ||||
| Shares from the conversion of redeemable
convertible preferred stock (based upon an assumed conversion price at December 31, 2020 of $ |
At December 31, 2019, there were the following potentially dilutive securities that were excluded from diluted net loss per share because their effect would be anti-dilutive (all shares adjusted to reflect a 25:1 reverse stock split effected on September 4, 2020):
| Shares from common stock options | ||||
| Shares from common stock warrants | ||||
| Shares from the conversion of debentures | ||||
| Shares that may be converted from Bridge Notes (based upon an assumed conversion price at December 31, 2019 of $ per share); | ||||
| Shares from the conversion of redeemable convertible preferred stock (not inclusive of cumulative dividends which may be converted to shares of common stock under certain conditions). |
Significant Estimates
U.S. Generally Accepted Accounting Principles (“GAAP”) requires the Company to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements, the reported amounts of revenues and expenses, cash flows and the related footnote disclosures during the period. On an on-going basis, the Company reviews and evaluates its estimates and assumptions, including, but not limited to, those that relate to the fair value of stock based compensation fair value of convertible bridge notes, and deferred the valuation allowance on deferred tax assets and contingencies. Actual results could differ from these estimates.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)”, requiring management to recognize any right-to-use-asset and lease liability on the statement of financial position for those leases previously classified as operating leases. The criteria used to determine such classification is essentially the same as under the previous guidance, but it is more subjective. The lessee would classify the lease as a finance lease if certain criteria at lease commencement are met. This ASU is effective for fiscal years beginning after December 15, 2018. Effective January 1, 2019 the Company adopted ASU 2016-02 which did not have an impact on the consolidated financial statements of the Company as the Company has no leases that meet the scope of ASC 842.
In June 2018, the FASB issued ASU No. 2018-07, Compensation – Stock Compensation (Topic 718), Improvements to Nonemployee Share-Based Payment Accounting, which is intended to simplify the accounting for nonemployee share-based payment transactions by expanding the scope of Topic 718 to include share-based payment transactions for acquiring goods and services from nonemployees. The guidance is effective for fiscal years and interim periods within those years beginning after December 15, 2018. Early adoption is permitted, but no earlier than an entity’s adoption date of ASC 606. Effective January 1, 2019 the Company adopted ASC 2018-07 and it did not have an impact on the Company’s consolidated financial statements.
| F-31 |
In August 2018, the FASB issued guidance that amends fair value disclosure requirements. The guidance removes disclosure requirements on the transfers between Level 1 and Level 2 of the fair value hierarchy in addition to the disclosure requirements on the policy for timing of transfers between levels and the valuation process for Level 3 fair value measurements. The guidance clarifies the measurement uncertainty disclosure and adds disclosure requirements for Level 3 unrealized gains and losses and significant unobservable inputs used to develop Level 3 fair value measurements. The guidance is effective for fiscal years beginning after December 15, 2019. Entities are permitted to early adopt any removed or modified disclosures upon issuance and delay adoption of the additional disclosures until the effective date. The Company is currently evaluating the impact of this new guidance on its consolidated financial statements and disclosures.
Reclassifications
Certain reclassifications of prior year amounts have been made to conform to the 2020 presentation. These reclassifications had no effect on net loss or loss per share as previously reported.
Concentration of Risk
The Company expects cash to be the asset most likely to subject the Company to concentrations of credit risk. The Company’s bank deposits may at times exceed federally insured limits. The Company’s policy is to maintain its cash with high credit quality financial institutions to limit its risk of loss exposure.
The Company had no revenue from its continuing operations in 2020 and 2019. Revenue included in discontinued operations was generated from one related customer in 2020 and two related customers in 2019.
NOTE 4 – SEPARATION AGREEMENT
On November 6, 2020, the Company entered into the Separation Agreement with its unconsolidated investee, EPH. The Company’s board of directors approved the Separation Agreement in support of the Company’s previously disclosed plan to secure new technologies and business opportunities in the broader biosciences sector, and to significantly reduce debt and liabilities of the Company and eliminate under-performing assets and agreements. The Separation Agreement resulted in the discontinuance of the Company’s management of businesses and assets focused on compost and soil manufacturing to focus solely on the development of its exclusively licensed pharmaceutical Technology, as well as other drug candidates that it may license or otherwise secure in the future. Pursuant to the Separation Agreement:
| ● | The Management Agreement, dated January 18, 2019, as amended, between EPH and the Company was terminated by mutual agreement of the parties. Fees from this agreement constituted most of the Company’s revenue over the prior two years. |
| ● | In
lieu of any severance or other termination payments due under the Management Agreement, EPH released the Company from a total
of $ | |
| ● | The
Company agreed to transfer to EPH its license agreement with Agrarian Technologies LLC
and Mulch Masters Inc. for the ABS soil enhancement product and all associated knowhow,
trade secrets and trademark/service marks. Accrued license fees in connection with this
license agreement were also assumed by EPH in the amount of $ | |
| ● | The prior officers and employees of the Company engaged in the Legacy Business were released from any non-competition, non-solicitation or other restricted covenant pursuant to their respective employment agreements. Effective October 1, 2020, several of these employees had already separated from the Company. | |
| ● | EPH received the right in its sole discretion to use the name “Q2Earth” in all jurisdictions of the United States and worldwide. |
Pursuant to ASC 205-20 Presentation of Financial Statements: Discontinued Operations and amended by ASU No. 2014-08, management has determined that the Separation Agreement results in the disposal of a component that represents a strategic shift in the Company’s business operations that will have a major effect on the Company’s operations and financial results. Therefore, the net income (loss) generated from this disposed component have been presented as discontinued operations for the years ended December 31, 2020 and 2019 on the statement of operations. Further, liabilities forgiven or assumed in connection with the Separation Agreement have been presented as liabilities held for disposal as of December 31, 2019 on the accompanying balance sheet (see Note 9 – Discontinued Operations).
NOTE 5 – EQUITY METHOD INVESTMENT
During
November 2018, the Company invested $
For
the year ended December 31, 2020, EPH generated $
Our prior Chairman and CEO of the Company, also serves as President of EPH; and Christopher Nelson, General Counsel and Director of the Company, also serves as General Counsel and Secretary of EPH. See Note 6 – Related Party Transactions for transactions with our equity method investment during the years ended December 31, 2020 and 2019.
| F-32 |
NOTE 6 – RELATED PARTY TRANSACTIONS
The Company currently has a License Agreement with IGL Pharma, Inc., an entity in which the Company’s Executive Chairman serves as President and holds a non-controlling equity interest.
The Company currently maintains an executive office in Florida, which is leased by an investment firm in which the Company’s General Counsel serves as an officer but does not hold any equity or voting rights. The Company has no formal agreement for this space and pays no rent.
During
the years ended December 31, 2020 and 2019, the Company received $
During
the years ended December 31, 2020 and 2019, the Company received $
During the year ended
December 31, 2020, the Company received $
During
the years ended December 31, 2020 and 2019, the Company incurred approximately $
In
2020, a total of approximately $
NOTE 7 – DEBENTURES, CONVERTIBLE BRIDGE NOTES, AND NOTES PAYABLE
Debentures
The
Company has Original Issue Discount Senior Secured Convertible Debentures (the “Debentures”) with two holders in
the aggregate amount of $
Convertible Bridge Notes
In
2017 and 2018, the Company issued a total of $
| F-33 |
As
of December 31, 2020, approximately $
Pursuant
to ASC 825-10-25-1, Fair Value Option, the Company made an irrevocable election at the time of issuance to report the Bridge Notes
at fair value, with changes in fair value recorded through the Company’s condensed consolidated statements of operations
as other income (expense) in each reporting period. The estimated fair value of the remaining outstanding Bridge Notes as of December
31, 2020 and 2019 was $
Paycheck Protection Program
On
April 14, 2020, the Company received $
Notes Payable
Between
June and November 2020, the Company received a total of $ under a promissory note with an unrelated third party
with multiple tranches with interest payable at annually. All outstanding principal and interest accrued and unpaid on the
note was due and payable twelve (12) months after the respective tranche date. As of December 31, 2020, the full
balance of $
See Note 6 – Related Party Transactions for additional notes payable with related parties.
NOTE 8 – FAIR VALUE MEASUREMENT
The Company measures fair value in accordance with a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described below:
| Level 1 | Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities; | |
| Level 2 | Quoted prices in markets that are not active, or inputs that are observable, either directly or indirectly, for substantially the full term of the asset or liability; and | |
| Level 3 | Prices or valuation techniques that require inputs that are both significant to the fair value measurement and unobservable (supported by little or no market activity). |
As disclosed in Note 7, the Bridge Notes are reported at fair value, with changes in fair value recorded through the Company’s consolidated statements of operations as other income (expense) in each reporting period.
| F-34 |
The following tables set forth the Company’s consolidated financial assets and liabilities measured at fair value by level within the fair value hierarchy at December 31, 2020 and 2019. Assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement.
| Fair value at | ||||||||||||||||
| December 31, 2020 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible Bridge Notes | $ | $ | $ | $ | ||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||
| Fair value at | ||||||||||||||||
| December 31, 2019 | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible Bridge Notes | $ | $ | $ | $ | ||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||
The following tables present a reconciliation of the beginning and ending balances of items measured at fair value on a recurring basis that use significant unobservable inputs (Level 3) and the related realized and unrealized gains (losses) recorded in the consolidated statement of operations during the periods.
| Year Ended December 31, 2020 | ||||
| Fair value, December 31, 2019 | $ | |||
| Accrued interest | ||||
| Conversions of debt and accrued interest to shares of common stock | ( |
) | ||
| Amortization of debt issuance costs | ||||
| Net unrealized loss on convertible bridge notes | ||||
| Fair value, December 31, 2020 | $ | |||
Year Ended December 31, 2019 | ||||
| Fair value, December 31, 2018 | $ | |||
| Issuances of debt | ||||
| Accrued interest | ||||
| Amortization of debt issuance costs | ||||
| Net unrealized gain on convertible bridge notes | ( | ) | ||
| Fair value, December 31, 2019 | $ | |||
The
Company’s convertible Bridge Notes are valued by using Monte Carlo Simulation methods and discounted future cash flow models.
Where possible, the Company verifies the values produced by its pricing models to market prices. Valuation models require a variety
of inputs, including contractual terms, market prices, yield curves, credit spreads, measures of volatility and correlations of
such inputs. These convertible Bridge Notes do not trade in liquid markets, and as such, model inputs cannot generally be verified
and do involve significant management judgment. Such instruments are typically classified within Level 3 of the fair value hierarchy.
The following assumptions were used to value the Company’s convertible Bridge Notes at December 31, 2020: dividend yield
of -
NOTE 9 – DISCONTINUED OPERATIONS
On November 6, 2020, the Company executed a Separation Agreement (see Note 4 – Separation Agreement), whereby the Company transferred its Legacy Business and the related assets and liabilities to EPH, a related party and equity method investee.
ASC 205-20 “Discontinued Operations” establishes that the disposal or abandonment of a component of an entity or a group of components of an entity should be reported in discontinued operations if the disposal represents a strategic shift that has (or will have) a major effect on an entity’s operations and financial results. As a result, the component’s results of operations have been reclassified as discontinued operations on a retrospective basis for all periods presented. Accordingly, the liabilities forgiven or assumed by EPH in connection with the Separation Agreement separately reported as “liabilities held for disposal” as of December 31, 2019. As of December 31, 2020, there were no assets held associated with this business. The results of operations of this component, for all periods, are separately reported as “discontinued operations” on the consolidated statements of operations.
As disclosed in Note 5, the Company retained its equity interest in EPH as of December 31, 2020. This equity interest has been accounted for as an equity method investment for all periods. There have been no transactions between the Company and EPH since the Separation Agreement.
| F-35 |
A reconciliation of the major classes of line items constituting the income (loss) from discontinued operations, net of income taxes as is presented in the consolidated statements of operations for the years ended December 31, 2020, and 2019 are summarized below:
Reconciliation of liabilities included in current liabilities held for disposal on the consolidated balance sheet:
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Carrying amounts of major classes of liabilities included as part of liabilities held for disposal | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Accrued interest - related parties | - | |||||||
| Notes payable - related parties | - | |||||||
| Total liabilities included in the liabilities held for disposal | $ | $ | ||||||
Reconciliation of revenue and expense items in discontinued operations on the consolidated statement of operations:
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| REVENUES | $ | $ | ||||||
| OPERATING EXPENSES | ||||||||
| Payroll and related expenses | ||||||||
| Professional fees | - | |||||||
| General and administrative | ||||||||
| Total Operating Expenses | ||||||||
| Financing costs including interest | ||||||||
| Gain on debt extinguishment | ( | ) | - | |||||
| INCOME (LOSS) FROM DISCONTINUED OPERATIONS | $ | $ | ( | ) | ||||
Reconciliation of cash flows from operating activities and financing activities on the consolidated statement of cash flow:
| Year ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Income (Loss) from Discontinued Operations | $ | $ | ( | ) | ||||
| Adjustments to reconcile net loss to net cash provided by discontinued operations: | ||||||||
| Gain on forgiveness or assumption of promissory notes and accrued expenses | ( | ) | - | |||||
| Changes in operating assets and liabilities | ||||||||
| Increase in accounts payable and accrued expenses | ||||||||
| Increase in contract liabilities - related party | - | ( | ) | |||||
| Increase in accrued interest - related party | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from promissory notes - related parties | ||||||||
| Repayments on promissory notes - related parties | ( | ) | - | |||||
| Net cash provided by financing activities | ||||||||
| Net cash provided by discontinued operations | $ | $ | ||||||
NOTE 10 – COMMON STOCK, PREFERRED STOCK AND WARRANTS
Common Stock
In 2020, the Company issued shares of common stock as follows:
| Stock based compensation for services | ||||
| Conversions of debentures and notes with unrelated parties | ||||
| Conversion of Bridge Notes | ||||
| Conversion of accrued salary and bonus, directors’ fees and notes with related parties | ||||
| Total Common Shares issued in 2020 |
In
total, $
In
2020,
The Company did not issue any common shares in 2019.
For the years ended December 31, 2020 and 2019, the Company recognized $ and $ of compensation expense for several service agreements.
Series A Redeemable Convertible Preferred Stock
The
Company has shares of Preferred Stock issued and outstanding as of December 31, 2020, which currently are convertible at $
per share of the Company’s common stock (the “Conversion Price”), which was adjusted to match the conversion
price of the Company’s Series B Preferred Stock. The Preferred Stock bears a
| F-36 |
The
Preferred Stock has price protection provisions in the case that the Company issues any shares of stock not pursuant to an “Exempt
Issuance” at a price below the Conversion Price. Exempt Issuances include: (i) shares of Common Stock or common stock equivalents
issued pursuant to the original merger of the company or any funding contemplated by that transaction; (ii) any common stock or
convertible securities outstanding as of the date of closing; (iii) common stock or common stock equivalents issued in connection
with strategic acquisitions; (iv) shares of common stock or equivalents issued to employees, directors or consultants pursuant
to a plan, subject to limitations in amount and price; and (v) other similar transactions. The Certificate of Designation contains
restrictive covenants not to incur certain debt, repurchase shares of common stock, pay dividends or enter into certain transactions
with affiliates without consent of holders of
Management
has determined that the Preferred Stock is more akin to a debt security than equity primarily because it contains a mandatory
The Preferred Stock carries a 6% per annum dividend calculated on the stated value of the stock and is cumulative and payable quarterly beginning July 1, 2016. These dividends are accrued at each reporting period. They add to the redemption value of the stock; however, as the Company shows an accumulated deficit, the charge has been recognized in additional paid-in capital.
Series B Preferred Stock
On
December 29, 2020, the Company filed an amendment to its Articles of Incorporation to authorize the issuance of up to shares
of Series B Convertible Preferred Stock (the “Series B Stock”), par value $0.001 per share, pursuant to a Certificate
of Designation. The Series B Preferred Shares provide the holders a
In
December 2020, the Company commenced a private offering of its Series B Stock for $
Series E-1 Preferred Stock
On
December 3, 2020 the Company filed an amendment to its Articles of Incorporation to authorize the issuance of up to shares
of Series E-1 Preferred Stock (the “Series E-1 Stock”) pursuant to a Certificate of Designation. The shares of Series
E-1 Stock are incentive-based, vesting and forfeitable securities that provide the holders the right in the aggregate to receive an
“earnout” equal to 20% of the total consideration received by the Company in the instance of a sale or sub-license of
its core licensed radiopharmaceutical Technology, or sale or merger of the Company, which is paid on a priority, senior basis. In
addition, the holders of the Series E-1 Stock can convert their vested preferred stock at anytime or after an event resulting in an
earnout payment, such as an acquisition of the Company, into an aggregate of
| F-37 |
Warrants
During
the year ended December 31, 2020, the Company issued no warrants and
| Number
of Warrants | Exercise
price per share | Average
remaining term in years | ||||||||||
| Warrants issued in connection with issuance of Series A Preferred Stock | $ | |||||||||||
During
the year ended December 31, 2018, the Company committed to issuing warrants to purchase
In 2016 to compensate officers, directors and other key service providers with equity grants, the Board approved the 2016 Omnibus Equity Incentive Plan (“2016 Plan”), which initially allowed for shares of common stock, stock options, stock rights (restricted stock units), or stock appreciation rights to be granted by the Board in its discretion. This authorized amount was increased to shares by Board resolution and amendment in 2017.
The
Company issued stock options in 2020, each to two of the Company’s independent directors, each to
one other independent director and one Board observer, and to a new director. The options issued to the directors and Board
observer were fully vested upon issuance, are exercisable at a price of $
Option Repricing
On January 6, 2020, the compensation committee of the Company’s Board of Directors, approved a one-time stock option repricing program (the “Option Repricing”) to permit the Company to reprice certain options to purchase the Company’s Common Stock held by its current directors, officers and employees (the “Eligible Options”), which actions became effective on January 6, 2020. Under the Option Repricing, Eligible Options with an exercise price at or above $per share (representing an aggregate of options, or of the total outstanding) were amended to reduce such exercise price to $0.50.
| F-38 |
The
impact of the Option Repricing was a one-time incremental non-cash charge of $
Total stock-based compensation for stock options issued and the one-time incremental charge for the Option Repricing for the year ended December 31, 2020 was $. There was stock-based compensation recognized in 2019 related to stock options.
A summary of the common stock options issued under the 2016 Plan and prior stock option plans for the year ended December 31, 2020 is as follows (shares and prices have been adjusted to account for a 25:1 reverse split):
Number Outstanding | Weighted Avg. Exercise Price | Weighted Avg. Remaining Contractual Life (Years) | ||||||||||
| Balance, December 31, 2019 | $ | |||||||||||
| Options issued | ||||||||||||
| Balance, December 31, 2020 | ||||||||||||
Exercisable/ Vested Options Outstanding | Weighted Avg. Exercise Price | Weighted Avg. Remaining Contractual Life (Years) | ||||||||||
| Balance, December 31, 2020 | $ | |||||||||||
Year Ended December 31, 2020 | ||||
| Risk free interest rate | % | |||
| Expected volatility | % | |||
| Expected dividend yield | % | |||
| Expected term in years | .0 | |||
Expected volatility is based on historical volatility of a group of 4 comparable companies, due to the low trading volume of the Company’s own stock. Short Term U.S. Treasury rates were utilized as the risk-free interest rate. The expected term of the options was calculated using the alternative simplified method codified as ASC 718 “Accounting for Stock Based Compensation,” which defines the expected life as the average of the contractual term of the options and the weighted average vesting period for all issuances.
| F-39 |
NOTE 12 – INCOME TAXES
A
reconciliation of the differences between the effective income tax rates and the statutory federal tax rates for the years ended
December 31, 2020 and 2019 (computed by applying the U.S. Federal corporate tax rate of
| 2020 | 2019 | |||||||
| Tax benefit at U.S. statutory rate | $ | ( | ) | $ | ||||
| State taxes, net of federal benefit | ( | ) | ||||||
| Change in fair value of convertible bridge notes and derivatives | ||||||||
| Other permanent differences | ||||||||
| Change in valuation allowance | ( | ) | ||||||
| $ | $ | |||||||
The tax effect of temporary differences that give rise to significant portions of the deferred tax assets and liabilities for the years ended December 31, 2020 and 2019 consisted of the following:
| 2020 | 2019 | |||||||
| Net operating loss carry-forward | $ | $ | ||||||
| Accrued expenses | ||||||||
| Stock based compensation | ||||||||
| Deferred revenue | - | |||||||
| Depreciation expense | - | |||||||
| Net deferred tax assets | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Total net deferred tax asset | $ | $ | ||||||
At December 31, 2020
and 2019, the Company had net deferred tax assets of $
The Company’s NOL and tax credit carryovers may be significantly limited under the Internal Revenue Code (“IRC”). NOL and tax credit carryovers are limited under Section 382 when there is a significant “ownership change” as defined in the IRC. During the year ended December 31, 2020 and in prior years, the Company may have experienced such ownership changes, which could impose such limitations.
The limitations imposed by the IRC would place an annual limitation on the amount of NOL and tax credit carryovers that can be utilized. When the Company completes the necessary studies, the amount of NOL carryovers available may be reduced significantly. However, since the valuation allowance fully reserves for all available carryovers, the effect of the reduction would be offset by a reduction in the valuation allowance.
NOTE 13 – COMMITMENTS AND CONTINGENCIES
The employment agreements for the Company’s new Executive Chairman and CEO each contain termination provisions whereby if they are terminated without cause or following a material change, as defined therein, they will receive salary through the date of termination plus an additional 12 months, bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest and healthcare benefits will continue for 12 months.
| F-40 |
The Company’s General Counsel’s employment agreement contains a 12 month severance payment in the instance of a termination without cause, as defined therein.
The
QSAM License Agreement requires multiple milestone-based payments including: $
Pursuant
to a services agreement signed in 2018, an additional
NOTE 14 - SUBSEQUENT EVENTS
On
January 8, 2021, the Company approved a modification of the Series B convertible preferred stock offering (the “Series
B Offering”) to provide investors in that offering (other than the lead investor) non-registered warrants to
purchase an aggregate of up to
On
January 27, 2021, the Company closed the Series B Offering and issued a total of shares at a price of $ per share,
raising an aggregate amount of $
On
January 27, 2021, one institutional investor converted its remaining portion of the Debenture in the amount of $
On February 1, 2021, the Board of Directors increased the number of stock options and other incentive shares allowed to be issued under the Company’s 2016 Omnibus Equity Incentive Plan, as amended, from to million shares.
On February 1, 2021,
the Company entered into a financial services consulting agreement providing for payment by the Company of cash compensation of
$ per month for eight months and warrants to purchase
On
February 8 and 16, 2021, one institutional investor converted a total of $ of its Series A Preferred stock into
On February 15, 2021, the Company appointed Charles J. Link Jr., M.D. to the Company’s Board of Directors. Dr. Link also agreed to serve the Company in a part-time, non-executive role as Medical Director. For his services, Dr. Link received shares of Series E-1 Incentive Preferred Stock, which vest in . Concurrently with the appointment, the Company accepted the resignation of Scott W. Whitney, a Board member since 2016.
Between
January 1, 2021 and March 22, 2021, the holders of the Company’s Bridge Notes converted the remaining $
On
March 23, 2021, the Company sold its common subordinated equity interests in EPH, its equity method investee, to an unaffiliated
party for $
| F-41 |
Shares of Common Stock

QSAM Biosciences Inc.
| PRELIMINARY PROSPECTUS |
ThinkEquity
, 2021
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses, other than the underwriting discounts and commissions, payable in connection with the sale and distribution of the securities being registered. All amounts are estimated except the SEC registration fee and the FINRA filing fee. Except as otherwise noted, all the expenses below will be paid by us.
| Amount | ||||
| SEC registration fee | $ | 2,247.98 | ||
| FINRA filing fee | $ | |||
| NASDAQ application and listing fee* | $ | 50,000 | ||
| Accountants’ fees and expenses* | $ | |||
| Legal fees and expenses* | $ | |||
| Transfer Agent’s fees and expenses* | $ | |||
| Printing and engraving expenses* | $ | |||
| Miscellaneous* | $ | |||
| Total expenses | $ | |||
| * | Amounts shown are estimates. |
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law provides, in general, that a corporation incorporated under the laws of the State of Delaware, such as the Company, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
Our Certificate of Incorporation, as amended, and Amended and Restated Bylaws provide that we will indemnify our directors, officers, employees and agents to the extent and in the manner permitted by the provisions of the Delaware General Corporation Law. Any repeal or modification of these provisions shall be prospective only, and shall not adversely affect any limitation on the liability of our directors or officers existing prior to the time of such repeal or modification. We are also permitted to apply for insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the Delaware General Corporation Law would permit indemnification.
| II-1 |
Item 15. Recent Sales of Unregistered Securities
In the three years preceding the filing of this registration statement, we have issued and sold the following securities that were not registered under the Securities Act of 1933, as amended:
During the year ended December 31, 2020, the Company made the following sales of unregistered securities:
| Purpose / Holder | Number of Shares | Total Price/Amount | ||||||
| Conversion into common stock of promissory notes among multiple note holders (1) | 13,312,175 | $ | 2,928,679 | |||||
| Conversion into common stock of related party notes (2) | 696,159 | $ | 153,155 | |||||
| Issuance of common stock to officers and directors in lieu of salary and bonus (3) | 1,277,226 | $ | 280,990 | |||||
| Common stock issued to related party for services (4) | 227,273 | $ | 50,000 | |||||
| Common stock issued to one service provider (5) | 800,000 | $ | 200,000 | |||||
| Common stock issued to one service provider (6) | 150,000 | $ | 30,000 | |||||
| Conversion of debt into Series B shares (7) | 156 | $ | 156,000 | |||||
| Sale of Series B Preferred Shares (8) | 125 | $ | 125,000 | |||||
| Grant of Series E-1 Preferred Shares to management (9) | 7,650 | - | ||||||
| (1) | Shares of common stock issued to 27 holders of the Company’s Bridge Notes. |
| (2) | Shares of common stock issued to Kevin Bolin, Joel Mayersohn, Scott Whitney (all current or former directors of the Company) and Checkmate Capital Group LLC or its affiliated entity (a 10% holder of the Company). |
| (3) | Shares of common stock issued to Kevin Bolin (former Chairman and CEO), Christopher Nelson (Director and General Counsel), Joel Mayersohn (Director), and Scott Whitney and Tristan Peitz (former directors), at a conversion price of $0.22 per share. |
| (4) | Shares of common stock issued to Checkmate Capital Group LLC or its affiliate. |
| (5) | Shares of common stock issued to Redstone Communications Inc. or its affiliate for investor relations services under contract. |
| (6) | Shares of common stock issued to Atlanta Capital Partners LLC for investor relations services under contract. |
| (7) | Shares of common stock issued to Checkmate Capital Group LLC or its affiliate. |
| (8) | Shares of common stock sold to two unaffiliated parties as part of Series B financing. |
| (9) | Series E-1 preferred shares issued to C. Richard Piazza (Executive Chairman), Douglas Baum (CEO and Director), Christopher Nelson (General Counsel and Director), and two non-executive management / advisors of the Company. |
Since January 1, 2021 through the date of this filing, the Company made the following sales of unregistered securities:
| Purpose / Holder | Number of Shares | Total Price/Amount | ||||||
| Conversion into common stock of promissory notes among multiple note holders (1) | 6,627,692 | $ | 1,447,315 | |||||
| Sale of Series B Shares to multiple investors (2) | 2,219 | $ | 2,219,000 | |||||
| Common stock warrants issued to investors in Series B Offering (3) | 6,743,575 | - | ||||||
| Common stock warrants issued to service provider (4) | 750,000 | $ | 120,000 | |||||
| Common stock issued to service provider for services (5) | 250,000 | $ | 132,500 | |||||
| Grant of Series E-1 Preferred Shares to director (6) | 850 | - | ||||||
| Conversion of Series A Preferred Stock with unrelated party | 750,000 | $ | 120,000 | |||||
| Common stock issued to service provider for services (8) | 700,000 | $ | 210,000 | |||||
| Conversion of Series B preferred shares and accrued dividends into common stock (9) | 6,525,378 | $ | 1,344,068 | |||||
| Common stock options issued to directors (10) | 644,000 | - | ||||||
| Series B warrant exercise (11) | 1,871,432 | $ | 467,858 | |||||
| Convertible Note Warrants (12) | 925,001 | $ | 555,000 | |||||
| Common shares issued in exchange for Series E-1 preferred shares (13) | 28,839,428 | $ | 8,651,829 | |||||
| (1) | Shares of common stock issued to six holders of the Company’s Bridge Notes. |
| (2) | Shares of common stock sold to 25 unaffiliated accredited investors as part of Series B financing. |
| (3) | 6,268,575 warrants issued to 27 investors who participated in Series B financing at an exercise price of $0.35, expiring July 8, 2021 (subsequently amended to October 15, 2021 with reduced $0.25 exercise price – see Note 11 below); and 475,000 warrants issued to Checkmate Capital Group LLC at an exercise price of $0.45, expiring January 15, 2022. |
| (4) | Warrants issued to Sterling Asset Management Inc. at an exercise price of $0.22 per share, expiring July 8, 2021 (subsequently amended to expire October 15, 2021; expired on October 15, 2021). |
| (5) | Common stock issued to Sterling Asset Management Inc. |
| (6) | Series E-1 preferred shares issued to Charles J. Link Jr. (Director) (subsequently exchanged for common shares – see Note 13 below). |
| (7) | Conversion of preferred shares by Alpha Capital Anstalt. |
(8) |
Common shares issued to Redstone Communications and affiliate for investor relations services pursuant to contract. |
| (9) | Common shares issued to nine unaffiliated parties upon conversion of Series B preferred shares and accrued unpaid dividends (see Note 2 above). |
| (10) | Options granted to directors, officers, and certain service providers of the Company under its Equity Incentive Plan. |
| (11) | Among 8 non-affiliate investors, does not include 142,857 unissued shares subject to a subscription receivable in the amount of $35,714. |
| (12) | Issued in connection with Convertible Promissory Notes in the total principal amount of $555,000 among six non-affiliated accredited investors. Warrants are exercisable prior to October 31, 2022 at $0.60 per share. |
| (13) | Issued pursuant to exchange agreement and plan of reorganization dated December 6, 2021, between the Company and six officers, directors and key employee holders of the Series E-1 Preferred Stock. |
We issued the securities reported hereunder to “accredited investors” as that term is defined in Rule 501 of Regulation D of the SEC, to “sophisticated investors”, or to directors and officers, each of whom had prior access to all material information about us prior to the offer and sale of these securities, and had the right to consult legal and accounting professionals, pursuant to registration exemptions provided in section 4(a)(2) and Rule 506 of Regulation D of the Securities Act.
| II-2 |
Item 16. Exhibits and Financial Statement Schedules
(a) Exhibits
| * | Filed herewith. |
| ** | To be filed by amendment. |
| II-3 |
Item 17. Undertakings
| (a) | The undersigned registrant hereby undertakes: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”);
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission (the “Commission”) pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
Provided, however, that Paragraphs (a)(1)(i), (ii), and (iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser: If the registrant is subject to Rule 430C (§230.430C of this chapter), each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of this chapter);
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(c) For the purpose of determining any liability under the Securities Act, the registrant will treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant under Rule 424(b)(1), or (4), or 497(h) under the Securities Act as part of this registration statement as of the time the Commission declared it effective.
(d) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-4 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, we have duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Austin, Texas, on the 17th day of December, 2021.
| QSAM Biosciences Inc. | ||
| By: | /s/ Douglas Baum | |
| Douglas Baum | ||
| Chief Executive Officer | ||
POWER OF ATTORNEY
The undersigned directors and officers of QSAM Biosciences, Inc. hereby constitute and appoint Douglas Baum and Christopher Nelson, and each of them, any of whom may act without joinder of the other, as the individual’s true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for the person and in his or her name, place and stead, in any and all capacities, to sign this Registration Statement and any or all amendments, including post-effective amendments to the Registration Statement, including a prospectus or an amended prospectus therein and any Registration Statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act, and all other documents in connection therewith to be filed with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact as agents or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature | Title | Date | ||
| /s/ Douglas Baum | Chief Executive Officer and Director (Principal Executive Officer) |
December 17, 2021 | ||
| Douglas Baum | ||||
| /s/ Adam King | Interim Chief Financial Officer | December 17, 2021 | ||
| Adam King | (Principal Accounting and Financial Officer) | |||
/s/ Christopher Nelson |
General Counsel and Director | December 17, 2021 | ||
| Christopher Nelson | ||||
| /s/ C. Richard Piazza | Executive Chairman | December 17, 2021 | ||
| C. Richard Piazza | ||||
| /s/ Joel Mayersohn | Director | December 17, 2021 | ||
| Joel Mayersohn | ||||
| /s/ Charles E. Link Jr. | Director | December 17, 2021 | ||
| Charles J. Link, Jr. |
| II-5 |