United States
Securities and Exchange Commission
Washington, D.C. 20549
FORM
For
the year ended:
or
For the period ended:
(Exact name of Registrant as specified in its Charter)
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
(Address of Principal Executive Offices)
(Registrant’s Telephone Number, including area code)
(Former name or former address, if changed since last report.)
Securities registered pursuant to Section 12(b) of the Act: None
Securities
registered pursuant to Section 12(g) of the Act:
Indicate
by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐
Indicate
by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during
the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject
to such filing requirements for the past 90 days.
Indicate
by check mark whether the Registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data
File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding
12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or emerging growth company:
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging Growth Company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements. Yes ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). Yes ☐ No ☒
Indicate
by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
The
aggregate market value of the voting and non-voting common stock held by non-affiliates computed by reference to the average bid and
asked price of such common stock on OTCQB of $4.47, as of the last business day of the Registrant’s most recently completed second
fiscal quarter, was approximately $
As of March 19, 2024, there were issued and outstanding shares of the registrant’s common stock.
Documents incorporated by reference: None.
QSAM Biosciences, Inc.
Index
| 2 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This document contains certain forward-looking statements that are subject to various risks and uncertainties. Forward-looking statements are generally identifiable by use of forward-looking terminology such as “may,” “will,” “should,” “potential,” “intend,” “expect,” “outlook,” “seek,” “anticipate,” “estimate,” “approximately,” “believe,” “could,” “project,” “predict,” or other similar words or expressions. Forward-looking statements are based on certain assumptions, discuss future expectations, describe future plans and strategies, contain financial and operating projections or state other forward-looking information. Our ability to predict results or the actual effect of future events, actions, plans or strategies is inherently uncertain. Although we believe that the expectations reflected in our forward-looking statements are based on reasonable assumptions, our actual results and performance could differ materially from those set forth or anticipated in our forward-looking statements. Factors that could have a material adverse effect on our forward-looking statements and upon our business, results of operations, financial condition, funds derived from operations, cash available for dividends, cash flows, liquidity and prospects include, but are not limited to, the factors referenced in this document, including those set forth below:
● |
our failure to close our merger agreement with Telix Pharmaceuticals Limited announced in the first quarter of 2024; | |
| ● | our lack of an operating history; | |
| ● | the net losses that we expect to incur as we develop our business; | |
| ● | obtaining FDA or other regulatory approvals or clearances for our technology; | |
| ● | implementing and achieving successful outcomes for clinical trials of our products; | |
| ● | convincing physicians, hospitals and patients of the benefits of our technology and to convert from current technologies and standards of care; | |
| ● | the ability of users of our products (when and as developed) to obtain third-party reimbursement; | |
| ● | any failure to comply with rigorous FDA and other government regulations; and | |
| ● | securing, maintaining and defending patent or other intellectual property protections for our technology. |
When considering forward-looking statements, you should keep in mind the risk factors and other cautionary statements in this document. Readers are cautioned not to place undue reliance on any of these forward-looking statements, which reflect our views as of the date of this document. The matters summarized below and elsewhere in this document could cause our actual results and performance to differ materially from those set forth or anticipated in forward-looking statements. Accordingly, we cannot guarantee future results or performance. Furthermore, except as required by law, we are under no duty to, and we do not intend to, update any of our forward-looking statements after the date of this document, whether as a result of new information, future events or otherwise.
MARKET DATA
Certain market and industry data included in this document is derived from information provided by third-party market research firms, or third-party financial or analytics firms that we believe to be reliable. Market estimates are calculated by using independent industry publications, government publications and third-party forecasts in conjunction with our assumptions about our markets. We have not independently verified such third-party information. The market data used in this document involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. While we are not aware of any misstatements regarding any market, industry or similar data presented herein, such data involves risks and uncertainties and are subject to change based on various factors, including those discussed below and set forth in the “Risk Factors” section of this document. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. Certain data are also based on our good faith estimates, which are derived from management’s knowledge of the industry and independent sources. Industry publications, surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but there can be no assurance as to the accuracy or completeness of included information. We have not independently verified any of the data from third-party sources nor have we ascertained the underlying economic assumptions relied upon therein. Statements as to our market position are based on market data currently available to us. While we are not aware of any misstatements regarding the industry data presented herein, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this document. Similarly, we believe our internal research is reliable, even though such research has not been verified by any independent sources.
| 3 |
PART I
ITEM 1. BUSINESS
In this Annual Report, references to the “Company,” “we,” “our,” “us” and words of similar import refer to QSAM Biosciences, Inc., the filer, a Delaware corporation. References to “QSAM” or the “Subsidiary” refer to QSAM Therapeutics Inc., a Texas corporation, our wholly-owned subsidiary and operating company.
Overview
We are developing next-generation nuclear medicines for the treatment of cancer. Our technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “QSAM Technology”), a clinical-stage bone targeting radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. We believe improvements in formulation and manufacturing from a prior FDA-approved drug utilizing the same radioisotope (Quadramet®) has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability. In early clinical testing, the QSAM Technology is demonstrating meaningful pain palliation effects in patients with metastatic prostate and breast cancer; and in future studies, may demonstrate disease modifying results in primary bone cancer patients, including children and young adults suffering from osteosarcoma.
In August 2021, the Food & Drug Administration (FDA) cleared our Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas. We initiated this trial at our first site (Houston, TX) in November 2021 and to date we have dosed five patients.
Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults; and in February 2022, the FDA granted Rare Pediatric Disease Designation for the same indication. In May 2020, CycloSam® was also utilized in a Single Patient Investigational New Drug for Emergency Use at the Cleveland Clinic. We believe the study we conducted at the Cleveland Clinic showed promising safety results in connection with a bone marrow ablation procedure, including patient tolerability at high dosages. To date, CycloSam® has completed animal studies in both small and large animals, including treating bone cancer in patient dogs at a university veterinary clinic.
Merger Agreement. On February 7, 2024, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Telix”), and certain subsidiaries of Telix established for the purpose of completing a reverse triangular merger with a second forward merger (collectively, the “Merger”). Pursuant to the Merger Agreement, QSAM stockholders will receive (i) $33.1 million in Telix ordinary shares (“Telix Shares”) or cash, less an adjustment amount equal to QSAM’s indebtedness and certain of its payables as of the Merger closing (the “Closing Consideration”), and (ii) contingent value rights (“CVRs”) to receive contingent future payments of up to $90 million in cash or Telix Shares, without interest, upon and subject to the achievement of four clinical and commercial milestones within ten years of closing.
Prior to closing the Merger, QSAM will effect a reverse stock split of its common stock in a ratio between 1:1000 and 1:2000. Each whole share of QSAM common stock outstanding after the reverse split will be entitled to receive Telix Shares and CVRs as in the Merger. Any remaining fractional shares of QSAM common stock resulting from the reverse split will be exchanged for cash and CVRs. As a result of the reverse split and the Merger, all QSAM stockholders will receive, for each share of QSAM common stock held prior to the reverse split, Telix Shares or cash with a value equal to such share of QSAM common stock’s pro rata share of the Closing Consideration and one CVR.
Telix Shares issued to QSAM stockholders will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), but will be issued pursuant to an exemption to the registration requirements thereunder. The Telix Shares will be subject to resale restrictions under Rule 144 of the Securities Act.
QSAM stockholders representing greater than a majority of the total voting stock of the Company have already approved the Merger. Closing, however, is subject to various conditions set forth in the Merger Agreement including, among others, the filing of a definitive information statement pursuant to Regulation 14C of the Securities Exchange Act of 1934, as amended (the “Information Statement”). The Merger and the reverse split cannot be closed until 20 days after the mailing of the Information Statement to QSAM stockholders. Upon the closing of the Merger, the Company will de-list from the OTC Markets and will file a Form 15 to cease reporting under the Securities Exchange Act of 1934, as amended. There is no guarantee that all closing conditions required to close the Merger will be achieved, or that the Merger will close on the terms described herein.
| 4 |
Background of the QSAM Technology
What is CycloSam®. CycloSam® is a targeted, bone seeking radiopharmaceutical that combines the beta-emitting radioisotope Samarium-153 (153Sm) with a chelating agent, DOTMP (1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetramethylenephosphonic acid). Samarium-153 is acquired from a nuclear reactor from a third party and the chelating agent is supplied in the form of kits. Chelating agents are organic compounds capable of linking together metal ions to form complex ring-like structures. This combination forms a stable complex which delivers a radioactive dose to sites of rapid bone mineral turnover such as bone cancers and tumors. CycloSam® has a physical half-life of 46 hours (radiation decreases by half in 46 hours) and emits both medium-energy beta particles that produce the therapeutic effect, and gamma photons that make it possible to take images of the skeleton and locate and characterize the size and nature of tumors. The use of radioisotopes to both diagnose and treat disease is called “theranostics” and is a rapidly growing area of medical discovery.
How CycloSam® Works – Mechanism of Action & Administration. CycloSam® utilizes a chelating agent called DOTMP that seeks out bone locations of high mineral turnover, typical in cancer cells and tumor growth. DOTMP is taken up by calcium turnover locations in bones and carries the radioactive “payload” along with it. The radioisotope Samraium-153 emits radiation as it decomposes in the form of beta particles. Approximately 50% of the radioactivity concentrates in bone mineral with a very high lesion-to-normal bone ratio. We believe this provides a radiation dose to the adjacent tumor cells. The absorbed radiation dose produces the presumed therapeutic effect to the tumor, killing the cancer cells or slowing their growth by damaging their DNA. Metastatic bone cancer patients who have been dosed with CycloSam® have also reported material reductions in pain lasting for several months after treatment. Our pre-clinical studies and single patient IND performed at the Cleveland Clinic has demonstrated that the remaining half of the administered activity is rapidly excreted through the kidneys.
Generally, radiation therapy does not immediately kill cancer cells and more than one treatment is expected to eradicate a tumor, dramatically reduce its size, or slow its growth. CycloSam® has a short half-life of 46 hours and is rapidly eliminated from the body. This avoids an undesirable radioactive buildup in healthy tissues and organs when used in multiple treatments, which we believe, is an important feature of CycloSam® over predecessor drugs. CycloSam® has also not demonstrated saturation of the bone sites in animal studies, which supports a multi-dosage treatment regimen.
The final drug product of CycloSam® is prepared from DOTMP kits and 153SmCl in 0.1 N HCl at a nuclear pharmacy local to the patient administration site. The final drug product is then delivered to the physician for use as an intravenous (IV) injection within 72 hours.
How is CycloSam® Made – Method of Manufacturing. CycloSam® uses a patented, low-specific-activity Samarium-153 which is produced in the lower flux region (beryllium reflector) of the nuclear reactor and can be accessed with a pneumatic tube on a daily basis. Once prescribed by radiation oncologists and nuclear medicine physicians, we order the radioisotopes from Missouri University Research Reactor (MURR) or the University of Texas at Austin (NETL) to be sent overnight to an onsite or nearby (to the patient) nuclear pharmacy to be compounded with a DOTMP “cold kit” and delivered to the treating physician for administration.
The DOTMP “cold kit” is patented in the U.S. and other jurisdictions, and was developed by IsoTherapeutics LLC, the inventors of CycloSam®. Although we believe the IsoTherapeutics’ cGMP manufacturing facility has the capacity to manufacture sufficient supply for our initial rollout, and such manufacturing is currently covered under our master services agreement, we plan to secure secondary manufacturing partners for the kits in the future. MURR has been our source of Samarium-153 used in our animal studies, our Single Patient IND for Emergency Use at the Cleveland Clinic, and for part of our current clinical trials. We also now use NETL for the supply of irradiated Samarium-153, especially as needed to dose patients at our Houston, TX clinical trial site. We plan to qualify additional suppliers of both the raw non-irradiated Samarium, and the irradiation of these materials, as part of our supply chain and general business risk diversification strategy.
| 5 |
What are CycloSam®’s anticipated competitive advantages. We believe CycloSam® has competitive advantages over current radiopharmaceutical offerings in the marketplace. Such potential competitive advantages include:
| ● | CycloSam®’s radioisotope, Samarium-153, emits beta particles that travel farther than alpha particles with what we believe is sufficient energy to slow the growth or decrease the size of target cancer cells. We believe beta particles penetrate bone matter deeper than the alpha emitting radiopharmaceuticals currently in the marketplace and may be more effective in treating tumors that form in or metastasize to bones, as well as the often debilitating pain that results from bone metastasis. | |
| ● | CycloSam®’s delivery agent, DOTMP, compared to other chelating agents such as EDTMP used in Quadramet®, has shown in animal and other pre-clinical testing to have a high bone binding affinity allowing for the maximum delivery of the radioactive “payload” adjacent to the tumor without saturation of the bone, as observed from our pre-clinical trials. | |
| ● | Our method of manufacturing Samarium-153 compared to Quadramet®, has shown in our pharmacopeial limits studies to produce a 30-fold reduction in levels of the long-lived radioactive impurity Europium-154. We believe this may mitigate toxicity issues with the patient. | |
| ● | Our initial studies show CycloSam® has fewer toxicities and a short 46-hour half-life that may allow for more frequent and repeated dosing of our radiopharmaceutical. |
The competitive advantages we believe to be important to CycloSam® are based on pre-clinical animal and other studies including our single patient IND at the Cleveland Clinic and the initial patients dosed in our Phase 1 safety trial. We cannot be sure that our technology will perform similarly in future clinical trials. Failure to achieve these competitive advantages could negatively affect our ability to achieve FDA approval as a new drug, or our ability to market CycloSam®.
License Agreement and Intellectual Property
License Agreement. The Company, through its wholly-owned subsidiary QSAM Therapeutics, entered into an exclusive worldwide Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) with IGL Pharma, Inc. (“IGL”) on April 20, 2020 with respect to the innovative work of Jim Simon, PhD and R. Keith Frank, PhD, at IsoTherapeutics on Samarium-153 DOTMP. IGL is an affiliated company with IsoTherapeutics. Until January 2024, our Executive Chairman also served as President of IGL. We amended the License Agreement on November 24, 2021, and then again on February 2, 2024 (the “Second Amendment”).
Our License Agreement with IGL, as modified in the first amendment in 2021, is for 20 years or until the expiration of the multiple patents covered under the license and requires multiple milestone-based payments including: up to $410,000 as CycloSam® advances through Phase 3 of clinical trials, and $2 million upon commercialization. IGL has also received 12,500 shares of the Company as additional compensation. Upon commercialization, IGL will receive an on-going royalty equal to 4.5% of Net Sales, as defined in the License Agreement, and 5% of any consideration we receive pursuant to a sublicense, sale of the asset, or sale of QSAM Therapeutics (the “IGL License Fee”). We will also pay for ongoing patent filing and maintenance fees, and we have certain requirements to defend the patents against infringement claims. The parties have agreed to mutual indemnification. Pursuant to the Second Amendment, which was entered into as a condition to the execution of the Merger Agreement, the parties: (i) made modifications to sublicense fees, royalties and other amounts payable to IGL; (ii) made modifications to the definitions of “Commercially Reasonable Efforts,” “Products” and “Patents” described in the License Agreement; and (iii) added an additional payment to IGL of $100,000, payable half upon the execution of the Second Amendment and the balance upon the closing of the Merger. The Second Amendment will only become effective upon the closing of the Merger, and shall become null and void if the closing does not occur.
| 6 |
Either party may terminate the License Agreement 30 days after notice in the event of an uncured breach, or immediately in the case of bankruptcy or insolvency of the other party. QSAM Therapeutics may terminate for any reason upon 30 days’ notice. In the case IGL terminates due to an uncured breach, IGL will repay to us 25% of our direct clinical costs to assume ownership of data and other information gained in that process.
In connection with the License Agreement, QSAM Therapeutics signed a two-year Consulting and Confidentiality Agreement (the “Consulting Agreement”) with IGL, which provided IGL with payments of $8,500 per month that continued through April 2022. We now contract directly with IsoTherapeutics for monthly consulting services at $8,500 per month under our Master Services Agreement. Pursuant to this arrangement, IsoTherapeutics provides us with additional consulting and advisory services from the technology’s founders to assist in the clinical development of CycloSam®. Until January 2024, our Executive Chairman served as President of IGL, and received a $500 per month fee from IGL.
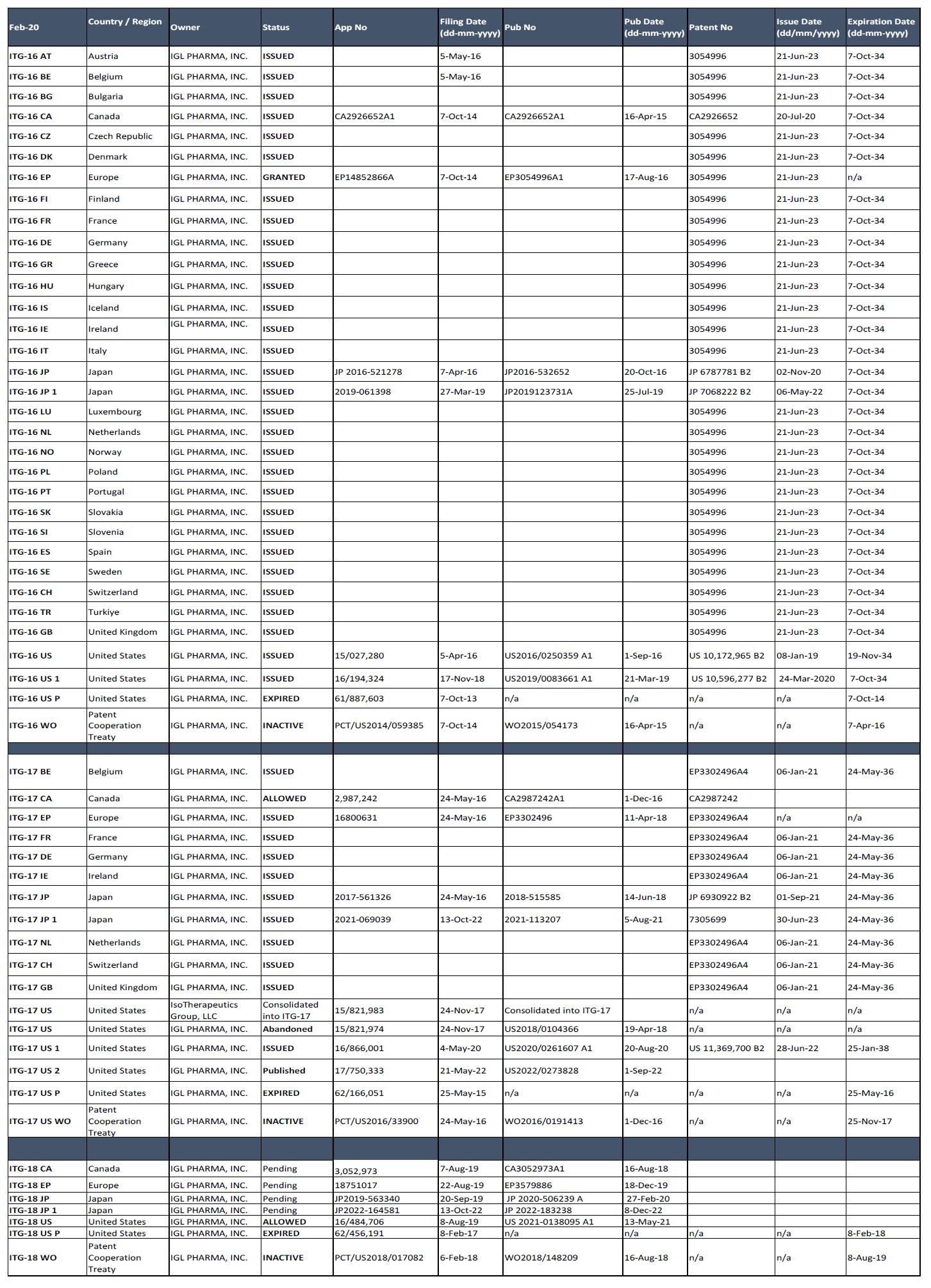
Patents. Pursuant to the License Agreement, our IP estate includes 14 total patents issued and pending across three distinct patent families that we believe provide protection for the use of CycloSam® as a radiopharmaceutical in the U.S. and internationally. Under the License Agreement, the Company holds multiple issued, allowed or granted patents in the US, Japan, Canada, and Europe (which have subsequently been granted in approximately 22 countries in Europe). The balance of these patent applications are pending. Notably, the patents cover the use of low-specific activity Samarium-153, allowing for daily supply of the highly toxicity-reduced formulation of the isotope, which we believe is the key to allowing for multi-dose regimens of CycloSam® that could have a positive therapeutic effect. Also, the CycloSam® kit that will be commercialized is protected by the patent estate that broadly protects DOTMP kit formulations for radioisotopes, potentially allowing for efficient distribution of the product and widespread use. Finally, methods relating to repeat dosing regimens for therapeutic radiopharmaceutical agents, which suggest increased efficacy based on prior research, is also covered under patent applications. Taken together, management believes that the patent family provides for a significant barrier to entry for a competitor as it is expected to prevent a generic product from being developed; however, we cannot guarantee that a competitor will not or cannot challenge our patents or otherwise circumvent our patents, or that we would have the resources to defend any patent infringement.
A list of our patents and status of prosecution is included in the following table:

| 7 |
Trademark. Pursuant to the License Agreement, the Company also has the right to use the registered trademark “CycloSam®” for the marketing and sale of the drug candidate. Pursuant to the Second Amendment, the trademark will be formally assigned to QSAM upon closing of the Merger.
Competition
The biotechnology and pharmaceutical industries are characterized by the rapid evolution of technologies and understanding of disease etiology, a strong emphasis on intellectual property and intense competition. We face substantial potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic research institutions, governmental agencies and public and private research institutions.
In addition to the current methods of care for cancer patients, the field of radiopharmaceuticals is deeply studied, and many parties are pursuing commercial and academic clinical trials. Early results from these trials have fueled continued interest in radiopharmaceuticals, which are pursued by several biotechnology companies as well as by large pharmaceutical companies.
We consider our competitors to be other companies developing targeted radiopharmaceuticals for the treatment of cancer. There are several companies developing targeted alpha-based radiopharmaceuticals for the treatment of cancer, including Bayer AG, or Bayer, Actinium Pharmaceuticals, Inc., RadioMedix, Inc, Orano Med, Telix, Fusion Pharmaceuticals Inc., and RayzeBio, Inc. These companies are targeting a wide range of solid and hematologic malignancies using various alpha emitting isotopes, including Radium-223, Actinium-225 and Thorium-227. The first approved alpha particle-based therapy is Bayer’s Xofigo, a salt of radium that is not currently attached to a targeting molecule, but naturally localizes to regions where cancer cells are infiltrating bone. Xofigo was approved in the United States by the FDA in 2013 for the treatment of bone metastases associated with prostate cancer.
There are several companies with approved or late clinical stage beta-based radiopharmaceuticals, including Novartis AG, Telix, and POINT Biopharma Global. Novartis received FDA approval for Pluvicto in 2022, a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer, and, in 2023, generated approximately $980 million in worldwide sales according to recent company filings. Pluvicto uses the beta-emitter Lutetium-177. Another competitive company, POINT Biopharma, has two indications using beta-emitting particles in Phase 3 trials, and which were recently licensed by Lantheus. The beta emitting isotopes used by these companies include Iodine-131, Lutetium-177, Strontium-89 and Yttrium-90. There are other beta particle-based radiopharmaceuticals in various stages of clinical development by companies including Ipsen S.A., Y-mAbs Therapeutics, Inc. and Clovis Oncology, Inc.
Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies and materials complementary to, or necessary for, our programs.
We could see a reduction or elimination in our commercial opportunity if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient to administer, are less expensive or with a more favorable label than our product candidates. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. The key competitive factors affecting the success of our product candidate, if approved, are likely to be their efficacy, safety, convenience, price, and the availability of reimbursement from government and other third-party payors.
| 8 |
Radiopharmaceutical Market
The global radiopharmaceuticals market size is projected to reach $13.67 billion by 2032, registering a CAGR of 10.2% during the forecast period 2023 to 2032, according to a November 2023 report from Precedence Research, a global provider of market insights. North America dominates the global radiopharmaceuticals market, which is attributed to the rising prevalence of chronic illnesses, extensive research and development initiatives, a supportive regulatory landscape, and the presence of leading pharmaceutical and radiopharmaceutical enterprises. Based on type, the radiopharmaceuticals market is bifurcated into diagnostic nuclear medicine and therapeutic nuclear medicine. The diagnostic nuclear medicine segment is holding a larger share of the market and is anticipated to register a higher CAGR during 2021–2028. By application, the market is segmented into oncology, cardiology, neurology, and others, with the oncology segment holding the largest market share in 2022.
Overall, we believe that the recent technological developments in radiopharmaceuticals, including promising new alpha-emitting candidates, will continue to provide supportive tailwinds to this sector. As a result, management believes that it is well positioned to capitalize on these future opportunities.
History of CycloSam® development and past studies and trials
The Company has an exclusive worldwide patent and technology License Agreement for CycloSam®. The QSAM Technology was developed at IsoTherapeutics Group, LLC (“IsoTherapeutics”) by its founders Jim Simone, PhD and R. Keith Frank, PhD (the “Inventors”). IsoTherapeutics subsequently transferred their technology to IGL Pharma, Inc., an affiliated entity and the Company’s current licensor. The Inventors also developed one of the first commercial radiopharmaceuticals on the market, Quadramet®, approved by the FDA in 1997 for pain palliation. Drs. Simone and Frank each have over 30 years of experience in radiopharmaceuticals, publishing more than 100 papers and authoring over 60 patents in the field. The Inventors spent much of their careers at Dow Chemical Company prior to divestiture of its radiopharmaceutical business. According to the Inventors, CycloSam® was developed to address the shortcomings of other radiopharmaceuticals, including Quadramet, such as toxicity, saturation effects, long-lived impurities, and supply-chain complexities.
Prior generation Quadramet vs Improvements in CycloSam®. CycloSam® is a second-generation bone-seeking radiopharmaceutical based on Quadramet. Although Quadramet was clinically proven to be effective for pain palliation associated with metastatic bone cancer, the toxicity of the drug made repeat doses undesirable and manufacturing complexities made daily availability highly challenging. Therefore, Quadramet’s use was always limited to pain control, not tumor reduction, elimination or disease modification. The loose binding affinity of Quadramet’s chelating agent, EDTMP, means the ratio of chelant to Samarium-153 is extremely high (~300:1). The resulting problem of bone saturation prohibits usage of Quadramet in high doses required for treatment of bone cancer or bone marrow ablation. Additionally, high levels of impurities from the Samarium-153 production, namely Europium-154, made repeated dosing of Quadramet undesirable. Lastly, Quadramet faced supply-chain and distribution limitations because the Samarium-153 it uses could only be accessed from the reactor once per week. We believe that because of these challenges, Quadramet demonstrated limited market success, and to our knowledge, was recently discontinued by its manufacturer and distributor. We believe CycloSam® overcomes these inherent limitations of Quadramet in terms of toxicity, usage, and availability.
The Vienna Protocol – Precedent of Efficacy. In August 2011, Dr. Helmut Sinzinger published a study in the Quarterly Journal of Nuclear Medicine and Molecular Imaging, which demonstrated that despite the described limitations of Samarium-153 EDTMP (Quadramet®), it could still be used to effectively treat bone metastasis.
The Vienna Protocol, as it was labeled, was based on a 550 patient study developed by Dr. Sinzinger to deliver therapeutic doses of Quadramet® on a periodic low dose basis balancing hematological toxicity and europium buildup with clinical results. The specific regimen used very low doses of the predecessor drug on an outpatient basis. The treatment was administered at three month intervals during the first year, followed by another five treatments at six month intervals, then five therapies at nine month intervals, and then annually indefinitely. The dosing schedule was driven by hematological concerns and constant monitoring was required.
During Dr. Sinzinger’s trials, a wide range of positive clinical responses were seen including arrested tumor growth and even regression of the cancer in the bone. Some patients were treated for over five years exhibiting significant clinical response. While effective, this regimen required significant time and safety precautions on the part of both the physician and patient both of which were considered overly burdensome. Although this study was well published and the efficacy results were promising, wide clinical adoption did not occur due to the overall effort that was required to deliver a true therapeutic dose while avoiding the toxicity issues. Quadramet was never approved by the FDA for the treatment of bone cancer, but rather, just for pain management associated with the disease.
| 9 |
Improvements of CycloSam® over Predecessor Drug
CycloSam® is a new, advanced generation Samarium-153 drug with a dramatically different clinical profile than Quadramet®. By producing the Samarium-153 in a different part of the nuclear reactor, the decay by-product Europium has shown in studies to be nearly non-existent, thus eliminating long-term buildup concerns [Source: IsoTherapeutics Group. (2021). Preparation and Stability of CycloSam® Sm-153-DOTMP. (Report No. QSM-1)]. Secondly, the superior binding affinity of the new chelating agent, DOTMP, means more energy can be delivered to the target, thus minimizing off-target concerns. Further, the method of harvesting the patented low specific activity Samarium-153 means it can be accessed on a daily basis, compared to weekly for Quadramet®, at a reduced cost. We believe that all of these clinical and manufacturing improvements were achieved without any reduction in either the tumor killing power of Samarium-153 or its ability to travel deep into the bone tumor.
Potential Market Indications for CycloSam®.
CycloSam’s therapeutic profile and presumed advantages over other radiopharmaceuticals, including Quadramet, translate to several potential key market indications, including treating pain associated with cancer that has metastasized to the bone and certain forms of primary bone cancer, such as osteosarcoma, which mostly affects children and young adults. The following table highlights the prevalences of these diseases in the United States on an annual basis:
| Market | Estimated New Cases Diagnosed Annually (US) | |||
| Bone Metastases (Breast, Prostate, Lung) | 400,000 | |||
| Other Primary Bone Cancers | 2,770 | |||
| Primary Bone Cancer – Osteosarcoma | 1,000 | |||
| Primary Bone Cancer – Ewing’s Sarcoma | 200 | |||
Source: American Cancer Society estimates of new cases reported each year in the United States. Data as of January 2023.
Bone metastases arise in about 5% of all types of cancer, 29% of patients with multiple myeloma (15,000), 16% of lung (37,000), 6% of prostate (48,000) and 7% of breast cancers (70,000). Roughly 70% of patients with bone metastases will experience bone pain, and many are at risk for skeletal-related events including fracture and spinal cord compression. The total annual cost for treatment of metastatic bone disease is approximately $12.7 billion or 17% of the total of $74 billion that was spent on direct medical costs of these cancers [Source: Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007 Jun 1;109(11):2334-42. doi: 10.1002/cncr.22678. PMID: 17450591]. In addition to metastatic bone cancers, according to the National Institute of Health SEER, there are approximately 14,000 people living with osteosarcoma in the US at any one time [Source: Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007 Jun;459:40-7. doi: 10.1097/BLO.0b013e318059b8c9. PMID: 17414166, and National Cancer Institute: Surveilance, E., and End Results Program Cancer Stat Facts: Bone and Joint Cancer, <https://seer.cancer.gov/statfacts/html/bones.html> (2020)] and their cost of care is estimated to exceed $100,000 per patient [Source: American Cancer Society. Key Statistics About Bone Cancer].
Metastatic bone cancer is currently incurable, and therefore palliation and arrest or deceleration of the progress of disease are important near-term goals. Quadramet® (Samarium-153-EDTP) and MetastronTM (89Sr chloride) were approved by the FDA for pain palliation resulting from osteoblastic bone metastases, but their widespread acceptance and use is hampered by concern about the perceived risk of myelosuppression when administered concurrently with chemotherapy. Xofigo, an alpha particle emitter, was approved in May 2013 and initially was expected to capture significant market share rapidly; however, certain safety concerns have limited the product’s applicability. Novartis’ Pluvicto, a beta-emitter, was approved by the FDA in late 2022 for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer. Novartis reported worldwide sales of Pluvicto in 2023 of approximately $980 million according to company filings, and reported in a recent conference that they expect sales to reach $2 billion in the coming years.
| 10 |
Osteosarcoma is the most common childhood and adolescent/young adult (ages 15-39) primary high-grade bone malignancy [Source: Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian J Med Paediatr Oncol. 2017;38(1):33-43. doi:10.4103/0971-5851.203513]. Patients can often have metastatic cancer at diagnosis, and metastasis to the lungs is often fatal for these patients. For patients who develop or present with metastatic cancer in this diagnosis, the 5-year survival rate is 66% [Source: Osteosarcoma - Childhood and Adolescence - Statistics.” Cancer.Net, 30 Sept. 2021, https://www.cancer.net/cancer-types/osteosarcoma-childhood-and-adolescence/statistics]. Osteosarcoma standard-of-care usually involves chemotherapy which has substantial negative side effects, or drastic surgeries such as limb salvage or amputation. Osteosarcoma is relatively resistant to External Beam Radiation Therapy (EBRT), and currently approved radiopharmaceutical therapeutics fall short due to myelotoxicity and long-lived radioactive impurities. There is a tremendous unmet need for a better treatment that is more efficacious against pediatric osteosarcoma and better tolerated by patients.
Preclinical and Clinical Studies
Preclinical Studies. Preclinical toxicology studies of CycloSam® in rats and dogs have shown that a single intravenous dose of non-radioactive Samarium-153 DOTMP elicited no significant systemic toxicity. Skeletal uptake has also been studied in rats over a wide range of doses to determine whether CycloSam® displays a similar saturation effect which has been observed in studies of Samarium-153 EDTMP (aka Quadramet). In the rat saturation study, no statistically significant difference was found in uptake as a function of increased dosage of CycloSam®.
In addition to rat and dog toxicological studies, a proof-of-concept study was conducted in ten dogs with spontaneously occurring bone cancer treated with 1-2 mCi/kg of CycloSam®. Treatment was well tolerated with seven dogs treated at a dose of 1 mCi/kg and one dog treated with 2 mCi/kg who did not experience a dose limiting toxicity. One dog treated with 2 mCi/kg and one dog treated with 2.3 mCi/kg experienced grade 4 asymptomatic thrombocytopenia and neutropenia; which refers to a manageable depressed level of platelets and neutrophils in the blood. Results from these preclinical studies suggested CycloSam® has potential as a therapeutic agent in the treatment of primary bone cancer and metastatic bone disease.
Safety/Tox Studies. Non-radioactive CycloSam® has been through a full-scale 14-day, acute toxicological study in both rats and dogs. This study was designed to determine the toxicokinetics of the product at four different dose levels that are higher than expected to be used in the current clinical trials. The studies showed no systemic toxicity in either of the species with a single intravenous administration of CycloSam® (non-radioactive). Some mild to moderate allergic-like responses were seen in dogs at the highest dose, which is much higher than would be expected for clinical use.
Non-clinical testing. Rat and rabbit pharmacology and targeted bone pharmacology studies have been undertaken and published in both patents and in the literature for Samarium-153-DOTMP and their preclinical results demonstrated significant skeletal uptake fractions. We believe these studies suggest CycloSam® has promise as a bone seeking radiopharmaceutical.
Clinical Pharmacology. The majority of non-clinical pharmacology studies with CycloSam® have been done in rats. When administered through the tail vein, much of the Samarium-153-DOTMP binds to the bone; the half-life is 46.3 hours, but the portion of the drug not bound to bone or calcified tissue is completely eliminated through the kidneys within 6 hours of administration. Two additional studies in dogs with osteosarcoma have also elicited promising results, and confirm bone uptake, preliminary safety, and preliminary clinical benefit against bone tumor. Preclinical results demonstrated significant skeletal uptake fractions.
Clinical Studies. We are currently enrolling patients in a Phase 1 multiple center, open label, dose escalation clinical trial intended to determine the maximum tolerated dose of CycloSam® in participants, and also assess early efficacy signals. Participants with bone cancer that has metastasized from the breast, lungs, prostate or other organs, as well as participants with cancer that has originated in the bone such as osteosarcoma and Ewing’s Sarcoma – diseases that mostly affect children and young adults — are eligible subject to the trial’s inclusion and exclusion criteria.
| 11 |
To date, we have completed two of four patient groupings (“cohorts”), with a total of up to 17 participants expected to be enrolled. The completed cohorts are comprised of five participants who received the lowest two dosages of CycloSam® in the study. The total dosage of the active radioisotope Samarium-153 to be received by the third cohort will be twice as high as the second cohort.
Safety data from the first five trial participants in our Phase 1 clinical trial showed no Serious Adverse Events (SAE’s), and no irreversible clinically significant adverse events. Importantly, there was no clinically significant white, red, or platelet blood cell suppression to date, no Grade 3 adverse events, and no irreversible adverse events. Some trial participants also had initial pain relief and reduction from baseline in pain intensity scores using the Visual Analog Scale (VAS) of pain intensity. Additionally, some trial participants had an initial reduction from baseline after treatment in bone tumor size as measured using Response Evaluation Criteria in Solid Tumors (RECIST) criteria in the followed bone tumor. Some trial participants also had an initial reduction in tumor Standard Uptake Values (SUV’s), an imaging measure of bone tumor activity. These results are preliminary and may not be indicative of future results in the trial.
In February 2022, the FDA cleared our amended protocol increasing the age criteria to participants 75 years old from the prior age limitation of 65. This amendment to the enrollment criteria expands the population of potential participants in QSAM’s Phase 1 study evaluating CycloSam® in the treatment of bone cancer.
In 2020, CycloSam® was studied for the first time in humans under a Single Patient Investigational New Drug (IND) for Emergency Use at the Cleveland Clinic. The patient, a 25 year-old male who suffered from myelodysplastic syndrome (MDS) and high-risk osteosarcoma, received a single low dose of 1 mCi/kg of CycloSam® on March 24, 2020 for dosimetry. This was followed seven days later on March 31, 2020 by a single high dose of 32 mCi/kg (1919 mCi) of CycloSam®. No injection site effects were noted at the time of injection. At 48 hours post-injection of the second dose there was no renal toxicity observed. The estimated dose delivered to the skeleton was 40 Gy with bone lesion uptake of 60 Gy. The abbreviation Gy stands for “gray”, which is a measurement of radiation reaching the target. In this instance, a 45 Gy is considered required to deliver the radiation to the target, and therefore, 60 Gy was considered very good. The patient received an allogeneic stem cell transfusion two weeks following high dose injection of Samarium-153 DOTMP; however, the stem cell transplant failed to fully engraft. The patient, who was terminally ill prior to the treatment, passed away on August 18, 2020, a month after bone marrow ablation and after additional procedures not using a radiopharmaceutical were performed, from complications of an infection unrelated to the infusion of CycloSam® according to the investigator.
The investigator concluded that high-dose CycloSam® can be given safely with no apparent renal toxicity and no unexpected adverse events attributable to Samarium-153 DOTMP. Skeletal targeting with sparing of other tissues was observed after the high dose. This was only a single patient human clinical trial, and the patient did not survive long enough for full observation, so additional safety and efficacy clinical trial data will have to be developed.
Contracted Research Organization. In January 2020, our licensor, IGL Pharma, entered into a Master Services Agreement (MSA) with a full-service Contract Research Organization (CRO) with over a 30 year history of service to pharmaceutical and biotechnology clients. The MSA was amended in February 2021 to add QSAM as a party and includes a fixed monthly retainer for regulatory and clinical trial consulting services as well as specific work orders for clinical trial execution services. The CRO has a full-time staff of project managers, statisticians, physicians, nurses and other regulatory and operational personnel to support our FDA interactions, filings and preclinical and clinical trial activities. Specifically, the CRO provides clinical trial management services, clinical study monitoring services, medical coding services, electronic data capture services, data management services, medical monitoring services, safety reporting and medical writing services.
Government Regulation and Product Approval
Clinical trials, the drug approval process, and the marketing of drugs are intensively regulated in the United States and in all major foreign countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and related regulations. Drugs are also subject to other federal, state, and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
| 12 |
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of biopharmaceutical products. These agencies and other federal, state, and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising, and promotion of product we develop in the future.
The FDCA and/or FDA’s policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of any candidate drug product or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Approval: The process required by the FDA before new drugs may be marketed in the United States generally involves the following:
| ● | nonclinical laboratory and animal tests; | |
| ● | chemistry, manufacturing, and control testing (CMC), validation and documentation of all synthesis, preparation and production processes for all kit ingredients and finished products; | |
| ● | submission of an Investigational New Drug (IND) application, which must become effective before clinical trials may begin; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; | |
| ● | pre-approval inspection of manufacturing facilities and clinical trial sites; and | |
| ● | FDA approval of a New Drug Application (NDA) which must occur before a drug can be marketed or sold. |
The testing and approval process requires substantial time and financial resources, and we cannot be certain that any approvals will be granted on a timely basis if at all.
We will need to successfully complete additional clinical trials in order to be in a position to submit an NDA to the FDA. Future trials may not begin or be completed on schedule, if at all. Trials can be delayed for a variety of reasons, including delays in:
| ● | obtaining regulatory approval to commence a study; | |
| ● | reaching agreement with third-party clinical trial sites and vendors and their subsequent performance in conducting accurate and reliable studies on a timely basis; | |
| ● | obtaining institutional review board approval to conduct a study at a prospective site; | |
| ● | recruiting subjects to participate in a study; and | |
| ● | supply of the drug. |
We must reach an agreement with the FDA on the proposed protocols for our future clinical trials, post-market safety monitoring, and on a Pediatric Development Plan in the United States. All new drugs now require the presentation to the FDA after Phase II clinical trials have ended of a Pediatric Development Plan outlining the strategy and steps to be taken by us to study CycloSam® in children as appropriate. A separate submission to the FDA must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each study subject. Regulatory authorities, an IRB, a data safety monitoring board, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk. Such risks may include unexpected or serious adverse events, or increased severity or occurrence rate of known potential adverse events.
| 13 |
FDA Post-Approval Requirements: Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping and reporting of adverse experiences with the drug and/or additional post-market clinical trials. Drug manufacturers are required to register their facilities with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain quality processes, manufacturing controls, and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality and purity characteristics that it purports to have. Under the federal Prescription Drug Marketing Act, the sampling and distribution and tracking of drugs is regulated. It is designed to discourage the sale of counterfeit, adulterated, misbranded, subpotent, and expired prescription drugs. Certain states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, fail to approve any NDA or other application, require us to recall a drug from distribution, shut down manufacturing operations or withdraw approval of the NDA for that drug. Noncompliance with cGMP or other requirements can result in issuance of warning letters, civil and criminal penalties, seizures, and injunctive action.
Labeling, Marketing and Promotion: The FDA closely regulates the labeling, marketing, and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to the safety and efficacy of a drug that are consistent with FDA approval and may only actively market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions, and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of drugs.
Pediatric Research Equity Act: The Pediatric Research Equity Act (“PREA”) amended the FDCA to authorize the FDA to require certain research into drugs used in pediatric patients. The intent of the PREA is to compel sponsors whose drugs have pediatric applicability to study those drugs in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The Secretary of Health and Human Services may defer or waive these requirements under specified circumstances. The FDA may decide that an NDA will be approved only following completion of additional pediatric studies.
Anti-Kickback and False Claims Laws: In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing, and scientific/educational grant programs must comply with the Anti-Kickback Statute, the False Claims Act, as amended, the privacy regulations promulgated under HIPAA, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
In the United States, we are subject to complex laws and regulations pertaining to healthcare “fraud and abuse,” including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act, and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
| 14 |
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multi-billion-dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. In addition, the federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws. The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), also created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the Anti-Kickback Statute a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, a similar federal requirement Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the “Affordable Care Act”) commonly referred to as the “Physician Payments Sunshine Act” requires manufacturers to track and report to the federal government certain payments and “transfers of value” made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, made in the previous calendar year. There are a number of states that have various types of reporting requirements as well. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
Patient Protection and Affordable Health Care Act: Historically in the United States, policy makers have attempted several healthcare reforms regarding the healthcare system that could expand access to healthcare, improve quality of healthcare, contain healthcare costs, prevent or delay approval of product candidates, restrict or regulate post-approval activities, and affect the profitable sale of drugs.
In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the ACA, was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. Since its enactment, there have been judicial and political challenges to certain aspects of the ACA. Most recently on June 17, 2021, the U.S. Supreme Court dismissed a judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how the healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact the ACA.
Congress and the Biden administration have generally indicated that they will continue to seek new legislative and/or administrative measures to control drug costs and improve access. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
| 15 |
Other Regulations: We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
The CycloSam® radioactive product is regulated by the federal Nuclear Regulatory Commission (NRC), and also by similar state regulatory agencies. The handling, packaging, shipping, transportation, and disposal of radioactive materials is highly regulated, and those regulations can change, and the Company would have to comply with all requirements, which could be costly. Additionally, if overnight delivery failures occur, the radioactive compounds cannot be used, and that can result in substantial increased costs and liabilities. The disposal of radioactive materials is also regulated by the Environmental Protection Agency (EPA), and also by similar state regulatory agencies and the Company would have to comply with all of those requirements, which could also be costly.
Radioactive waste from the medical sector is an environmental concern from a global perspective. However, most studies have concluded that radioactive material from the medical sector does not present a significant long term waste management problem when compared to wastes generated from nuclear fuel cycle operations. This is primarily due to the characteristics of biomedical waste, such as its short half-life and low radiotoxicity. For instance, Samarium-153 has a half-life of approximately 46 hours. Biomedical waste also typically contains low energy emitters, such as beta and gamma isotopes, and is generally of low total and specific activity. Further considerations are the volumes of this waste and any other hazardous properties associated with the waste such as biological and chemical risks.
Regardless of the relatively low risks in the preparation, use and disposal of medical isotopes, entities that handle such materials should implement an effective program for biomedical radioactive waste management based on the principles of waste prevention and minimization, while providing for the protection of personnel and the environment, consistent with the requirements of applicable regulatory authorities. This assessment should include an analysis of the total radionuclide inventory and pattern of use, waste types and amounts generated and the potential routes for disposal.
We seek to assure that the nuclear reactor facilities that produce our Samarium-153, as well as the nuclear pharmacies that prepare doses for treatment, have proper and effective waste management procedures in place applicable to the risks presented by the actual material. Further, doctors and trial sites who handle radioactive materials must be educated on the dangers of handling hazardous substances and the proper methods of disposing radioactive or formally radioactive waste, similar to the handling of other medical and bio wastes.
Smaller Reporting Company
We are subject to the reporting requirements of Section 13 of the Exchange Act, and subject to the disclosure requirements of Regulation S-K of the SEC, as a “smaller reporting company.” That designation will relieve us of some of the informational requirements of Regulation S-K.
Sarbanes/Oxley Act
Except for the limitations excluded by the JOBS Act discussed under the preceding heading “Emerging Growth Company,” we are also subject to the Sarbanes-Oxley Act of 2002. The Sarbanes/Oxley Act created a strong and independent accounting oversight board to oversee the conduct of auditors of public companies and strengthens auditor independence. It also requires steps to enhance the direct responsibility of senior members of management for financial reporting and for the quality of financial disclosures made by public companies; establishes clear statutory rules to limit, and to expose to public view, possible conflicts of interest affecting securities analysts; creates guidelines for audit committee members’ appointment, compensation and oversight of the work of public companies’ auditors; management assessment of our internal controls; prohibits certain insiders from trading during pension fund blackout periods; requires companies and auditors to evaluate internal controls and procedures; and establishes a federal crime of securities fraud, among other provisions. Compliance with the requirements of the Sarbanes/Oxley Act will substantially increase our legal and accounting costs.
| 16 |
Exchange Act Reporting Requirements
Section 14(a) of the Exchange Act requires all companies with securities registered pursuant to Section 12(g) of the Exchange Act, like we are, to comply with the rules and regulations of the SEC regarding proxy solicitations, as outlined in Regulation 14A. Matters submitted to shareholders at a special or annual meeting thereof or pursuant to a written consent will require us to provide our shareholders with the information outlined in Schedules 14A (where proxies are solicited) or 14C (where consents in writing to the action have already been received or anticipated to be received) of Regulation 14, as applicable; and preliminary copies of this information must be submitted to the SEC at least 10 days prior to the date that definitive copies of this information are forwarded to our shareholders.
We are also required to file annual reports on Form 10-K and quarterly reports on Form 10-Q with the SEC on a regular basis, and will be required to timely disclose certain material events (e.g., changes in corporate control; acquisitions or dispositions of a significant amount of assets other than in the ordinary course of business; and bankruptcy) in a Current Report on Form 8-K.
Number of Total Employees and Number of Full Time Employees
As of the date of this Annual Report, we have four full-time employees. Our CFO is currently part-time.
In 2024, and subject to adequate funding and assuming the Merger is not consummated, we seek to provide our employees with health care coverage and other benefits to help attract and maintain our workforce. We are not currently obligated to provide health insurance, however, we believe this is an important addition to our benefits package. All employees receive at least three weeks of paid time off per year. We have historically provided incentive stock options and other equity incentives to officers, directors and key employees to provide ownership and alignment of interests with our shareholders. We also use in certain instances performance-based vesting for stock options and restricted stock, whereby we set milestones to reflect important value creating initiatives of the Company. As a company, we seek diversity and inclusion in our workplace.
Available Information
We maintain an internet website at www.qsambio.com. We make available on or through our website, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practical after we electronically file such material with, or furnish it to, the Securities and Exchange Commission. We do not intend the address of our website to be an active link or to otherwise incorporate the contents of our website into this Annual Report. You may also find all of the reports that we have filed electronically with the SEC at their Internet site www.sec.gov.
Item 1a. Risk Factors
General Risks Related to our Business and Technology
A pandemic, epidemic or outbreak of an infectious disease, such as COVID-19, or coronavirus, may materially and adversely affect our business and our financial results.
In the recent past, the COVID-19 pandemic and efforts to control its spread have significantly impacted the movement of people, goods and services worldwide, including the conduct of our business operations. The COVID-19 pandemic has materially affected segments of the global economy and, if it were to reoccur, may affect our operations by causing a period of business disruption, supply chain issues, including the potential interruption of our clinical trial activities and delays or disruptions in the supply of our products and product candidates. In addition, there could be a potential effect of COVID-19 or any future pandemic, epidemic or outbreak of an infectious disease to the business at FDA or other health authorities, which could result in delays of reviews and approvals, including with respect to our product candidates.
| 17 |
The recurrence of the COVID-19 pandemic, or the spread of any other such disease, globally could also adversely impact our clinical trial operations, including our ability to recruit and retain patients and principal investigators and site staff who, as healthcare providers, may have heightened exposure to disease if an outbreak occurs in their geography. COVID-19, or another infectious disease, could also negatively affect our manufacturing operations, which could result in delays or disruptions in the supply of our product candidates.
Although the immediate impacts of the COVID-19 pandemic have been assessed and mitigated, the ultimate extent of the impact of this or any future pandemic, including as a result of possible subsequent outbreaks of Coronavirus or of new variants thereof and measures taken in response thereto, will depend on future developments, which remain uncertain and cannot currently be predicted. While health and safety restrictions have been lifted and vaccines are available, certain adverse consequences of the pandemic continue to impact the macroeconomic environment and may persist for some time. Any negative impact on our business, financial condition, results of operations and cash flows cannot be reasonably estimated at this time, but the outbreak of any future pandemic could lead to extended disruption of economic activity and the impact on our business, financial condition, results of operations and cash flows could be material.
Given the dynamic nature of variants of COVID-19 and the unpredictability of future pandemics, epidemics or the outbreak of infectious disease, it is difficult to predict its full impact on our business, but if we or any of the third parties with whom we engage were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively impacted, which could have a material adverse effect on our business and our results of operation and financial condition.
Unfavorable global economic, geopolitical conflicts and other political conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy, the global financial markets and global political conditions. The United States and global economies are facing inflation, higher interest rates and there remains the potential of a recession. Furthermore, a severe or prolonged economic downturn, including a recession or depression resulting from another pandemic or political disruption, such as the war between Ukraine and Russia or political conflict between China and Taiwan or Israeli military actions in Gaza and the potential for a wider conflict in the Middle East, could result in a variety of risks to our business, including increased costs as well as our ability to raise additional capital on acceptable terms. A material portion of the world’s supply of pharmaceutical grade Samarium-152 is produced in Russia, and future economic sanctions or other hostilities between Ukraine and Russia could disrupt that supply. A weak or declining economy or political disruption, including any international trade disputes and disruptions in international trade and commerce, could also strain our manufacturers or suppliers, possibly resulting in supply disruption, or cause delays in completion of clinical trials. Any of the foregoing could seriously harm our business, and we cannot anticipate all of the ways in which the political or economic climate and financial market conditions could seriously harm our business.
Drug development is a long and inherently uncertain process with a high risk of failure at every stage of development.
Drug development is a highly uncertain scientific and medical endeavor, and failure can unexpectedly occur at any stage of clinical development. Typically, there is a high rate of attrition for product candidates in preclinical and clinical trials due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. Pre-clinical studies and clinical trials are long, expensive and highly uncertain processes that can take many years. It will take us several years to complete our clinical trials and the time required for completing, testing and obtaining approvals is uncertain. The start or end of a clinical trial is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparator drug or required prior therapy, clinical outcomes, or financial constraints. The FDA and other U.S. and foreign regulatory agencies have substantial discretion, at any phase of development, to terminate clinical trials, require additional clinical development or other testing, delay, condition or withhold registration and marketing approval and mandate product withdrawals, including recalls. Additionally, we may also amend, suspend or terminate clinical trials at any time if we believe that the participating patients are being exposed to unacceptable health risks. Results attained in our early human clinical trial may not be indicative of results in later clinical trials. Our failure to demonstrate adequately the safety and efficacy of a product under development would delay or prevent marketing approval, which could adversely affect our operating results and credibility. The failure of one or more of our product candidates could have a material adverse effect on our business, financial condition and results of operations.
| 18 |
The future of our business and operations depends on the success of our development and commercialization programs.
Our business and operations entail a variety of serious risks and uncertainties and are inherently risky. The development programs on which we focus involve novel approaches to treating bone cancer and related diseases. Our product candidate is in clinical development, and in some respects, involve technologies with which we have limited prior experience. We are subject to the risks of failure inherent in the development and commercialization of product candidates based on new technologies. There is some precedent for the successful commercialization of products based on our technologies, but there are still a number of technological challenges that we must overcome to complete our clinical trials and development efforts. We may not be able to successfully develop our product candidates. We must successfully complete clinical trials and obtain regulatory approvals for potential commercial products. Once approved, if at all, commercial product sales are subject to general and industry-specific local and international economic, regulatory, technological and policy developments and trends. Delays, higher costs or other weaknesses in the manufacturing process or any of our contracted manufacturing organizations could hinder the development and commercialization of our product pipeline. The oncology space in which we operate presents numerous significant risks and uncertainties that may be expected to increase to the extent it becomes more competitive or less favored in the commercial healthcare marketplace.
We currently have no in-house sales, marketing or distribution capabilities and have limited experience in marketing products. We may in the future develop an in-house marketing and sales team, which would require significant capital expenditures, management resources and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. If we decide against absorbing marketing and sales responsibilities in-house, we will need to collaborate with third-parties. However, there can be no assurance that such collaborations will be successful or even if they are, they will be profitable for the Company after expending capital resources in fees and expenses. There can be no assurance that we will be able to develop in-house sales and distribution capabilities or establish or maintain relationships with third-party collaborators to commercialize any product.
If we do not obtain regulatory approval for our product candidates on a timely basis, or at all, or if the terms of any approval impose significant restrictions or limitations on use, our business, results of operations and financial condition will be adversely affected. Setbacks in clinical development programs could have a material adverse effect on our business.
Regulatory approvals are necessary to market product candidates and require demonstration of a product’s safety and efficacy through extensive pre-clinical and clinical trials. We may not obtain regulatory approval for product candidates on a timely basis, or at all, and the terms of any approval (which in some countries includes pricing and reimbursement approval) may impose significant restrictions, limitations on use or other commercially unattractive conditions. The process of obtaining FDA and foreign regulatory approvals often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. We have had only limited experience in filing and pursuing applications and other submissions necessary to gain marketing approvals. Products under development may never obtain marketing approval from the FDA or other regulatory authorities necessary for commercialization.
We or regulators may also amend, suspend or terminate clinical trials if we or they believe that the participating patients are being exposed to unacceptable health risks, and after reviewing trial results, we may abandon projects which we previously believed to be promising for commercial or other reasons unrelated to patient risks. During this process, we may find, for example, that results of pre-clinical studies are inconclusive or not indicative of results in human clinical trials, clinical investigators or contract research organizations do not comply with protocols or applicable regulatory requirements, or that product candidates do not have the desired efficacy or have undesirable side effects or other characteristics that preclude marketing approval or limit their potential commercial use if approved. In such circumstances, the entire development program for that product candidate could be adversely affected, resulting in delays in trials or regulatory filings for further marketing approval and a possible need to reconfigure our clinical trial programs to conduct additional trials or abandon the program involved. Conducting additional clinical trials or making significant revisions to a clinical development plan would lead to delays in regulatory filings. If clinical trials indicate, or regulatory bodies are concerned about, actual or possible serious problems with the safety or efficacy of a product candidate, we may stop or significantly slow development or commercialization of affected products. As a result of such concerns, the development programs for our product candidates may be significantly delayed or terminated altogether.
| 19 |
The results of our early-stage clinical trials may not be predictive of the results of the later-stage clinical trials. In addition, initial success in clinical trials may not be indicative of results obtained when such trials are completed. There can be no assurance that any of our current or future clinical trials will ultimately be successful or support further clinical development of any of our product candidates. There is a high failure rate for drugs and biologics proceeding through clinical trials.
If the results of any of our clinical trials are not satisfactory or we encounter problems and/or delays enrolling patients, clinical trial supply issues, setbacks in developing drug formulations or in clinical trials, including raw material supply, manufacturing, stability or other difficulties, or issues complying with protocols or applicable regulatory requirements, the entire development program for our product candidates could be adversely affected in a material manner. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses and many companies that believed their product candidates performed satisfactorily in preclinical studies or clinical trials nonetheless failed to obtain FDA approval or approval from foreign regulatory authorities.
Our business is highly dependent on our product candidate, CycloSam®, and a failure to obtain regulatory approval or successfully commercialize our product could adversely affect our financial condition and results of operations.
In April 2020, the Company, through its wholly-owned subsidiary QSAM Therapeutics entered into an exclusive worldwide patent and technology license agreement and trademark assignment with respect to CycloSam® and obtained exclusive commercial rights to the patent portfolio developed by IGL Pharma Inc. (“IGL”) (the “Original Agreement”). The agreement was mutually amended on November 24, 2021 (collectively with the Original Agreement referred to hereinafter as the “License Agreement). The License Agreement was amended a second time as of February 2, 2024; however, this amendment will not take effect until and unless the Company closes its Merger with Telix, provided however, we were obligated to pay IGL $50,000 upon the signing of that second amendment. The License Agreement as it currently stands is terminable in the event of a material breach by us that is not cured within a predefined period of time after notice of the breach is provided to us. If the License Agreement is terminated, the Company will not have further rights to CycloSam® and will be unable to continue its regulatory approval process or if already commercialized, benefit from the future sales of CycloSam®. Further, the Company may be liable for damages for the breach and may suffer additional losses, which could adversely impact our financial condition. The Company’s sole focus at this time is to develop CycloSam® and it does not possess licenses to other product candidates. If we do not obtain regulatory approval for CycloSam® or fail to successfully commercialize CycloSam®, we currently have no fall back options to continue our business operations unless we secure licenses of or develop alternative drug candidates. There is no assurance that either will occur.
We must design and conduct successful clinical trials for our product candidates to obtain regulatory approval. We rely on third parties to conduct our clinical trials, which reduces our control over their timing, conduct and expense and may expose us to conflicts of interest. Clinical trial results may be unfavorable or inconclusive, and often take longer and cost more than expected.
We have limited internal resources for conducting clinical trials, and we rely on or obtain the assistance of others to design, conduct, supervise, or monitor some or all aspects of some of our clinical trials. In relying on these third parties, we have less control over the timing and other aspects of clinical trials than if we conducted them entirely on our own. Problems with the timeliness or quality of the work of a contract research organization or clinical data management organization may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay our trials and contractual restrictions may make such a change difficult or impossible. These third parties may also have relationships with other entities, some of which may be our competitors. In all events, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA and other foreign regulatory authorities require us to comply with good clinical practices for conducting and recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements.
| 20 |
To obtain regulatory approval of our product candidates we must demonstrate through preclinical studies and clinical trials that they are safe and effective. Adverse or inconclusive clinical trial results concerning any of our product candidates that regulators find deficient in scope, design or one or more other material respects, could require additional trials, resulting in increased costs, significant delays in submissions of approval applications, approvals in narrower indications than originally sought, or denials of approval, none of which we can predict. As a result, any projections that we publicly announce of commencement and duration of clinical trials are not certain. Clinical trial delays may occur as a result of slower than anticipated enrollment. Delays can be caused by, among other things, deaths or other adverse medical events; regulatory or patent issues; interim or final results of ongoing clinical trials; failure to enroll clinical sites as expected; competition for enrollment from other clinical trials; scheduling conflicts with participating clinicians and institutions; disagreements, disputes or other matters arising from collaborations; our inability to obtain necessary funding; or manufacturing problems.
Even if our product candidates obtain marketing approval, our ability to generate revenue will depend upon public perception of radiopharmaceuticals and will be diminished if our products are not accepted in the marketplace, or if we select pricing strategies for our products that are less competitive than those of our competitors, or fail to obtain acceptable prices or an adequate level of reimbursement for products from third-party payers or government agencies.
Adverse events in clinical trials of our product candidates or in clinical trials of others developing similar products and the resulting negative publicity, as well as any other adverse events in the field of radiopharmaceuticals that may occur in the future, could result in a decrease in demand for our products or any product candidates that we may develop. If public perception is influenced by claims that radiopharmaceuticals or specific therapies within radiopharmaceuticals are unsafe, our products or product candidates may not be accepted by the general public or the medical community.
The commercial success of our products will depend upon their acceptance by the medical community and third-party payers as clinically useful, cost effective and safe. Market acceptance of approved products is affected by a wide range of factors including the timing of regulatory approvals, product launches and the presence of generic, over-the-counter or other competitors; the pricing of the product and relative prices of competing products; product development efforts for new indications; the availability of reimbursement for the product; our ability to obtain sufficient commercial quantities of the product; success in arranging for necessary sublicense or distribution relationships; and general and industry-specific local and international economic pressures. If health care providers believe that patients can be managed adequately with alternative, currently available therapies, they may not prescribe our products, especially if the alternative therapies are viewed as more effective, as having a better safety or tolerability profile, as being more convenient to the patient or health care providers or as being less expensive. Third-party insurance coverage may not be available to patients for any products we develop. For pharmaceuticals administered in an institutional setting, the ability of the institution to be adequately reimbursed from government and health administration authorities, private health insurers and other third-party payers could also play a significant role in demand for our products. Significant uncertainty exists as to the reimbursement status of newly-approved pharmaceuticals. Government and other third-party payers increasingly are attempting to contain healthcare costs by limiting both coverage and the level of reimbursement for new drugs and by refusing, in some cases, to provide coverage for uses of approved products for indications for which the FDA has not granted labeling approval. In most foreign markets, pricing and profitability of prescription pharmaceuticals are subject to government control. In the U.S., we expect that there will continue to be a number of federal and state proposals to implement similar government control and that the emphasis on managed care in the U.S. will continue to put pressure on the pricing of pharmaceutical products. Cost control initiatives could decrease the price that we can receive for any products in the future and adversely affect our ability to successfully commercialize our products. If any of our product candidates do not achieve market acceptance, we will likely lose our entire investment in that product candidate.
We are subject to extensive and ongoing regulation, which can be costly and time consuming, may interfere with marketing approval for our product candidates, and can subject us to unanticipated limitations, restrictions, delays and fines.
Our business, products and product candidates are subject to comprehensive regulation by the FDA and comparable authorities in other countries, and include the Sunshine Act under the Patient Protection and Affordable Care Act (“PPACA”). These agencies and other entities regulate the pre-clinical and clinical testing, safety, effectiveness, approval, manufacture, labeling, marketing, export, storage, recordkeeping, advertising, promotion and other aspects of our products and product candidates. We cannot guarantee that approvals of product candidates, processes or facilities will be granted on a timely basis, or at all. If we experience delays or failures in obtaining approvals, commercialization of our product candidates will be slowed or stopped. In addition to these uncertainties, there have been several attempts and public announcements by members of the U.S. Congress to repeal the PPACA and replace it with a curtailed system of tax credits and dissolve an expansion of the Medicaid program. For example, Tax Cuts and Jobs Act of 2017 was enacted in 2017, which, among other things, eliminated the individual mandate requiring most Americans (other than those who qualify for a hardship exemption) to carry a minimum level of health coverage, and became effective January 1, 2019. There is considerable uncertainty regarding the future of the current PPACA framework, and any changes will likely take time to unfold. As such, we cannot predict what effect the PPACA or other healthcare reform initiatives that may be adopted in the future will have on our business.
| 21 |
Even if we obtain regulatory approval for a product candidate, the approval may include significant limitations on indicated uses for which the product could be marketed or other significant marketing restrictions.
If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be subject to forced removal of a product from the market, product seizure, civil and criminal penalties and other adverse consequences.
Our products may face regulatory, legal or commercial challenges even after approval.
Even if a product receives regulatory approval:
| ● | It might not obtain labeling claims necessary to make the product commercially viable (in general, labeling claims define the medical conditions for which a drug product may be marketed, and are therefore very important to the commercial success of a product), or may be required to carry warnings that adversely affect its commercial success. | |
| ● | Approval may be limited to uses of the product for treatment or prevention of diseases or conditions that are relatively less financially advantageous to us than approval of greater or different scope or subject to an FDA imposed Risk Evaluation and Mitigation Strategy (“REMS”) that imposes limits on the distribution or use of the product. While we may develop a product candidate with the intention of addressing a large, unmet medical need, the FDA or other foreign regulatory authorities may only approve the use of the drug for indications affecting a relatively small number of patients, thus greatly reducing the market size and our potential revenues. | |
| ● | Side effects identified after the product is on the market might hurt sales or result in mandatory safety labeling changes, additional pre-clinical testing or clinical trials, imposition of a REMS, product recalls or withdrawals from the market, reputational harm to us, and lawsuits (including class-action suits). | |
| ● | Efficacy or safety concerns regarding a marketed product, or manufacturing or other problems, may lead to a recall, withdrawal of marketing approval, marketing restrictions, reformulation of the product, additional pre-clinical testing or clinical trials, changes in labeling, imposition of a REMS, warnings and contraindications, the need for additional marketing applications, declining sales or other adverse events. These potential consequences may occur whether or not the concerns originate from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not they are scientifically justified. If products lose previously received marketing and other approvals, our business, results of operations and financial condition would be materially adversely affected. | |
| ● | In certain foreign jurisdictions, drug products cannot be marketed until pricing and reimbursement for the product is also approved. In the United States, reimbursement approval is not required, but if not available, that may severely limit the sales, and the Center for Medicare & Medicaid Services may require additional clinical studies, more than the FDA demands. | |
| ● | We will be subject to ongoing FDA obligations and continuous regulatory review, and might be required to undertake post-marketing trials to verify the product’s efficacy or safety or other regulatory obligations. |
| 22 |
We are increasingly dependent on information technology, and potential cyberattacks, security problems, or other disruption and expanding social media vehicles present new risks.
We rely on information technology networks and systems, including the internet, to process, transmit, and store electronic information, and to manage or support a variety of business processes, including financial transactions and records, billing, and operating data. We may purchase some of our information technology from vendors, on whom our systems will depend, and we rely on commercially available systems, software, tools, and monitoring to provide security for processing, transmission, and storage of confidential operator and other patient data. We depend upon the secure transmission of this information over public networks. Our networks and storage applications could be subject to unauthorized access by hackers or others through cyberattacks, which are rapidly evolving and becoming increasingly sophisticated, or by other means, or may be breached due to operator error, malfeasance or other system disruptions. In some cases, it will be difficult to anticipate or immediately detect such incidents and the damage they cause. We cannot assure that such breaches, failures or interruptions will not occur or, if they do occur, that they will be adequately addressed by us or the third parties on which we rely. We may not be insured against all types of losses as a result of third-party failures, and insurance coverage may be inadequate to cover all losses resulting from breaches, systems failures or other disruptions. Any significant breakdown, invasion, destruction, interruption, or leakage of information from our systems could harm our reputation and business.
In addition, the use of social media could cause us to suffer brand damage or information leakage. Negative posts or comments about us on any social networking website could damage our or our brands’ reputations. Employees or others might disclose non-public sensitive information relating to our business through external media channels, including through the use of social media. The continuing evolution of social media will present us with new challenges and risks.
Risks Related to our Financial Position and Operating History
We have a limited operating history and are operating at a loss, and there is no guaranty that we will become profitable.
We began operations in late 2020 and anticipate that we will operate at a loss for some time. Since we have limited operating history and no history of profitability, we have limited financial results upon which you may judge our potential. Further, our ability to become profitable depends upon our ability to generate revenue. We have recorded no revenue from operations since inception. We do not expect to generate significant product revenue unless or until we successfully complete clinical development and obtain regulatory approval of, and then successfully commercialize, at least one of our product candidates; or alternatively, out license or sell our drug candidates. Both of these scenarios are highly uncertain. In the future, we may experience under-capitalization, development delays, set-backs with our drug development programs, lack of funding options, setbacks and many of the problems, delays and expenses encountered by any early stage business, many of which are beyond our control. These include, but are not limited to:
| ● | our lack of an operating history; | |
| ● | the net losses that we expect to incur as we develop our business; | |
| ● | obtaining FDA or other regulatory approvals or clearances for our technology; | |
| ● | implementing and achieving successful outcomes for clinical trials of our products; | |
| ● | convincing physicians, hospitals and patients of the benefits of our technology and to convert from current technology and standards of care; | |
| ● | the ability of users of our products (when and as developed) to obtain third-party reimbursement; | |
| ● | any failure to comply with rigorous FDA and other government regulations; and | |
| ● | securing, maintaining and defending patent or other intellectual property protections for our technology. |
Because our history is limited and we are subject to intense competition, any investment in us would be inherently risky.
Because we are a company with limited operational history and no profitability, our business activity is early-staged and subject to numerous risks. The pharmaceutical development business is highly competitive with many companies having access to the similar products and markets. Many of them have greater financial resources and longer operating histories than we have and can be expected to compete within the business in which we engage and intend to engage. There can be no assurance that we will have the necessary resources to become or remain competitive. We are subject to the risks which are common to all companies with a limited history of operations and profitability. Therefore, investors should consider an investment in us to be an extremely risky venture.
| 23 |
There is substantial doubt as to our ability to continue as a going concern.
The Report of our Independent Registered Public Accounting Firm issued in connection with our audited consolidated financial statements for the calendar years ended December 31, 2023 and 2022 expressed substantial doubt about our ability to continue as a going concern because of our recurring operating losses and our lack of liquidity and working capital. A going concern opinion means that there is substantial doubt that the Company can continue as an ongoing business for the next 12 months. Even if we do raise additional funding, if we fail to successfully deploy our funds, fail to implement our business plan or commercialize our drug as planned, or fail to raise additional capital when required, there can be no assurance that we will not again lose our ability to continue as a going concern.
We will require additional financing.
Pharmaceutical development is inherently costly and requires significant capital. We expect our expenses to increase significantly as we enter into the next stage of our drug development including steps such as preclinical studies, clinical trials, research and development, and FDA marketing approvals. If we do obtain FDA approval, we expect to incur substantial costs in commercialization of the product. In addition, we will continue to incur costs to operate as a public company. Accordingly, we will need to obtain additional financing in connection with our operations. There can be no assurance that additional funds will be available when and if needed, or on acceptable terms to the Company. If we are unable to obtain such financing, or if the terms thereof are too costly, we may be forced to curtail or cease operations until such time as alternative financing may be arranged, which could have a materially adverse impact on our planned operations and our shareholders’ investment.
Based upon our current operating plan, we believe that current cash will enable us to fund our operations through the second quarter of 2024 if the Merger does not close. Our operating plans and other demands on our cash resources may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings or other capital sources, including potentially additional collaborations, licenses and other similar arrangements. In addition, our ability to continue as a going concern is dependent on our ability to raise additional capital in order to implement our current business plan. This is particularly uncertain in the current environment of rising inflation and increasing interest rates. If the market conditions are favorable or given our strategic considerations, even if we believe we have sufficient funds for our current or future operating plans, we may raise additional capital. Attempting to secure additional financing may divert our management from our day-to-day activities, which may adversely affect our ability to develop our product candidates.
Our success will be dependent on our management, and the continued service of key employees.
Our success is dependent upon the decision making of our directors and executive officers. We believe that our success depends on the continued service of our key employees and our ability to hire additional key employees when and as needed. Although we currently intend to retain our existing management, we cannot assure you that such individuals will remain with us. Further, we cannot assure that we will be able to find and recruit new employees on terms acceptable to the Company. We have fixed term employment agreements with our four key employees – Messrs. Baum, Piazza and Nelson and Namrata Chand — but have not obtained key man life insurance on the lives of any of them. The unexpected loss of the services of one or more of our key executives, directors and advisors, or the inability to find new key employees within a reasonable period of time could have a material adverse effect on the economic condition and results of operations of the Company.
Risk Related to the Pending Merger
Failure to complete the Merger with Telix would adversely affect the stock price and future business and financial results of the Company.
There can be no assurance that the Merger will be completed. If the Merger is not completed, the ongoing business of the Company would be adversely affected and we will be subject to a variety of risks and possible consequences associated with the failure to complete the Merger, including the following:
| ● | upon termination of the Merger Agreement under specified circumstances, the $2 million option payment paid by Telix to the Company upon execution of the term sheet for Telix’s acquisition of QSAM (the “Option Payment”) will convert into shares of QSAM common stock at a price of $6.70 per share, which would have a dilutive effect; | |
| ● | we will incur certain significant transaction costs, including legal, accounting, financial advisor, filing, printing and mailing fees, regardless of whether the Merger closes; | |
| ● | under the Merger Agreement, the Company is subject to certain restrictions on the conduct of its business prior to the closing of the Merger, which may adversely affect its ability to execute certain of its business strategies; | |
| ● | we may lose key employees during the period in which QSAM and Telix are pursuing the Merger, which may adversely affect the Company in the future if we are not able to hire and retain qualified personnel to replace departing employees; and | |
| ● | the proposed Merger, whether or not it closes, will divert the attention of our management and other key employees from ongoing business activities, including the pursuit of other opportunities that could be beneficial to us as an independent company. |
| 24 |
If the Merger is not completed, these risks could materially affect our business and financial results and our stock price, including to the extent that the current market price of QSAM common stock is positively affected by a market assumption that the Merger will be completed.
We will need additional capital to meet our current obligations and continue to operate our business if the Merger is not completed in a timely fashion or at all.
If we had adequate funding in a timely manner, QSAM management believes that we can complete the current Phase 1 safety trial of approximately 17 patients in 2024. We have estimated that an additional $3 million to $4 million will be required to complete this phase of our study. The next step in our clinical trial program, if cleared by the FDA, is expected to be a study designed to show both safety and efficacy in the treatment of bone tumors utilizing a multi-dose regimen of CycloSam®. Management estimates that an additional $12 million to $14 million will be required to complete the first portion of this critical phase of our study over the next 24 to 30 months.
Advancement of current and future plans for our technology requires significant additional funding, which would most likely result in the issuance of more common stock, preferred stock or debt in subsequent raises. There is no guaranty that we would be successful in raising such funding on terms acceptable to our shareholders, if at all, and if we were not successful, we may be required to slow down or cease our clinical trials, or in a worst-case scenario, shut down the Company. Our independent registered public accounting firm has issued a going concern opinion for the year ended December 31, 2023. This means that our auditors believe there is substantial doubt that we can continue as an on-going business for the next 12 months. Management expects expenses to increase in 2024 as our drug technology advances through clinical trials, and as a result, we will need to raise significant additional capital to support these operations. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. If the Merger does not close, the circumstances around such failure to close could create uncertainty and a lack of confidence among potential funders, which would make raising capital significantly more difficult. Any funds raised will likely result in material dilution to our current shareholders (apart from dilution caused by conversion of the Option Payment to shares of QSAM common stock to Telix) or be in the form of debt which poses other risks of default. Our current cash or cash equivalents can enable us to fund our operations through the second quarter of 2024. If we are not successful in completing this Merger prior to June 30, 2024, or raising additional capital, or providing other options to support operations and our trials, we may need to delay clinical trials, reduce overhead, or in the most extreme scenario, shut down operations.
We will incur substantial transaction fees and Merger-related costs in connection with the Merger.
We expect to incur non-recurring transaction fees, which include legal and advisory fees and substantial Merger-related costs associated with completing the Merger. Additional unanticipated costs may be incurred in the course of the integration of the businesses of Telix and QSAM. The companies cannot be certain that the realization of other benefits related to the integration of the two businesses will offset the transaction and Merger-related costs in the near term, or at all.
Risks Related to Working with Third Parties
We have been and expect to continue to be dependent on collaborators for the development, manufacturing and sales of certain products and product candidates, which expose us to the risk of reliance on these collaborators.
In conducting our operations, we currently depend, and expect to continue to depend, on numerous collaborators. In addition, certain clinical trials for our product candidates may be conducted by government-sponsored agencies, and consequently will be dependent on governmental participation and funding. These arrangements expose us to the same considerations we face when contracting with third parties for our own trials.
| 25 |
If any of our collaborators breach or terminate their agreement with us or otherwise fail to conduct successfully and in a timely manner the collaborative activities for which they are responsible, the preclinical or clinical development or commercialization of the affected product candidate or research program could be delayed or terminated. We generally do not control the amount and timing of resources that our collaborators devote to our programs or product candidates. We also do not know whether current or future collaboration partners, if any, might pursue alternative technologies or develop alternative products either on their own or in collaboration with others, including our competitors, as a means for developing treatments for the diseases or conditions targeted by our collaborative arrangements. Our collaborators are also subject to similar development, regulatory, manufacturing, cyber-security and competitive risks as ours, which may further impede their ability to successfully perform the collaborative activities for which they are responsible. Setbacks of these types to our collaborators could have a material adverse effect on our business, results of operations and financial condition.
We are dependent upon third parties for a variety of functions. These arrangements may not provide us with the benefits we expect.
We rely on third parties to perform a variety of functions. We are party to numerous agreements which place substantial responsibility on clinical research organizations, consultants and other service providers for the development of our product candidates. We also rely on medical and academic institutions to perform aspects of our clinical trials of product candidates. We may not be able to enter into new arrangements without undue delays or expenditures, and these arrangements may not allow us to compete successfully. Moreover, if third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct clinical trials in accordance with regulatory requirements or applicable protocols, our product candidates may not be approved for marketing and commercialization or such approval may be delayed. If that occurs, we or our collaborators will not be able, or may be delayed in our efforts, to commercialize our product candidates.
Our relationships with customers and third-party payers are or may become subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, program exclusion, contractual damages, reputational harm and diminished profits and future earnings.
Health care providers, physicians and third-party payers play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payers and customers will or already do require us and them to comply with broadly applicable fraud and abuse and other health care laws and regulations, including both federal and state anti-kickback and false claims laws, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products that obtain marketing approval. Efforts to ensure that business arrangements comply with applicable health care laws and regulations involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If such operations are found to be in violation of any of these laws or other applicable governmental regulations, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of related operations. If physicians or other providers or entities involved with our products are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs, which may adversely affect us.
If we or our partners are unable to obtain sufficient quantities of the materials needed to make our products or product candidates, development of our products or product candidates or commercialization of our approved products could be slowed or stopped.
We have utilized Missouri University Research Reactor (“MURR”) and University of Texas at Austin (“NETL”) to procure Samarium-153, a primary ingredient used in manufacturing of CycloSam®, for our recently conducted clinical studies. Samarium-153 is critical to manufacturing of CycloSam® and to our supply-chain process both during our clinical trials and if the product is commercialized. MURR and NETL have verbally committed to supply us with Samarium-153 in the future but we do not have definitive agreements with either group to provide such ongoing materials if we commercialize our drug candidate. If we fail to partner with MURR or NETL or secure other partners for supply of Samarium-153 or if our arrangements with a supplier do not satisfy our requirements in the future, it will directly and adversely impact the production of CycloSam®. IsoTherapuetics currently produces all of our needed supply of DOTMP cold kits, and if they are not able to continue to supply such kits, or if we cannot obtain kits from other sources, our clinical trials may be delayed.
| 26 |
We or our partners may not be able to obtain the materials necessary to make a particular product or product candidate in adequate volume and quality. If any materials needed to make a product or product candidate is insufficient in quantity or quality, if a supplier fails to deliver in a timely fashion or at all or if these relationships terminate, we or our partners may not be able to fulfill manufacturing obligations for our products or product candidates, either on our own or through third-party suppliers. A delay or disruption of supplies of our products or product candidates would have a material adverse effect on our business as a whole. Our existing arrangements with suppliers may result in the supply of insufficient quantities of our product candidates needed to accomplish our clinical development programs or commercialization, and we may not have the right and in any event, do not currently have the capability to manufacture these products if our suppliers are unable or unwilling to do so. Some of these raw materials or their starting materials, components or ingredients may come from foreign countries, including countries such as Russia, where there exist trade and commercial restrictions, all of which can present significant supply chain issues. There is no broad market for pharmaceutical grade Samarium-152 (the non-radioactive version of the material used for our product candidate), and securing such raw materials from a limited number of sources, many of which are in foreign countries, has been and will likely continue to be difficult and expensive. Although we believe we have secured enough Samarium-152 for our clinical trials, we may not be able to secure enough Samarium-152 for commercial operations, which could materially restrict our ability to market CycloSam® and recoup our development expenses. We currently arrange for supplies of critical raw materials used in production of our product candidates from single sources. We do not have long-term contracts with any of these suppliers. Any delay or disruption in the availability of materials would slow or stop product development and commercialization of the relevant product.
Manufacturing resources could limit or adversely affect our ability to commercialize products.
We or our partners may engage third parties, including nuclear reactor sites, to manufacture our product candidates. We or our partners may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturing organizations or CMOs at acceptable costs.
In order to commercialize our product candidates successfully, we need to be able to manufacture or arrange for the manufacture of products in commercial quantities, in compliance with regulatory requirements, at acceptable costs and in a timely manner. Manufacture of our product candidates can be complex, difficult to accomplish even in small quantities, difficult to scale-up for large-scale production and subject to delays, inefficiencies and low yields of quality products. The manufacture of radiopharmaceuticals is relatively complex and requires significant capital expenditures. We continue to rely on CMOs for our product candidates. The cost of manufacturing our product candidates may make them prohibitively expensive. If adequate supplies of any of our product candidates or related materials are not available on a timely basis or at all, our clinical trials or commercialization of our product candidates could be seriously delayed, since these materials are time consuming to manufacture and cannot be readily obtained from third-party sources. We continue to be dependent on a limited number of highly specialized manufacturing and development partners, including single source manufacturers for certain of our product candidates. If we were to lose one or more of these key relationships, it could materially adversely affect our business. Establishing new manufacturing relationships, or creating our own manufacturing capability, would require significant time, capital and management effort, and the transfer of product-related technology and know-how from one manufacturer to another is an inherently complex and uncertain process.
Failure of any manufacturer of our various product candidates to comply with applicable regulatory requirements could subject us to penalties and have a material adverse effect on supplies of our product candidates.
Third-party manufacturers are required to comply with current goods manufacturing practice regulations or cGMP or similar regulatory requirements outside of the U.S. If manufacturers of our product candidates cannot successfully manufacture material that conforms to the strict regulatory requirements of the FDA and any applicable foreign regulatory authority, they may not be able to supply us with our product candidates. If these facilities are not approved for commercial manufacture, we may need to find alternative manufacturing facilities, which could result in delays of several years in obtaining approval for a product candidate. We do not control the manufacturing operations and are completely dependent on our third-party manufacturing partners or contractors for compliance with the applicable regulatory requirements for the manufacture of some of our product candidates. Manufacturers are subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMP and similar regulatory requirements. Failure of any manufacturer of any of our product candidates to comply with applicable cGMP or other regulatory requirements could result in sanctions being imposed on our collaborators or us, including fines, injunctions, civil penalties, delays, suspensions or withdrawals of approvals, operating restrictions, interruptions in supply and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates and have a material adverse impact on our business, financial condition and results of operations.
| 27 |
If the use of hazardous and biological materials by us or third parties, such as CROs or CMOs, in a manner that causes injury or violates applicable law, we may be liable for damages.
Our research and development activities may involve the controlled use of potentially hazardous substances, including radioactive, chemical and biological materials, by us or third parties, such as contract research organizations or CROs and CMOs. Exposure to high levels of radiation can cause acute health effects such as skin burns and acute radiation syndrome (“radiation sickness”). It can also result in long-term health effects such as cancer and cardiovascular disease. We and such third parties are subject to federal, state, and local laws and regulations in the United States governing the use, manufacture, storage, handling, and disposal of medical and hazardous materials. Although we believe that our and such third-parties’ procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. In the event of any such contamination or injury, we may incur liability or local, city, state, or federal authorities may curtail the use of these materials and interrupt our business operations. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development and manufacturing efforts, which could harm our business prospects, financial condition, or results of operations. We plan to maintain insurance coverage in the future for injuries resulting from hazardous materials; however, future claims may exceed the amount of our coverage. Also, we do not have insurance coverage for pollution cleanup and removal. Currently the costs of complying with such federal, state, provincial, local and foreign environmental regulations are not significant, and consist primarily of waste disposal expenses. However, they could become expensive, and current or future environmental laws or regulations may impair our research, development, production and commercialization efforts. Further, although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological, hazardous or radioactive materials.
Unexpected disruptions could seriously harm our future revenue and financial condition and increase our expenditures.
Our operations, and those of our CROs, CMOs and other contractors and consultants, could be subject to events like earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce and process our product candidates to meet our demands. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption.
Risks Relating to Our Intellectual Property
The validity, enforceability and commercial value of our patents and other intellectual property rights are highly uncertain.
We license a number of issued patents and other patent applications that have not yet been issued. We must obtain, maintain and enforce patent and other rights to protect our intellectual property. The patent position of biotechnology and pharmaceutical firms is highly uncertain and involves many complex legal and technical issues. There are many laws, regulations and judicial decisions that dictate and otherwise influence the manner in which patent applications are filed and prosecuted and in which patents are granted and enforced, all of which are subject to change from time to time. There is no clear policy involving the breadth of claims allowed, or the degree of protection afforded, under patents in this area. Accordingly, patent applications owned by or licensed to us may not result in patents being issued. Even if we own or license a relevant issued patent, we may not be able to preclude competitors from commercializing drugs that may compete directly with one or more of our products or product candidates, in which event such rights may not provide us with any meaningful competitive advantage. In the absence or upon successful challenge of patent protection, drugs may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products.
| 28 |
It is generally difficult to determine the relative strength or scope of a biotechnology or pharmaceutical patent position in absolute terms at any given time. The issuance of a patent is not conclusive as to its validity or enforceability, which can be challenged in litigation or via administrative proceedings. The License Agreement from which we derive or license intellectual property provide for various royalty, milestone, sublicensing and other payments, and include other provisions like patent prosecution and enforcement, insurance, indemnification and other obligations and rights, and is subject to certain reservations of rights. While we generally have the right to defend and enforce patents licensed to or by us, either in the first instance or if the licensor or licensee chooses not to do so, we must usually bear the cost of doing so.
Patents have a limited life and expire by law.
In addition to uncertainties as to scope, validity, enforceability and changes in law, patents by law have limited lives. Upon expiration of patent protection, our drug candidates and/or products may be subject to generic competition, which could adversely affect pricing and sales volumes of the affected products. Many of our licensed patents expire in 2034 unless they can be extended through legal procedures provided by the USPTO.
We depend on intellectual property licensed from third parties and unpatented technology, trade secrets and confidential information. If we lose any of these rights, including by failing to achieve milestone requirements or to satisfy other conditions, our business, results of operations and financial condition could be harmed.
Our core product candidate is derived from intellectual property licensed from a third party. We could lose the right to patents and other intellectual property licensed to us if the related License Agreement is terminated due to a breach by us or otherwise. Our ability to commercialize products incorporating licensed intellectual property would be impaired if the related License Agreements were terminated. In addition, we are required to make substantial cash payments, achieve milestones and satisfy other conditions, including filing for and obtaining marketing approvals and introducing products, to maintain rights under our intellectual property license. Due to the nature of this agreement and the uncertainties of development, we may not be able to achieve milestones or satisfy conditions to which we have contractually committed, and as a result may be unable to maintain our rights under the license. If we do not comply with our License Agreement, the licensor may terminate it, which could result in our losing our rights to, and therefore being unable to commercialize, related products.
We also rely on unpatented technology, trade secrets and confidential information. Third parties may independently develop substantially equivalent information and techniques or otherwise gain access to our technology or disclose our technology, and we may be unable to effectively protect our rights in unpatented technology, trade secrets and confidential information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with us. These agreements may, however, not provide effective protection in the event of unauthorized use or disclosure of confidential information. Any loss of trade secret protection or other unpatented technology rights could harm our business, results of operations and financial condition.
If we infringe third-party patent or other intellectual property rights, we may need to alter or terminate a product development program.
There may be patent or other intellectual property rights belonging to others that require us to alter our products, pay licensing fees or cease certain activities. If our products infringe patent or other intellectual property rights of others, the owners of those rights could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. We may not prevail in any action brought against us, and any license required under any rights that we infringe may not be available on acceptable terms or at all.
| 29 |
Research, development and commercialization of a biopharmaceutical product often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes may depend on subsequent discoveries and test results and cannot be predicted with certainty at the outset. There are numerous third-party patents in our field, and we may need to obtain a license under a patent in order to pursue the preferred development route of one or more of our products or product candidates. The need to obtain a license would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate the program altogether.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to take legal action to enforce our patents or our licensors’ patents against such infringing activity. Such enforcement proceedings can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that one or more of our patents is invalid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the compositions or activities in question. An adverse result could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not being issued. Defense against these assertions, non-infringement, invalidity or unenforceability regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Post-grant proceedings provoked by third parties or brought by the United States Patent and Trademarks Office may be brought to determine the validity or priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or post-grant proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as those within the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Risks Related to our common stock
Liquidity risks associated with our common stock.
Our stock currently trades on OTCQB and our shares of common stock are thinly traded. While management would like to pursue a listing on NASDAQ Capital Market or other national exchange for its common stock in the future if the Merger does not close, there can be no assurance that (1) such plan will be achieved, (2) an active trading market will be developed or sustained, (3) the liquidity of such market will increase, (4) our stockholders will be able sell their shares of common stock, or (5) the price that our stockholders may obtain for their common stock will be greater than the public offering price. If an active market for our common stock with meaningful trading volume does not develop or is not maintained, the market price of our common stock may decline materially and you may not be able to sell your shares. Under the Merger, shares of Telix received by QSAM shareholders will be restricted under Rule 144 of the Securities Act and, as a result, may not be sold for a period of time after the closing.
The price of our common stock may fluctuate significantly, which could lead to losses for stockholders.
The securities of public companies can experience extreme price and volume fluctuations, which can be unrelated or out of proportion to the operating performance of such companies. We expect our common stock price will be subject to similar volatility. Any negative change in the public’s perception of the prospects of our Company or companies in our market could also depress our common stock price, regardless of our actual results. Factors affecting the trading price of our common stock may include:
| * | Failure to close the Merger; | |
| * | Regulatory actions; |
| 30 |
| * | Variations in our operating results; | |
| * | Announcements of technological innovations, new products or product enhancements, strategic alliances or significant agreements by us or by our competitors; | |
| * | Recruitment or departure of key personnel; | |
| * | Litigation, legislation, regulation or technological developments that adversely affect our business; | |
| * | Changes in the estimates of our operating results or changes in recommendations by any securities analysts that elect to follow our common stock; and | |
| * | Market conditions in our industry, the industries of our customers and the economy as a whole. |
The application of the “penny stock” rules could adversely affect the market price of our common stock and increase your transaction costs to sell those shares.
The open-market trading of our common stock is currently subject to the “penny stock” rules. The penny stock rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than accredited investors. For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser’s written consent to the transaction before the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the Commission relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability or decrease the willingness of broker-dealers to sell our common stock, and may result in decreased liquidity of our common stock and increased transaction costs for sales and purchases of our common stock as compared to other securities. Penny stocks generally do not have a very high trading volume. Therefore, as long as our shares of common stock are subject to the penny stock rules, the holders of such shares of common stock may find it more difficult to sell their securities.
We do not intend to pay dividends.
We have not paid any cash dividends on our common stock since inception and we do not anticipate paying any cash dividends in the foreseeable future. Earnings, if any, that we may realize will be retained in the business for further development and expansion.
Concentration of Stock Ownership and Control.
Our executive officers and directors currently control approximately 26% of the common stock of the Company inclusive of vested options; David H. Clarke, a major investor in our last three offerings and a consultant to the Company, and certain entities that he controls holds 16.5% of the Company; and Checkmate Capital and its affiliates, one of our former debt holders and lead investors in the Series B round, control approximately 7% of the Company. The Company has employee options that could result in further dilution. We may conduct funding rounds in the future which may utilize our common stock or preferred stock. In this regard, management, prior investors and future investors may control a significantly large amount of equity, and as a result, these stockholders acting together will be able to influence many matters requiring stockholder approval including the election of directors and other significant corporate transactions. This concentration of ownership may have the effect of delaying, preventing or deterring a change in control, and could deprive our stockholders of an opportunity to receive a premium for their shares of common stock as part of a sale of our company and may affect the market price of our stock.
| 31 |
We will need to grow the size of our organization, and we may experience difficulties in managing this growth.
As of December 31, 2023, we had four full-time employees and one part-time employee. As our drug development and commercialization plans and strategies develop, and assuming the Merger does not close, we expect to need additional operational, sales, marketing, managerial, financial and other personnel, as well as additional resources to expand our operations.
We currently rely, and for the foreseeable future will continue to rely, in large part on certain third-party organizations, advisors and consultants to provide certain services, including substantially all aspects of regulatory approval, clinical trial management and manufacturing. There can be no assurance that the services of independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. In addition, if we are unable to manage our outsourced activities effectively or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all. If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, or we are not able to effectively build out new facilities to accommodate this expansion, we may not be able to successfully implement our business plan to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
We are a smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are a smaller reporting company as defined in the Exchange Act, and we will remain a smaller reporting company until the fiscal year following the determination that our voting and non-voting common stock held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenue is more than $100 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter. Smaller reporting companies are able to provide simplified executive compensation disclosure and have certain other reduced disclosure obligations, including, among other things, being required to provide only two years of audited financial statements and not being required to provide selected financial data, supplemental financial information or risk factors.
We may choose to take advantage of the available exemptions for smaller reporting companies. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our shares price may be more volatile.
If we fail to comply with the rules and regulations under the Sarbanes-Oxley Act, our operating results, our ability to operate our business and investors’ views of us may be harmed.
Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls. Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Our failure to maintain the effectiveness of our internal controls in accordance with the requirements of the Sarbanes-Oxley Act could have a material adverse effect on our business. We could lose investor confidence in the accuracy and completeness of our financial reports, which could have an adverse effect on the price of our common stock. In addition, our efforts to comply with the rules and regulations under the Sarbanes-Oxley or new or changed laws, regulations, and standards may differ from the activities intended by regulatory or governing bodies due to ambiguities related to practice. Regulatory authorities may investigate transactions disclosed in our “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and if legal proceedings are initiated against us, it may harm our business.
| 32 |
We have historically identified certain material weaknesses in our internal control over financial reporting and if we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired.
In the course of preparing our financial statements, we have historically identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness related to limited accounting personnel and resources resulting into lack of segregation of duties, lack of experience in accounting for equity transactions, and lack of internal controls.
We plan to take additional steps to continue to improve our accounting function; and we must be able to confirm that such remedial measures have been operating effectively for a sufficient period of time. Further, we cannot assure you that any such actions we have taken or will take will prevent or avoid potential future material weaknesses. Our current controls and any new controls that we develop may become inadequate because of changes in conditions in our business. Further, weaknesses in our disclosure controls and internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our common stock.
Our financial statements may be materially affected if our estimates prove to be inaccurate as a result of our limited experience in making critical accounting estimates.
Financial statements prepared in accordance with GAAP require the use of estimates, judgments, and assumptions that affect the reported amounts. Actual results may differ materially from these estimates under different assumptions or conditions. These estimates, judgments, and assumptions are inherently uncertain, and, if they prove to be wrong, then we face the risk that charges to income will be required. In addition, because we have limited to no operating history and limited experience in making these estimates, judgments, and assumptions, the risk of future charges to income may be greater than if we had more experience in these areas. Any such charges could significantly harm our business, financial condition, results of operations, and the price of our securities. See “Note 3 – Summary of Significant Accounting Policies” under notes to our consolidated financial statements for a discussion of the accounting estimates, judgments, and assumptions that we believe are the most critical to an understanding of our business, financial condition, and results of operations.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None. Not required for smaller reporting companies.
ITEM 1C. CYBERSECURITY
Cybersecurity Risk Management and Strategy
The Company does not have its own cybersecurity policy but relies on the policies and procedures of its Contract Research Organizations (CROs) and Software as a Service (SaaS) contractors that handle its data and software. We are committed to protecting the confidentiality, integrity, and availability of our information assets and complying with applicable laws and regulations regarding cybersecurity.
Cybersecurity Risks and Incidents
The Company faces various cybersecurity risks and threats that could potentially affect its operations, reputation, financial condition, and competitive position. These risks and threats include, but are not limited to, unauthorized access, use, disclosure, modification, or destruction of our data, systems, or networks; denial of service attacks; malware infections; phishing or social engineering attacks; ransomware attacks; loss or theft of devices or media containing our data; human error or negligence; natural disasters; power outages; or sabotage. Our data and systems may also be subject to cybersecurity breaches or incidents at its CROs, vendors, partners, or other third parties that we interact with or rely on.
| 33 |
We have not experienced any material cybersecurity breaches or incidents to date, but we cannot guarantee that we will not suffer any such breaches or incidents in the future. We may not be able to detect, prevent, or respond to all cybersecurity risks and threats in a timely or effective manner. We may also incur significant costs and liabilities as a result of any cybersecurity breaches or incidents, such as legal claims, regulatory fines, remediation expenses, reputational damage, loss of business opportunities, or competitive disadvantage. We may also face litigation, investigations, or enforcement actions by governmental authorities, customers, shareholders, or other parties arising from any cybersecurity breaches or incidents.
Cybersecurity Policies and Procedures
The Company does not have its own cybersecurity policy, but it contracts with CROs that handle all of its data and software. Our CRO’s data systems are 21 CFR 11 (Part 11) compliant, which means that they have implemented controls to ensure the reliability and integrity of electronic records and signatures. Our CRO also runs industry standard antivirus and antimalware software on their networks and have written procedures for cybersecurity management, incident response, backup and recovery, and employee training. We have reviewed the cybersecurity policies and procedures of our CRO and require them to report any cybersecurity breaches or incidents that may affect our data or systems.
All of the software that we use is Commercial Off the Shelf Software (COTS) and Microsoft, Dropbox, and Google cloud services. We do not develop, modify, or customize any software for our own use. We rely on the cybersecurity measures and practices of our software and cloud service providers and update our software and systems regularly to address any known vulnerabilities or issues. We also limit the access and use of our software and cloud services to authorized personnel and encourage them to use strong passwords and multifactor authentication. We do not store any sensitive or confidential data on our own devices or media, but use password-protected cloud storage.
Cybersecurity Oversight and Governance
The Company’s management is responsible for overseeing and managing our cybersecurity risks and activities as part of its overall risk assessment portfolio. Our management regularly evaluates and reviews the Company’s cybersecurity posture and performance and reports to the board of directors on any material cybersecurity matters or developments. Our management also coordinates with our CROs, vendors, partners, and other third parties to ensure that they comply with our cybersecurity expectations and requirements and to address any cybersecurity issues or concerns that may arise.
The Company’s board of directors is responsible for overseeing and approving our cybersecurity strategy and policies. Our board of directors receives updates from management on the Company’s cybersecurity status and initiatives and provides guidance and feedback on the cybersecurity goals and objectives. Our board of directors also monitors the Company’s cybersecurity risks and exposures and ensures that the company has adequate cybersecurity resources and capabilities to protect its data and systems.
ITEM 2. PROPERTIES
The Company currently conducts its business from its office in Austin, Texas. The Company’s office space in Austin is leased month-to-month at a rate of $255 per month.
ITEM 3. LEGAL PROCEEDINGS
We are not a party to any pending legal proceeding and, to the knowledge of our management, no federal, state or local governmental agency is presently contemplating any proceeding against us. No director, executive officer, affiliate of ours, or owner of record or beneficially of more than five percent of our common stock is a party adverse to the Company or has a material interest adverse to us in any proceeding.
ITEM 4. MINE SAFETY DISCLOSURES
None; not applicable.
| 34 |
part II
ITEM 5: MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
There is a limited “established trading market” for our shares of common stock. No assurance can be given that a robust market for our common stock will develop or be maintained. If a robust public market ever develops in the future, the sale of shares of our common stock that are deemed to be “restricted securities” pursuant to Rule 144 of the SEC by members of management or others may have a substantial adverse impact on any such market.
Our common stock is quoted on OTCQB under the symbol “QSAM”. Set forth below are the high and low closing bid prices for our common stock for each quarter of the years 2022 and 2023, reflecting a 40:1 reverse stock split that took place in March 2022. These bid prices were obtained from OTC Markets Group, Inc. All prices listed herein reflect inter-dealer prices, without retail mark-up, mark-down or commissions and may not represent actual transactions.
| Period | High | Low | ||||||
| January 1, 2022 through March 31, 2022 | $ | 14.00 | $ | 6.00 | ||||
| April 1, 2022 through June 30, 2022 | $ | 9.00 | $ | 4.26 | ||||
| July 1, 2022 through September 30, 2022 | $ | 6.00 | $ | 3.50 | ||||
| October 1, 2022 through December 31, 2022 | $ | 5.96 | $ | 4.25 | ||||
| January 1, 2023 through March 31, 2023 | $ | 5.00 | $ | 3.90 | ||||
| April 1, 2023 through June 30, 2023 | $ | 6.20 | $ | 3.90 | ||||
| July 1, 2023 through September 30, 2023 | $ | 5.00 | $ | 4.15 | ||||
| October 1, 2023 through December 31, 2023 | $ | 7.00 | $ | 4.65 | ||||
Holders
The number of record holders of our common stock as of March 1, 2024 is 342. This figure does not include beneficial owners who may hold their shares in “street name”.
Dividends
We have not declared any cash dividends with respect to our common stock, and do not intend to declare dividends in the foreseeable future. Our future dividend policy cannot be ascertained with any certainty, and if and until we determine to engage in any business or we complete any acquisition, reorganization or merger, no such policy will be formulated. There are material restrictions limiting our ability to pay dividends on our securities, including state law.
| 35 |
Recent Sales of Unregistered Securities
The following table sets forth the sales of unregistered securities by the Company in 2023 and through the date of this filing:
| Purpose / Holder | Number of Shares of Common Stock | Total
Price/Amount | ||||||
| Issuances in 2023 | ||||||||
| Sale of common stock and warrants for cash in private placement (1) | 69,832 | $ | 314,239 | |||||
| Exercise of warrants issued in private placement (2) | 365,001 | $ | 1,095,001 | |||||
| Conversion of promissory notes into common stock (3) | 164,446 | $ | 519,712 | |||||
| Common stock issued under three service agreements (4) | 144,900 | $ | 850,929 | |||||
| Common stock issued to employees and board of directors (5) | 271,142 | $ | 1,827,502 | |||||
| Sale of common stock for cash in private placement (6) | 226,472 | $ | 850,000 | |||||
| Conversion of Series A Preferred Stock into common stock (7) | 80,000 | $ | 240,000 | |||||
| Cashless exercise of service warrant into common stock (8) | 8,571 | $ | - | |||||
| Issuances in 2024 | ||||||||
| Conversion of Series A Preferred Stock into common stock (9) | 169,133 | $ | 507,399 | |||||
| Exchange of Series B Preferred Stock for common stock (10) | 658,968 | $ | 1,977,318 | |||||
| Common stock issued for services (11) | 3,000 | $ | 18,900 | |||||
| (1) | Issued in PIPE offering pursuant to an exemption from registration under Section 4(a)(2) of the Securities Act of 1933, as amended, to seven accredited investors who also received 69,832 warrants exercisable for 24-months. |
| (2) | Exercise of common stock warrants received in PIPE offering by 19 accredited investors. |
| (3) | Eight convertible note holders converted all principal and accrued interest on outstanding convertible debt. |
| (4) | Issued to Redstone Communications (30,000 shares in April 2023 and 30,000 shares in August 2023) and Force Family Office (16,000 shares in April 2023 and 16,000 shares in July 2023), for investor relations and other similar services; and GSB Holdings Inc. (30,000 in June 2023 and 6,400 shares in September 2023) for general consulting services. |
| (5) | Issued in June 2023 to Richard Piazza, Executive Chairman (72,276 shares); Douglas Baum, CEO (72,276 shares); Christopher Nelson, EVP and General Counsel (46,365 shares); Adam King, CFO (22,950 shares); Namrata Chand, VP (36,365 shares), Adriann Sax, Director (10,455 shares), and Charles Link Jr., Director (10,455 shares), for employee compensation and annual bonuses in lieu of cash, and board of director bonuses in lieu of cash. All shares are subject to performance and/or time vesting terms and forfeiture if such terms are not timely achieved. |
| (6) | Issued pursuant to an exemption from registration under Section 4(a)(2) of the Securities Act of 1933, as amended, to four accredited investors in private placement, one of whom is a family member of our Executive Chairman. |
| (7) | Issued to one holder of Series A Preferred Stock. |
| (8) | Cashless exercise of service warrant by Checkmate Capital Group, exercised per the terms of their Warrant Agreement. In the exercise, Checkmate forfeited and retired 41,429 warrants. |
| (9) | Issued to two holders of Series A Preferred Stock. |
| (10) | Issued to 14 holders of Series B Preferred Stock. |
| (11) | Issued to Redstone Communications for general consulting services. |
We issued all securities reported to persons who were “accredited investors” as that term is defined in Rule 501 of Regulation D of the SEC, or to “sophisticated investors” or key employees; and each such person had prior access to all material information about us prior to the offer and sale of these securities, and had the right to consult legal and accounting professionals. We believe that the offer and sale of these securities were exempt from the registration requirements of the Securities Act, pursuant to Sections 4(a)(2) and Rule 506 of Regulation D of the SEC.
Purchases of Equity Securities by Us and Affiliated Purchasers
None.
Securities Authorized for Issuance under Equity Compensation Plans
See Item 11 for detailed information and tables.
ITEM 6: [RESERVED]
| 36 |
ITEM 7: MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
A. Plan of Operation
We are developing next-generation nuclear medicines for the treatment of cancer and other diseases. Our initial technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “Technology”), a clinical-stage bone targeting therapeutic radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. We believe improvements in formulation and manufacturing from a prior FDA-approved drug (Quadramet®) utilizing the same radioisotope has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability.
In August 2021, the Food & Drug Administration (FDA) cleared our Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the breast, lung, prostate, and other organs. We initiated this trial at our first site (Houston, TX) in November 2021, and dosed our first patient in this open-label, dose escalating study in April 2022. As of March 15, 2024, we have dosed a total of five patients, which includes completion of the first two cohort groupings in this study. This phase of our clinical trials is expected to conclude in 2024, subject to timely funding, at which time we expect to seek approval from the FDA to commence Phase 2 efficacy trials of CycloSam®.
Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults, and in January 2022, the FDA granted us Rare Pediatric Disease Designation for that indication. Although patients with osteosarcoma or Ewing’s sarcoma are eligible to participate in our initial Phase 1 trial, we anticipate filing separate indication-specific protocol(s) to our IND in the future, subject to funding, to commence clinical trials specifically for these primary, pediatric bone cancers. Pursuing clinical trials and a possible treatment for osteosarcoma is an important mission for the Company. We are also looking at how CycloSam® can be used to treat the debilitating pain from cancer that has metastasized to the bone and may seek to initiate a separate study for that important indication in the near future.
Clinical trials, the drug approval process, and the marketing of drugs are intensively regulated in the United States and in all major foreign countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and related regulations. Drugs are also subject to other federal, state, and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
Merger Agreement. On February 7, 2024, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Telix”), and certain subsidiaries of Telix established for the purpose of completing a reverse triangular merger with a second forward merger (collectively, the “Merger”). Pursuant to the Merger Agreement, QSAM stockholders will receive (i) $33.1 million in Telix ordinary shares (“Telix Shares”) or cash, less an adjustment amount equal to QSAM’s indebtedness and certain of its payables as of the merger closing (the “Closing Consideration”), and (ii) contingent value rights (“CVRs”) to receive future payments of up to $90 million upon the achievement of four clinical and commercial milestones within ten years of closing.
Prior to closing the Merger, we will effect a reverse stock split of our common stock in a ratio between 1:1000 and 1:2000. Each whole share of QSAM common stock outstanding after the reverse split will be entitled to receive Telix Shares and CVRs in the Merger. Any remaining fractional shares of QSAM common stock resulting from the reverse split will be exchanged for cash and CVRs. As a result of the reverse split and the Merger, all of our stockholders will receive, for each share of QSAM common stock held prior to the reverse split, Telix Shares or cash with a value equal to such share of QSAM common stock’s pro rata share of the Closing Consideration and one CVR.
| 37 |
Telix Shares issued to our stockholders will not be registered under the Securities Act of 1933, as amended (the “Securities Act”), but will be issued pursuant to an exemption to the registration requirements thereunder. The Telix Shares will be subject to resale restrictions under Rule 144 of the Securities Act.
Our stockholders representing greater than a majority of the total voting stock of the Company have already approved the merger. Closing, however, is subject to various conditions set forth in the Agreement including, among others, the filing of a definitive information statement pursuant to Regulation 14C of the Securities Exchange Act of 1934, as amended (the “Information Statement”). The merger cannot be closed until 20 days after the mailing of the Information Statement to our stockholders. Upon the closing of the Merger, we will de-list from the OTC Markets and will file a Form 15 to cease reporting under the Securities Exchange Act of 1934, as amended. There is no guarantee that all closing conditions required to close the Merger will be achieved, or that the Merger will close on the terms described herein.
The Company filed a Form 8-K with the SEC on February 8, 2024, providing a more detailed description of the Merger, the Merger Agreement, the CVR Agreement and other transactions and agreements to be conducted pursuant to and in connection with the Merger, as well as filing the Merger Agreement as an exhibit thereto. Shareholders are encouraged to review that Form 8-K filing.
B. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Results of Operations for the years ended December 31, 2023 and 2022
For the years ended December 31, 2023 and 2022, we recorded no revenue from operations.
For the year ended December 31, 2023, we recorded a net loss from operations of $4,393,126, a decrease of $1,088,165 (19.9%) from our net loss from operations of $5,481,291 in 2022. Basic and diluted net loss per share was $1.69 and $3.61 for the years ended December 31, 2023 and 2022, respectively. The primary reasons for the decrease in the net loss for 2023 over 2022 were as follows:
(1) FY 2023 decrease in compensation and related expenses of $1,898,283 is driven by a decrease in Employee Stock Option Expense of $1,213,589, a decrease in wages and taxes of $672,674, and a decrease in Board of Directors Fee expenses of $11,909.
(2) FY 2023 decrease in General & Administrative costs of $28,617 is driven primarily by a decrease in filling fees of $63,426, offset by an increase in travel of $14,974 and franchise tax of $12,554, and an increase in patient marketing of $12,500.
(3) FY 2023 increase in Other Expenses of $430,507 is driven primarily by inducement interest of $397,928 resulting from the modification of exercise prices of warrants and conversion prices of preferred stock in 2023, a decrease in the gain on conversion of convertible debt of $54,288, and offset by a decrease in loan interest of $28,562.
(4) FY 2023 increase in Professional Fees of $270,647 is primarily driven by an increase in stock based consulting expense of $370,142, offset by a decrease in investor relations service expense of $137,945.
(5) FY 2023 increase in Research and Development of $137,581 is driven by an increase in vendor expenses related to the clinical trial enrolling multiple patients during 2023.
| 38 |
Financial Condition, Liquidity and Capital Resources
For the year ended December 31, 2023, cash increased by $1,161,111 from $225,276 as of December 31, 2022 to $1,386,387 at the end of 2023. This increase was primarily the result of cash provided by financing activities of $4,009,247, inclusive of a $2,000,000 option fee paid by Telix upon the signing of a term sheet for the Merger, as further described below (the “Telix Option Fee”), offset by cash used in operating activities of $2,848,136.
Net cash used by operating activities was $2,848,136 for the year ended December 31, 2023, which reflected our net loss during the period of $4,393,126, non-cash adjustments of $1,678,920, a net decrease in operating liabilities of $204,415, and a net increase in operating assets of $70,485. The majority of non-cash adjustments consists of $392,405 of stock-based compensation to employees and directors, $852,257 of stock-based compensation for services, $36,332 of amortization of debt issuance costs, and inducement expense of $397,928 related to the repricing of warrants and preferred stock. Net cash provided by financing activities was $4,009,247 for the year ended December 31, 2023, which reflected the proceeds received from the issuance of common stock of $914,246, the Telix Option Fee of $2,000,000, and proceeds from warrant exercises of $1,095,001. Net cash used in investing activities during the year ended December 31, 2023 consisted of $0.
At December 31, 2023, our cash totaled $1,386,387. Our cash is currently held at large U.S. banks.
On November 14, 2023, we signed a non-binding term sheet with Telix with respect to the Merger. Upon execution of that term sheet, Telix paid us $2,000,000 as an Option and Pre-Closing Collaboration Fee, among other reasons, to assure 60 days of exclusivity as they completed their due diligence and the parties negotiated the definitive Merger Agreement (the “Telix Option Fee”). If the Merger does not close, the Telix Option Fee will be automatically converted to QSAM common stock at a price of $6.70 per share. The exclusivity period granted in return for the Telix Option Fee was extended on January 11, 2024 for an additional 30 days for no additional consideration. On February 7, 2024, we signed the definitive Merger Agreement with Telix, and are currently working to close the Merger in the first half of 2024. We believe we have sufficient capital to continue operations through the estimated closing date of the Merger and to cover significantly all closing expenses. As part of the Merger Agreement, Telix agreed to assume up to $500,000 of our closing expenses. Our current focus and strategy is to complete the Merger in a timely manner. If the Merger does not close, or closing is delayed significantly beyond the estimated timeframe, we will need to raise additional capital to support operations through the coming year. There is no guarantee that we can complete any future capital offerings on terms suitable to the Company and its shareholders if at all. As a result, there is substantial doubt about our ability to operate as a going concern. See “Note 2 – Basis of Presentation and Going Concern” in our consolidated financial statements.
We have limited operations and are not currently generating any revenues from our business operations. Our independent registered public accounting firm has issued a going concern opinion for the year ended December 31, 2023. This means that our auditors believe there is substantial doubt that we can continue as an on-going business for the next 12 months, assuming the Merger does not close. Our consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties. The ability of the Company to continue as a going concern is dependent on the success of management’s plans, which is not guaranteed. The Company has supported operations through the issuance of common stock, preferred stock and debt over the last 12 months. This includes the recent sale of common stock in the fourth quarter of 2023, the common stock and warrant offering completed in March 2023, the Series B Preferred Stock offering in the first quarter of 2021, the exercise of warrants issued in connection with the Series B offering, and also the convertible debt offering conducted in the fourth quarter of 2021. If the Merger does not close, management expects expenses to increase in 2024 as our drug technology advances through clinical trials, and as a result, we will need to raise additional capital to support these operations. Further, if the Merger does not close, we will still have significant legal and other closing expenses to pay which could jeopardize our ability to continue to operate the Company. There is no assurance that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. Management believes that it has cash to support operations into the second quarter of 2024. If the Merger does not close and we are not successful in raising additional capital, we may need to delay clinical trials, reduce overhead, or in the most extreme scenario, shut down operations.
| 39 |
Since 2020, we have completed approximately $7 million in total equity and debt funding in multiple offerings, not including the Telix Option Fee, as follows:
Common Stock Offering. In the third quarter of 2023, the Company issued 176,470 shares of common stock to three accredited investors, one of whom is a family member of our Executive Chairman, for a total of $600,000 in funding. In December 2023, a fourth investor purchased 50,000 shares of common stock for $250,000, which funds were received in early January 2024. We issued these shares pursuant to an exemption to the registration requirements thereunder, and more specifically, Section 4(a)(2) of the Securities Act.
Common Stock and Warrant Unit Offering. On March 31, 2023, we closed a total of $2.86 million in a common stock and warrant unit offering, which consisted of 381,500 shares of common stock at $4.50 and 381,500 two-year common stock warrants (the “Warrants”) which were all exercised. The Warrants originally had an exercise price of $6.00 per share, but on March 31, 2023, our Board approved a reduction of the price to $3.00 as an inducement to immediate exercise of the Warrants. All Warrant holders exercised at this reduced price, which raised an additional $1.14 million for the Company, inclusive of $342,669 in subscription receivables which were received in April 2023. We issued these shares and warrants pursuant to an exemption to the registration requirements thereunder, and more specifically, Section 4(a)(2) of the Securities Act.
Series B Financing. In January 2021, the Company closed a Series B Convertible Preferred Stock private placement (the “Series B Offering”) and issued a total of 2,500 shares at a price of $1,000 per share, raising an aggregate amount of $2.5 million, inclusive of $156,000 in debt conversion. The Series B Offering, which commenced in 2020, was led by Checkmate Capital Group, LLC, a California based investment firm focused on biotechnology and other technology investments. As of December 31, 2023, after the conversion of 991 shares of Series B Stock plus $53,061 of accrued dividends in 2022 for 164,134 shares of common stock, there were 1,509 shares of Series B Stock outstanding.
In October 2023, all 14 remaining holders of Series B Stock signed Exchange Agreements with the Company providing for the automatic exchange of all their remaining shares of Series B Stock plus all accrued dividends for common stock at a price of $3.00 per share in the instance the Company listed its shares on Nasdaq or signed an agreement to be acquired. In connection with the signing of the Merger Agreement, on February 6, 2024, all 1,509 shares of Series B Stock plus $468,364 in accrued dividends were exchanged for 658,968 shares of common stock. Currently no shares of Series B Stock remain outstanding.
Warrant Conversion. In connection with the Series B Offering, we issued in 2021 approximately 150,000 common stock warrants, which were originally exercisable prior to July 8, 2021 at an exercise price of $14.00 per share, and later modified by our Board to expire on October 15, 2021 and be exercisable at $10.00 per share. As of October 15, 2021, seven holders of the Series B warrants exercised those warrants and received a total of 46,786 common shares for total consideration to the Company of $467,858.
Convertible Note Financing. In the fourth quarter of 2021, we entered into convertible note purchase agreements with eight accredited investors, pursuant to which we issued an aggregate of $605,000 of 6% annual interest convertible notes (the “Notes”). The Notes were due to mature on December 31, 2023 and were convertible into shares of common stock of the Company in the event of future equity financing of $5 million or greater, Nasdaq uplisting, or at the discretion of the noteholders. In connection with the Notes offering, the Company issued warrants to the noteholders, with each warrant convertible into one share of common stock at an exercise price of $24.00 per share beginning from the date of the warrant until October 31, 2022, all of which expired without exercise. In the fourth quarter of 2022, two note holders converted $132,932 of principal and interest on their notes into 22,155 shares of common stock, at a reduced conversion price approved by the Board of $6.00. In the first quarter of 2023, the remaining holders converted $519,712 of principal and interest on their notes into 148,621 shares of common stock, at a reduced conversion price approved by the Board of $3.50, and the two holders who converted in 2022 received an additional 15,825 shares to “make whole” their prior conversion at a higher price. Due to the inducement to the reduced conversion price, the Company recorded $397,937 of inducement interest expense to other income and expenses. The fair value of the shares issued for the conversion as of March 31, 2023 was $594,484 based on the stock price of $4.00 as of March 31, 2023.
During the year ended December 31, 2023 and 2022, the Company recorded interest expense related to the amortization of the discount of $0 and $36,322, respectively. As of December 31, 2023 and December 31, 2022, unamortized debt discount was $0 and $36,322, respectively.
| 40 |
Prior Offering
Series A Preferred Stock Financing. The Company raised $600,000 in a Series A 6% Convertible Preferred Stock (the “Series A Stock”) from two accredited investors in November 2015 and January 2016, respectively. The Series A Stock bears a 6% dividend per annum, calculable and payable per quarter in cash or additional shares of common stock as determined in the Certificate of Designation. The Series A Stock was originally convertible at $6.50 per share at the discretion of the holders and contains price protection provisions in the instance that we issue shares at a lower price, subject to certain exemptions. The price has been reset several times since the issuance of the Series A Stock. As of December 31, 2023, as a result of the 2023 private placement offering and warrant exercise inducement, the conversion price was $3.00 per share. Series A Stockholders also received other rights and protections including piggy-back registration rights, rights of first refusal to invest in subsequent offerings, security over our assets (secondary to our debt holders), and certain negative covenant guaranties that we will not incur non-ordinary debt, enter into variable pricing security sales, redeem or repurchase stock or make distributions, and other similar warranties. The Series A Stock has no voting rights until converted to common stock.
The Series A Stock was redeemable on December 31, 2023, pursuant to a March 31, 2023 modification agreement signed by both Series A Stockholders. In November 2023, the two Series A Stockholders signed conditional conversion agreements with the Company providing that in the instance the Company lists on Nasdaq or is acquired, all remaining shares of Series A Stock plus all accrued dividends would be automatically converted to common stock at $3.00 per share, and extending the redemption period to December 31, 2024. As of December 31, 2023, 240 shares of Series A Stock remained outstanding plus $268,800 of accrued dividend payments. As of February 8, 2024, the Series A Stockholders unilaterally converted all remaining shares of Series A Stock plus all dividends into 170,184 shares of common stock. There are currently no shares of Series A Stock outstanding.
All shares and promissory notes in these offerings were sold pursuant to an exemption from the registration requirements of the Securities Exchange Commission under Section 4(a)(2) of the Securities Act and/or Regulation D to accredited or sophisticated investors who completed questionnaires confirming their status. Unless otherwise described in this Annual Report, reference to “restricted” common stock means that the shares have not been registered and are restricted from resale pursuant to Rule 144 of the Securities Act of 1933, as amended.
Cash and Working Capital
We have incurred negative cash flows from operations since inception. As of December 31, 2023, we had an accumulated deficit of $40,158,601 and negative working capital of $672,348.
Critical Accounting Policies
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the revenue and expenses incurred during the reported periods. On an ongoing basis, we evaluate our estimates and judgments. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known.
Accrued Research and Development Expenses
We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on the facts and circumstances known to us at that time. Our expense accruals for contract research, contract manufacturing and other contract services are based on estimates of the fees associated with services provided by the contracting organizations. Payments under some of the contracts we have with such parties depend on factors such as successful enrollment of patients, site initiation and the completion of clinical trial milestones. In accruing service fees, we estimate the time-period over which services will be performed and the level of effort to be expended in each period. If possible, we obtain information regarding unbilled services directly from these service providers. However, we may be required to estimate these services based on other information available to us. If we underestimate or overestimate the activity or fees associated with a study or service at a given point in time, adjustments to research and development expenses may be necessary in future periods. Subsequent changes in estimates may result in a material change in our accruals.
| 41 |
Stock-Based Compensation
We recognize stock-based compensation expense for grants of stock option awards, restricted stock units and restricted stock under our Incentive Plan to employees, nonemployees and nonemployee members of our board of directors based on the grant-date fair value of those awards. The grant-date fair value of an award is generally recognized as compensation expense over the award’s requisite service period. In addition, in the past we have granted performance-based stock option awards and restricted stock grants, which vest based upon our satisfying certain performance conditions. Potential compensation cost, measured on the grant date, related to these performance options will be recognized only if, and when, we estimate that these options will vest, which is based on whether we consider the options’ performance conditions to be probable of attainment. Our estimates of the number of performance-based options that will vest will be revised, if necessary, in subsequent periods.
We use the Black-Scholes model to compute the estimated fair value of stock option awards. Using this model, fair value is calculated based on assumptions with respect to (i) expected volatility of our common stock price, (ii) the periods of time over which employees and members of the board of directors are expected to hold their options prior to exercise (expected term), (iii) expected dividend yield on the common stock, and (iv) risk-free interest rates. Stock-based compensation expense also includes an estimate, which is made at the time of grant, of the number of awards that are expected to be forfeited. This estimate is revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Our financial statements are prepared in conformity with U.S. generally accepted accounting principles (GAAP). Disclosures regarding our Critical Accounting Policies are provided in Note 3 – Summary of Significant Accounting Policies of the footnotes to our consolidated financial statements.
Off-Balance Sheet Arrangements
The Company did not engage in any “off-balance sheet arrangements” (as that term is defined in Item 303(a)(4)(ii) of Regulation S-K) as of December 31, 2023.
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not required for smaller reporting companies.
ITEM 8: FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and the reports of our independent registered accounting firm required pursuant to this item are included in Item 15 of this report and are presented beginning on page F-1.
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A: CONTROLS AND PROCEDURES
In connection with the preparation of this Annual Report, management, under the supervision and with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, have evaluated the effectiveness of the Company’s disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), as of the end of the period covered by this Annual Report. In designing and evaluating the disclosure controls and procedures, our management recognizes that any controls and procedures are designed only to provide reasonable assurance, and no matter how well designed and operated, there can be no assurance that disclosure controls and procedures will operate effectively in all circumstances. Based on this evaluation, our Chief Executive Officer and our Chief Financial Officer concluded that as of December 31, 2023, the Company’s disclosure controls and procedure were not effective based on the criteria in Internal Control – Integrated Framework issued by the COSO, version 2013, as discussed below.
| 42 |
Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Securities Exchange Act Rule 13a-15(f). The Company carried out an evaluation under the supervision and with the participation of the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of the Company’s internal control over financial reporting. The Company’s management used the framework in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations (COSO) to perform this evaluation. As a result of this assessment, management identified a material weaknesses in internal control over financial reporting. A material weakness is a control deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. The identified material weakness is disclosed below:
| ● | Due to the size of the Company and available resources, there are limited personnel to assist with the accounting and financial reporting function, which results in a lack of segregation of duties. |
As a result of the material weakness in internal control over financial reporting described above, management concluded that, as of December 31, 2023, the Company’s internal control over financial reporting was not effective based on the criteria in Internal Control – Integrated Framework issued by the COSO. Management notes that the Company signed a merger agreement with Telix on February 7, 2024, and expects the transaction to close in the first half of 2024 and as such, the Company will have the available resources in order to hire additional personnel to expand the finance and accounting department in order to mitigate the material weakness noted above if required at such time.
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. We were not required to have, nor have we, engaged our independent registered public accounting firm to perform an audit of internal control over financial reporting pursuant to the rules of the Securities and Exchange Commission that permit us to provide only management’s report in this annual report.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during 2023 other than noted above that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B: OTHER INFORMATION
There was no other information required to be disclosed in the fourth quarter of 2023 that was not filed by the Company in a Current Report on Form 8-K.
ITEM 9C: DISCLOSURES REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
None.
| 43 |
PART III
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Identification of Directors and Executive Officers
The following table sets forth the names of all of our current directors and executive officers. The Directors will serve until the next annual meeting of the shareholders or until their successors are elected or appointed and qualified, or their prior resignation or termination.
| Name | Positions Held | Age | Date
of Election or Designation | Date
of Termination or Resignation | ||||||
| C. Richard Piazza | Executive Chairman | 77 | November 2020 | * | ||||||
| Douglas R. Baum | Chief Executive Officer & Director | 57 | January 2020 (1) | * | ||||||
| Adam King | Chief Financial Officer | 39 | December 2021 | * | ||||||
| Christopher Nelson | General Counsel & Executive VP | 54 | July 2014 (2) | * | ||||||
| Charles J. Link, Jr. | Director | 64 | February 2021 | * | ||||||
| Adriann Sax | Director | 63 | January 2022 | * | ||||||
(1) |
Mr. Baum was appointed director in January 2020 and CEO in November 2020. | |
| (2) | Mr. Nelson served in multiple roles since 2014 including, CEO, President and Director. He has held the role of General Counsel since 2015 and EVP since 2023. |
Business Experience
C. Richard Piazza, Ph.D. – Executive Chairman. Mr. Piazza was appointed as a member and the Executive Chairman of the board of the Company in November 2020. Mr. Piazza also served from 2017 until January 2024 as President and CEO of IGL Pharma Inc., the licensor of CycloSam®, and a consultant to IsoTherapeutics Group, LLC, the inventors of the technology. Mr. Piazza also currently serves on the board of directors of NovaScan LLC, a privately-held cancer detection and diagnostics company. Prior to his work with IGL Pharma, from 2014 to 2016, he was CEO of SynVivo, Inc., a cancer diagnostics company. Mr. Piazza has more than 48 years of healthcare experience in both medical devices and pharmaceutical/biotech and has led several technology companies to market success including numerous FDA approvals in both sectors. Previously, he served in general management positions in both public and private international companies including Ohmeda, Smith & Nephew Pharmaceuticals, Marquest and VitaGen (world’s first bioartifical liver). Over his career, he has provided advisory services to some of world’s leading institutions including MD Anderson Cancer Center, Baylor College of Medicine, University of California San Diego, University of Chicago and Kings College Hospital (London). In 2019, he co-founded QSAM Therapeutics, Inc. with Douglas Baum, our CEO. Mr. Piazza obtained a BS in Economics and a BS in Speech Pathology from the State University of New York and MA & PhD in Economics from the University of Buffalo and Leeds University.
We believe Mr. Piazza is qualified to serve on our board of directors due to his significant experience in the pharmaceutical and biotech industry, and his experience serving on the boards and in senior management positions in several publicly traded companies.
Douglas R. Baum – Chief Executive Officer & Director. Mr. Baum was appointed to the board of the Company in January 2020 and to the position of CEO in November 2020. He brings to the Company over 30 years of experience in the bioscience and biotech industries, including development, FDA/EMA approval and commercialization of multiple drugs and medical devices. Over his long senior executive tenure, he has overseen 15 product approvals through the FDA and EMA and raised over $85 million in capital to fund breakthrough technologies. Between 2017 and 2020, Mr. Baum consulted with multiple medical schools, biotech and pharmaceutical companies; and between 2012 and 2017, he served as President, Chief Executive Officer and Director of Xeris Pharmaceuticals Inc. (currently, NASDAQ: XERS). Previously, he served as Executive Vice President and Chief Operating Officer of Macuclear Inc., and other executive level positions with clinical trial research firms including SCIREX and Premier Research Group, Inc. He holds a Master’s of Science in Technology Commercialization and BBA in International Business and Marketing from the University of Texas.
| 44 |
Mr. Baum’s extensive experience in the biotech industry as a senior level executive and vast exposure to the lifecycle of healthcare products from trials to commercialization makes him qualified to serve on our board of directors.
Christopher Nelson – General Counsel and Executive Vice President – Business Development. Mr. Nelson has been General Counsel of the Company since 2015, and was also one of its directors from 2016 to January 2022, and President from 2016 to November 2020. In 2023, Mr. Nelson also assumed the expanded role of EVP – Business Development. In these roles, he has overseen corporate and governance legal matters, finance and business development for the Company. He has also served since 2016 as Managing Director of GreenBlock Capital LLC in Palm Beach, Florida, a boutique mergers and acquisitions advisory firm specializing in bio-technology, ag-technology and similar sector business combination transactions; and from 2019 to 2022, as General Counsel for Earth Property Holdings, LLC, a private equity-backed company engaged in soil health and compost manufacturing in Texas and Florida. Mr. Nelson has practiced law in Florida for over 28 years, and during that time has represented many start-up, early stage and established businesses seeking financing, acquisitions and general growth management counseling. Early in his career, Mr. Nelson was an associate with Greenberg Traurig PA, and with Akerman Senterfitt PA, both in Miami, Florida. At both firms he served in their corporate and securities practice, representing NYSE and NASDAQ companies. Mr. Nelson received a BA from Princeton University, and JD from University of Miami School of Law, and is an active member of the Florida Bar.
Adam King, CPA – Chief Financial Officer. Mr. King currently serves as CFO for the Company, a part-time position he has held since December 6, 2021. Mr. King is the founder and CEO of King Consulting Group, where he provides a range of financial and reporting services for clients that include Vice President of Finance for large private equity-backed international companies to CFO of small start-ups. Before founding King Consulting Group in January 2021, Mr. King was the CFO for Netsertive, a venture-backed digital marketing company in Research Triangle Park, North Carolina. From 2016 to 2018, he was the Office Managing Audit Director for BDO’s Greenville, SC office, in addition to Audit Director in Raleigh, NC, and Boston. While at BDO, Mr. King worked with various clients, from Tech and Life Science start-ups to large billion-dollar publicly traded companies. Before his time at BDO, he served as the Director of Revenue Assurance and Internal Controls at Bandwidth.com and an Audit Manager at Ernst & Young. Mr. King holds a Bachelor of Science in Accounting from Elon University and is a CPA in Raleigh, NC. In October 2019, Mr. King and his wife filed for personal bankruptcy under chapter 13 of the United States Bankruptcy Code due to extensive medical expenses incurred by the family in connection with their child’s medical diagnosis and treatment. The repayment plan was completed in full in March 2022.
Charles J. Link, Jr. – Director. Dr. Link was appointed to the Board in February 2021, and brings decades of biotech and drug development experience to the Company. He currently serves on the executive committee of the Board of Directors at NovaScan Inc., a clinical-stage company focused on cancer detection. Dr. Link is also the founder and Executive Chairman of privately-held Syncromune Inc., a cancer immunotherapy company and a founder of biotech startup ChainLink Pharma. Dr. Link served on the Board of Directors for Viewpoint Molecular Targeting, a clinical stage company developing alpha-particle radiopharmaceuticals until its recent merger into a NASDAQ-listed corporation to form Perspective Therapeutics. Previously, Dr. Link was the CEO, CSO, Chairman, and founder of NewLink Genetics, a NASDAQ-listed immunotherapy company focused on developing novel immuno-oncology product candidates, from 1999 until his retirement in 2019. During his tenure at NewLink, Dr. Link led a series of collaborative transactions totaling hundreds of millions of dollars with Merck, Roche and the United States government. He also oversaw the collaboration with Merck to develop EVERBO, the first Ebola vaccine to receive FDA approval. Prior to founding NewLink Genetics, Dr. Link was an attending physician at the National Cancer Institute. He has authored more than 100 peer-reviewed papers. Dr. Link received an M.D. from Stanford University, and he attended the U.S. Air Force Academy.
Dr. Link’s experience as CEO and Chairman of a NASDAQ-listed biotechnology company where he led several multi-party drug development programs and successfully raised significant funding in the capital markets, as well as his service as an oncology physician and senior government researcher, qualifies him to serve on our board of directors.
| 45 |
Adriann Sax – Director. Ms. Sax was appointed Director in January 2022. She has a distinguished 30+ year career in biotech and life sciences, serving in leadership, operational and business development roles with a focus on oncology for both Fortune 100 and start-up companies. Since May 2020, she has served as CEO and co-founder of Vetigenics LLC, an animal health biotech company, where she has secured partnerships with Merck, obtained federal grants, and was named 2021 Start-up of the Year by the Penn Center for Innovation at the University of Pennsylvania. From 2019 to 2020, she served as CEO and Director of Orsenix LLC, a clinical staged oncology biotech, and from 2014 through 2019, as Entrepreneur in Residence at Fortress Biotech. Previously she was EVP and Chief Commercial Officer at Kadmon Corp., a division of Sanofi Company, and before that served in various leadership capacities at large pharmaceutical companies, notably Vice President at Bristol Myers Squibb, Executive Director at Merck & Co., and Executive Vice President in charge of Business Development and Strategic Planning at King Pharmaceuticals, leading to its $6.5 Billion acquisition by Pfizer. Ms. Sax holds an MBA from the Keller Graduate School and a BS in Animal Science from the University of Delaware. She is an active advisor and board member for many industry associations, academic institutions, and nonpublic company boards.
Ms. Sax’s extensive experience in biotech and life sciences, serving in leadership, operational, business development, and board of director roles for both Fortune 100 and start-up companies qualifies her to serve on our board of directors.
Other Key Employees and Advisors
Namrata Chand – VP Operations. Age 44. Ms. Chand has held the position of VP - Operations with the Company since November 2020. Ms. Chand brings 20 years of experience and a diverse foundation in administration, marketing, operations and business development within non-profit and for-profit sectors. Initially focusing her career in large organizations, she held leadership positions in marketing and corporate relations at Nestle, Beam Suntory, Aetna and BFAS, a leading national animal welfare organization, to build brand awareness, improve operational efficiency and drive overall growth. Ms. Chand later entered the life science space and joined La Jolla Capital Partners in 2011 where she assisted small to midsize healthcare companies through various stages of development with special emphasis on capital formation, operations, supply chain, clinical trials, marketing and business development. Most recently, Ms. Chand led Investor Relations and Business Development for Ryca International, an innovative dental product company that entered into a joint venture with a leading global MedTech company. In addition to leading operations at the Company, Ms. Chand serves on the Advisory Board of a number of early-stage biotech companies.
Barry Sugarman – Scientific Advisor. Age 66. Mr. Sugarman has held the position of senior regulatory and scientific advisor with the Company since November 2020. Mr. Sugarman has over 30 years of experience spanning public and private companies in the pharmaceutical, medical device, dietary supplement and cosmetic industries. Mr. Sugarman has considerable direct experience in pharmaceutical product development, manufacturing, clinical trials, regulatory affairs, FDA and government relations, marketing, and distribution; as well as Good Manufacturing Practices (GMP’s), Good Clinical Practices (GCP’s), Good Laboratory Practices (GLP’s), and International Conference for Harmonization (ICH) requirements. He is an author and co-author of numerous FDA filings and approvals including Investigational New Drug Applications, New Drug Applications, Abbreviated New Drug Applications, and Medical Device Applications 510(k)’s. Mr. Sugarman is a member of the Regulatory Affairs Professional Society, American Association of Pharmaceutical Scientists, Association of Clinical Research Professionals, and the National Association of Corporate Directors. He is a co-author of “Prompt, Accurate Diagnosis of Pediatric Cancer and Leukemia for Pediatricians, Orthopedists, and Family Practitioners” (2007).
Richard “Keith” Frank – Scientific Advisor. Age 68. Dr. Frank has served as scientific advisor to the Company since April 2020. Since 2006, he has served as CEO, President and co-founder of IsoTherapeutics LLC, a radiopharmaceutical R&D and contract manufacturing company that invented CycloSam® and provides services for both large and small biotechnology companies. He also serves as Chairman of IGL Pharma, Inc., the Company’s licensor of CycloSam®. Prior to these positions, Dr. Frank spent over 20 years in numerous senior scientific positions at Dow Chemical Company. At Dow, he was a collaborator in the development of bone-seeking radiopharmaceuticals Quadramet (Sm-153-EDTMP) and STR (Ho-166-DOTMP). Additionally, Dr. Frank was the lead inventor of Iotrex™ for use in the GliaSite® Radiation Therapy System. He also initiated and was the technical leader of Dow’s ChelaMedSM Radiopharmaceutical Services offering.
| 46 |
Jaime “Jim” Simon – Scientific Advisor. Age 70. Dr. Simon has served as scientific advisor to the Company since April 2020. Since 2006, he has served as Vice President and Chief Science Officer, and co-founder of IsoTherapeutics LLC, a radiopharmaceutical R&D and contract manufacturing company that invented CycloSam® and provides services for both large and small biotechnology companies. Prior to co-founding IsoTherapeutics, Dr. Simon spent over 25 years as a senior scientist at Dow Chemical Company where his initial proposals led to the creation of Dow’s radiopharmaceutical group. At Dow, Dr. Simon was the lead inventor for all bone agent patents including Sm-153-EDTMP and Ho-166-DOTMP. Dr. Simon has been involved in numerous FDA submissions for clinical trials, and has coordinated the radioisotope activities at the University of Missouri Research Reactor (isotope production), the University of Missouri Veterinary School (dog studies), and The Harry S. Truman Veterans Administration Hospital (human clinical studies).
Family Relationships
There are no family relationships between any of the Company’s directors or executive officers or any person nominated or chosen by the Company to become a director or executive officer.
Directorships
Dr. Link served as Chairman of the Board of Directors of NewLink Genetics, a NASDAQ-listed company from 1999 until his retirement in 2019. None of our other directors is a director of a company or has been in the last five years the director of any other reporting company or registered investment company.
Involvement in Certain Legal Proceedings
Except as disclosed under Mr. King’s biographical information pertaining to Mr. King’s and his wife’s filing of personal bankruptcy under the United States Bankruptcy Code in 2019, during the past 10 years, none of our present or former directors, executive officers or persons nominated to become directors or executive officers or control person of our Company:
| ● | has filed a petition under federal bankruptcy laws or any state insolvency laws, nor had a receiver, fiscal agent or similar officer appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing; | |
| ● | was convicted in a criminal proceeding or named subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); | |
| ● | was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him or her from or otherwise limiting the following activities: |
Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
Engaging in any type of business practice; or
Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
| ● | was the subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in the preceding bullet point, or to be associated with persons engaged in any such activity; |
| 47 |
| ● | was found by a court of competent jurisdiction in a civil action or by the SEC to have violated any Federal or State securities law, and the judgment in such civil action or finding by the SEC has not been subsequently reversed, suspended, or vacated; | |
| ● | was found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated; | |
| ● | was the subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of: |
any Federal or State securities or commodities law or regulation; or
any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
any law or regulation prohibiting mail or wire fraud in connection with any business activity; or
| ● | was the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, or any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Section 16(a) Beneficial Ownership Reporting Compliance
Our shares of common stock are registered under the Exchange Act, and therefore the officers, directors and holders of more than 10% of our outstanding shares (“Insiders”) are subject to the provisions of Section 16(a), which requires them to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and our other equity securities. The Insiders are required by SEC regulations to furnish us with copies of all Section 16(a) reports they file. Based solely upon review of the copies of such forms furnished to us during the fiscal year ended December 31, 2023, we have determined that all Insiders except the following timely filed their reports under Section 16(a):
| 1. | Adam King was delayed in filing his Form 3 in connection with assuming the position of Chief Financial Officer with the Company. Mr. King was further delayed in filing his Form 4 reporting (i) the award of certain stock options in connection with his hiring, (ii) the award of restricted stock under a stock award agreement, and (iii) the award of shares upon conversion of deferred salary; | |
| 2. | Checkmate Capital Group, LLC, Paschall Charles Thomas, Checkmate Strategic Capital Holdings, LLC Checkmate Strategic Capital 2, LLC (filing as a “group” as defined in Section 13(d) of the Exchange Act) were delayed in filing two Form 4s, one in connection with the acquisition of warrants and another in connection with conversion of those warrants. |
Corporate Governance
Audit Committee. Our Audit Committee is currently comprised of Dr. Charles Link and Adriann Sax. We have determined that both Dr. Link and Ms. Sax are independent under Rule 10A-3 of the Exchange Act, and have the financial experience to serve of the Audit Committee; however, pursuant to OTCQB rules, the Company is not currently required to have an audit committee. We may appoint one additional independent director who would be qualified as a “financial expert” as required under NASDAQ rules to serve as the Audit Committee Chair, if necessary, however since the Company is not required to have an audit committee under the SEC or OTCQB rules, its audit committee currently does not have a “financial expert”, as that term is defined under the SEC rules.
Under the Audit Committee’s charter, the Audit Committee is responsible for assisting with the Board’s oversight of: (1) the quality and integrity of the Company’s financial statements and related disclosure; (2) the Company’s compliance with legal and regulatory requirements relating to financial and accounting matters; (3) the independent auditor’s qualifications, performance and independence; (4) the integrity of the internal controls at the Company; and (5) the administration of the Company’s conflicts of interest and related party transactions policies and procedures. The Committee is also responsible for providing an avenue for effective communication among the Audit Committee, the independent auditor, management and the Board.
| 48 |
Compensation Committee. Our Compensation Committee is currently comprised of Douglas Baum and Dr. Link, of whom only Dr. Link is an independent director under the board independence standards. The Compensation Committee formally instituted its governance procedures in 2017, and actively oversees all executive-level salary and compensation matters for the Company.
Under the Compensation Committee’s charter, the purpose of the Compensation Committee is to assist the Board in carrying out its responsibilities relating to compensation and benefits for the Company’s directors, officers and employees. The Compensation Committee is also responsible for performing the duties relating to executive compensation provided for in the rules of the national securities exchange on which the Company’s stock may be listed. The Committee is also responsible for preparing any compensation committee reports required in the Company’s annual report and proxy materials by the rules of the SEC. The Compensation Committee also oversees the performance of the Audit Committee.
Nominating and Corporate Governance Committee. We have not yet established a Nominating and Corporate Governance Committee (“Nominating Committee”). Up to this time, we believe that we have been able to effectively manage the issues normally considered by a Nominating Committee through the Board of Directors.
Code of Ethics
On January 13, 2022, we adopted a revised code of ethics for our principal executive and financial officers, directors and employees. Our code of ethics, as amended, was posted to our website (www.qsambio.com) on January 13, 2022, updating a prior version that was originally posted as of November 2015. On January 13, 2022, we also adopted a Policy on Insider Trading. Pursuant to new rules promulgated by the SEC, we adopted an amendment to the Policy on Insider Trading effective March 14, 2024.
ITEM 11: EXECUTIVE COMPENSATION
The following table sets forth information regarding compensation earned in or with respect to our fiscal year 2023 and 2022 for the following persons (“Names Executive Officers”):
| (i) | our principal executive officer, or other individual serving in a similar capacity during the fiscal year 2023 and 2022; | |
| (ii) | our two most highly compensated executive officers other than our principal executive officer who were serving as executive officers at December 31, 2023, and 2022; and | |
| (iii) | up to two additional individuals for whom disclosure would have been required but for the fact that the individual was not serving as an executive officer at December 31, 2023. |
Summary Executive Compensation Table
Name and Principal Position | Year | Salary
($) (a) | Bonus
($) (b) | Stock
Awards ($) (c) | Option
Awards ($) (d) | Non-Equity Incentive Plan Compensation ($) (e) | Nonqualified
Deferred Compensation ($) (f) | All
Other Compensation ($) (g) | Total Earnings ($) (h) | |||||||||||||||||||||||||||
| C. Richard Piazza, | 2023 | $ | 125,000 | - | $ | 361,380 | - | - | - | - | $ | 486,380 | ||||||||||||||||||||||||
| Executive Chairman (1) | 2022 | $ | 163,021 | - | $ | 188,949 | $ | 116,500 | - | - | - | $ | 468,470 | |||||||||||||||||||||||
| Douglas Baum, | 2023 | $ | 125,000 | - | $ | 361,380 | - | - | - | - | $ | 486,380 | ||||||||||||||||||||||||
| CEO and Director (2) | 2022 | $ | 170,833 | - | $ | 180,733 | $ | 116,500 | - | - | - | $ | 468,066 | |||||||||||||||||||||||
| Christopher Nelson, | 2023 | $ | 100,000 | - | $ | 231,825 | - | - | - | - | $ | 331,825 | ||||||||||||||||||||||||
| General Counsel (3) | 2022 | $ | 133,333 | - | $ | 131,442 | $ | 116,500 | - | - | - | $ | 381,275 | |||||||||||||||||||||||
| Adam King, | 2023 | - | - | $ | 114,748 | - | - | - | $ | 282,000 | $ | 396,748 | ||||||||||||||||||||||||
| CFO (4) | 2022 | - | - | - | $ | 117,000 | - | - | $ | 208,200 | $ | 325,200 | ||||||||||||||||||||||||
| (a) | Salaries include those amounts paid and accrued as an expense on the books of the Company. | |
| (c)(d) | Stock and Option Awards are calculated based on the face value of awards as of the date of grant. |
| 49 |
| (g) | Other Compensation is comprised of consulting fees. |
| (1) | Per a November 14, 2022 amendment to his employment agreement, Mr. Piazza accepted as payment in full for $239,583 in deferred salary that had accrued through September 30, 2022, a cash amount of $47,917 and 53,241 shares of common stock, subject to restrictions and forfeiture. On June 23, 2023, the Board approved an issuance of 72,276 shares of common stock as additional compensation in the amount of $361,380 subject to vesting and forfeiture as provided in his issuance agreement. As of December 31, 2023, these shares were issued and outstanding, but had not yet vested. | |
| (2) | Per a November 14, 2022 amendment to his employment agreement, Mr. Baum accepted as payment in full for $229,167 in deferred salary that had accrued through September 30, 2022, a cash amount of $45,833, which was not paid as of December 31, 2022, and 50,926 shares of common stock, subject to restrictions and forfeiture. On June 23, 2023, the Board approved an issuance of 72,276 shares of common stock as additional compensation in the amount of $361,380 subject to vesting and forfeiture as provided in his issuance agreement. As of December 31, 2023, these shares were issued and outstanding, but had not yet vested. | |
| (3) | Per a November 14, 2022 amendment to his employment agreement, Mr. Nelson accepted as payment in full for $166,667 in deferred salary that had accrued through September 30, 2022, a cash amount of $33,333, which was not paid as of December 31, 2022, and 37,037 shares of common stock, subject to restrictions and forfeiture. On June 23, 2023, the Board approved an issuance of 46,365 share of common stock as additional compensation in the amount of $231,825 subject to vesting and forfeiture as provided in his issuance agreement. As of December 31, 2023, these shares were issued and outstanding, but had not yet vested. | |
| (4) | Mr. King was appointed to the position of interim CFO in December 2021, and was compensated as an independent contractor during 2023 and 2022. He serves as CFO in a part-time role. On June 23, 2023, the Board approved an issuance of 20,910 shares of common stock as additional compensation in the amount of $104,550 subject to vesting and forfeiture as provided in his issuance agreement. As of December 31, 2023, these shares were issued and outstanding, but had not yet vested. Mr. King also agreed and the board approved to convert two invoices for services in the amount of $10,200 to 2,040 shares of fully vested common stock. |
Executive Employment Agreements
C. Richard Piazza – Executive Chairman. Mr. Piazza signed his employment agreement with the Company on November 1, 2020, with an effective date of November 6, 2020. This agreement was amended and restated on December 6, 2021, and then amended again on November 14, 2022. The term is three years from December 2021, with extensions at the agreement of the parties. His base salary, as amended, is $300,000 per year, however, that base salary will not commence until the Company successfully raises a minimum of $7.5 million in a single offering. He is currently receiving an annualized base salary of $125,000 until the Company raises $2 million, at which time the annualized base salary will be increased to $160,000, and then increased again to $225,000 upon the Company raising $5 million. Pursuant to the November 14, 2022 amendment, Mr. Piazza accepted as payment in full for $239,583 in deferred salary that had accrued through September 30, 2022, a cash amount of $47,917 and 53,241 shares of common stock, subject to restrictions and forfeiture.
Under the amended agreement, Mr. Piazza is entitled to receive regular company benefits, including annual bonuses and salary increases, participation in management incentive plans involving cash or stock bonuses ranging from 25% and 125% of base salary, vacations, sick leave and PTO. Mr. Piazza is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 1.75% of the consideration received by the Company, which will be triggered by the Merger closing. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Piazza shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Piazza will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately, and base salary and healthcare benefits will continue for 24 months. Mr. Piazza also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
| 50 |
Douglas Baum, CEO. Mr. Baum signed his employment agreement with the Company on November 1, 2020, with an effective date of November 6, 2020. This agreement was amended and restated on December 6, 2021, and then amended again on November 14, 2022. The term is three years from December 2021, with extensions at the agreement of the parties. His base salary, as amended, is $300,000 per year, however, that base salary will not commence until the Company successfully raises a minimum of $7.5 million in a single offering. He is currently receiving an annualized base salary of $125,000 until the Company raises $2 million, at which time the annualized base salary will be increased to $160,000, and then increased again to $225,000 upon the Company raising $5 million. Pursuant to the November 14, 2022 amendment, Mr. Baum accepted as payment in full for $229,167 in deferred salary that had accrued through September 30, 2022, a cash amount of $45,833, which was paid in December 2023, and 50,926 shares of common stock, subject to restrictions and forfeiture.
Under the amended agreement, Mr. Baum is entitled to receive regular company benefits, including annual bonuses and salary increases, participation in management incentive plans involving cash or stock bonuses ranging from 25% and 125% of base salary, vacations, sick leave and PTO. Mr. Baum is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 1.75% of the consideration received by the Company, which is triggered by the Merger closing. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Baum shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Baum will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately and salary and healthcare benefits will continue for 24 months. Mr. Baum also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
Christopher Nelson, General Counsel and EVP. Mr. Nelson initially signed his employment agreement in 2017, which was renewed on a year-to-year basis on April 1 each year. On December 6, 2021, Mr. Nelson signed an amended and restated employment agreement with the Company providing for a term of three years, with extensions at the agreement of the parties. This agreement was amended again on November 14, 2022. His base salary, as amended, is $225,000 per year, however, that base salary will not commence until the Company successfully raises a minimum of $7.5 million in a single offering. He is currently receiving an annualized base salary of $100,000 until the Company raises $2 million, at which time the annualized base salary will be increased to $125,000, and then increased again to $170,000 upon the Company raising $5 million. Pursuant to the November 14, 2022 amendment, Mr. Nelson accepted as payment in full for $166,667 in deferred salary that had accrued through September 30, 2022, a cash amount of $33,333, which was paid in December 2023, and 37,037 shares of common stock, subject to restrictions and forfeiture.
Under the amended agreement, Mr. Nelson is entitled to receive regular company benefits, including annual bonuses and salary increases, vacations, sick leave and PTO. As amended per a July 26, 2023 Director’s resolution, Mr. Nelson is also entitled to receive a transaction bonus in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 1% of the consideration received by the Company, which is triggered by the Merger closing. If he is terminated for cause, as defined in the agreement, or he leaves the employment of the Company on his own volition, Mr. Nelson shall receive salary and benefits that have accrued up to the date of termination. If he is terminated without cause or following a material change, as defined in the agreement, Mr. Nelson will receive salary through the date of termination plus a pro-rated portion of bonus that would be earned during the full year when the termination became effective (or a lump sum of 50% of the full target bonus), all stock options shall vest immediately and salary and healthcare benefits will continue for 18 months. Mr. Nelson also agreed to a 12 month non-compete / non-solicitation, and signed a separate Proprietary Information and Inventions Agreement with his employment agreement which assigns to the Company any intellectual property developed by him during his employment.
Proprietary Information and Inventions Agreement
All officers, directors, and other key employees and consultants have signed a Proprietary Information and Invention Agreement (“PIIA”) with the Company that provides in material part the following:
| ● | All inventions and discoveries made by them during their employment or using Company resources shall be assigned to the Company. | |
| ● | They will not interfere in customer relationships during the term of employment plus 12 months after termination for any reason. |
| 51 |
| ● | They will not solicit other employees of the Company during the term of employment plus 12 months after termination for any reason. | |
| ● | They will not compete with the business of the Company during the term of employment plus 12 months after termination for any reason. |
Outstanding Option and Stock Awards
The following table presents information concerning unexercised options and unvested restricted stock awards for the named executive officers outstanding as of December 31, 2023.
Outstanding Option and Stock Awards
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||||
| Name (a) | Number of Securities Underlying Unexercised Options (#) Exercisable (b) | Number of Securities Underlying Unexercised Options (#) Unexercisable (c) | Equity Incentive Plan Awards Number of Securities Underlying Unexercised Unearned Options (#) (d) | Option Exercise Price ($) (e) | Option Expiration Date (f) | Number of Shares or Units of Stock That Have Not Vested (#) (g) | Market Value of Shares or Units of Stock That Have Not Vested ($) (h) | Equity Incentive Plan Awards: Number of Unearned Shares, Vested Units or Other Rights That Have Not Vested (#) (i) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) (j) | |||||||||||||||||||||||||
| C. Richard Piazza | 3,125 | - | - | $ | 14.40 | 8/21/31 | - | - | - | - | ||||||||||||||||||||||||
| Executive Chairman | 12,500 | - | $ | 10.00 | 3/3/32 | - | - | - | - | |||||||||||||||||||||||||
| 72,276 | $ | 361,380 | ||||||||||||||||||||||||||||||||
| Douglas Baum | 200 | - | - | $ | 20.00 | 1/14/2025 | - | - | - | - | ||||||||||||||||||||||||
| CEO and Director | 3,125 | - | - | $ | 14.40 | 8/24/31 | - | - | - | - | ||||||||||||||||||||||||
| 12,500 | - | - | $ | 10.00 | 3/3/32 | - | - | - | - | |||||||||||||||||||||||||
| 72,276 | $ | 361,380 | ||||||||||||||||||||||||||||||||
| Christopher Nelson | 255 | - | - | $ | 20.00 | 7/30/24 | - | - | - | - | ||||||||||||||||||||||||
| General Counsel | 60 | - | - | $ | 20.00 | 11/17/25 | - | - | - | - | ||||||||||||||||||||||||
| 2,875 | - | - | $ | 14.40 | 8/24/31 | - | - | - | - | |||||||||||||||||||||||||
| 12,500 | - | - | $ | 10.00 | 3/3/32 | - | - | - | - | |||||||||||||||||||||||||
| 46,365 | $ | 231,825 | ||||||||||||||||||||||||||||||||
| 52 |
Securities Authorized for Issuance under Equity Compensation Plans
Equity Compensation Plan Information.
| Plan Category | Number of Securities to be issued upon exercise of outstanding options, stock appreciation rights and common stock awards* | Weighted-average exercise price of outstanding options, stock appreciation rights and common stock awards | Number of securities remaining available for future issuance under equity compensation plans excluding securities reflected in column (a) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 149,700 | $ | 10.27 | 131,198 | ||||||||
| Equity compensation plans not approved by security holders | 911 | $ | 35.65 | 3,086 | ||||||||
| Total | 150,611 | 134,284 | ||||||||||
| * | Information provided as of December 31, 2023. |
Director Compensation
On January 21, 2022, the Board approved a plan of compensation for independent directors, which provides: an annual retainer of $30,000; additional annual fees of $20,000, $15,000 and $10,000 for serving as Chair of the Audit Committee, Compensation Committee and Nominating & Governance Committee, respectively; and annual fees of $7,500, $5,000 and $3,500 for serving as members of the Audit Committee, Compensation Committee and Nominating & Governance Committee, respectively. Upon appointment to our Board, non-employee directors receive 6,250 stock options, exercisable for 10 years at a price equal to the closing price of our common stock on the date of appointment, and vesting 50% in 12 months and the balance in 24 months. Additional annual option and restricted stock awards are granted at the discretion of the Compensation Committee. All cash fees are annual and accrue quarterly.
On May 23, 2023, the Board approved annual bonus payments for employees and directors payable in common stock of the Company. Pursuant to these resolutions, each of Charles Link and Adriann Sax, independent directors, received 10,455 shares of common stock. These shares vest 50% upon the closing of Company’s Nasdaq uplisting if within 3 years, 50% in eight quarterly installments commencing on the sooner of Nasdaq uplisting or 12 months after issuance, and all vest upon a sale or merger of the Company. In August 2023, the Board approved a transaction bonus to Dr. Link in the instance any of the Company’s assets are sold or sublicensed or if the Company or its subsidiary is acquired, equal to 0.75% of the consideration received by the Company, which is triggered by the Merger closing.
In 2022, the Board approved the following additional stock and stock options issuances to past and present directors:
| ● | On January 15, 2022, Joel Mayersohn received a stock grant of 10,000 common shares for his prior services on the Board. | |
| ● | On January 25, 2022, Adriann Sax received a grant of 6,250 stock options exercisable at $8.00, and vesting half on January 25, 2023, and the balance on January 25, 2024. The options are exercisable for 10 years following the grant date. | |
| ● | On March 3, 2022, Adriann Sax received a grant of 18,750 stock options exercisable at $10.00, and vesting half on March 3, 2023, and the balance on March 3, 2024. The options are exercisable for 10 years following the grant date. | |
| ● | On February 21, 2022, Dr. Link received a grant of 25,000 stock options exercisable at $8.00, and all vested in 2022. The stock options are exercisable for 10 years following the date of grant. |
The following table provides all fees paid to all directors in 2023, but excludes fees paid to the inside directors, Messrs. Baum and Piazza, for their services in 2023 in their respective officer capacities. Messrs. Baum and Piazza did not receive additional fees specifically for their services as directors of the Company.
| Name | Fees Earned or Paid in Cash ($) (1) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Charles J. Link | $ | 82,133 | $ | 52,275 | $ | - | - | $ | - | $ | 134,408 | |||||||||||||||||
| Adriann Sax | $ | 87,083 | $ | 52,275 | $ | - | - | $ | - | $ | 139,358 | |||||||||||||||||
| 53 |
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding the beneficial ownership of the Company’s common stock as of March 15, 2024, unless otherwise noted below for the following:
| ● | each person, or group of affiliated persons, who we know to beneficially own more than 5% of our Common Stock; | |
| ● | each of our named executive officers; | |
| ● | each of our directors; and | |
| ● | all our executive officers, and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to such securities. Except as otherwise indicated, all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. Common stock subject to options exercisable on or within 60 days after March 15, 2024 are deemed outstanding for the purpose of computing the percentage ownership of the person holding those options but are not deemed outstanding for computing the percentage ownership of any other person.
The beneficial ownership of voting securities of the Company is based on 4,445,469 shares of QSAM’s Common Stock issued and outstanding as of March 15, 2024.
| Name of beneficial owner | Amount beneficially owned(1) | Percent of Class Beneficially Owned(5) | ||||||
| 5% or Greater Stockholders | ||||||||
| David H. Clarke | 733,972 | (2) | 16.5 | % | ||||
| Checkmate Capital Group LLC, Checkmate Strategic Capital 2, LLC, Checkmate Strategic Capital Holdings LLC, and Charles Thomas Paschall | 313,291 | (3) | 7.0 | % | ||||
| Strategic Planning Assets Limited | 255,370 | (4) | 5.7 | % | ||||
| Named Executive Officers and Directors | ||||||||
| C. Richard Piazza | 393,487 | 8.8 | % | |||||
| Douglas Baum | 392,674 | 8.8 | % | |||||
| Christopher Nelson | 197,690 | 4.4 | % | |||||
| Adam King | 35,450 | 0.8 | % | |||||
| Charles J. Link, Jr. | 108,929 | 2.4 | % | |||||
| Adriann Sax | 35,455 | 0.8 | % | |||||
| All Officers and Directors (as a group, 6 persons) | 1,163,685 | 26.0 | % | |||||
(1) The number of shares beneficially owned includes any shares over which the person has sole or shared voting power or investment power and also any shares that the person can acquire within 60 days of March 15, 2024, through the exercise of any stock options or other rights. Unless otherwise indicated, each person has sole investment and voting power (or shares such power with his or her spouse) over the shares set forth in the table. For each person, the number of shares that is included in the table because the person has options to acquire the shares is set forth below.
| Name | Option Shares | |||
| C. Richard Piazza | 15,625 | |||
| Douglas Baum | 15,825 | |||
| Christopher Nelson | 15,690 | |||
| Adam King | 12,500 | |||
| Charles J. Link Jr. | 26,375 | |||
| Adriann Sax | 25,000 | |||
(2) The information is based on a Schedule 13G filed with the SEC on February 15, 2024. Mr. Clarke is the Vice-President and Director of GSB Holdings, Inc. (“GSB”) and makes investment decisions on its behalf. Further, Mr. Clarke makes investment decisions on behalf of Bounty Hunter, LLC (“Bounty Hunter”), in his capacity as its managing director and certain shares held of record by his grandson, Mr. August Gaines (“Gaines”). As such, Mr. Clarke may be deemed to be the beneficial owner of the shares of the Company held by each of GSB (621,744), Bounty Hunter (58,672) and Gaines (13,334). Except for the 40,222 shares that Mr. Clarke owns, all the remaining shares are owned by GSB, Bounty Hunter and Gaines. Mr. Clarke disclaims beneficial ownership with respect to shares of common stock of the Company not held by him. The address for each holder is 14179 Laurel Trail, Wellington, FL 33414.
(3) The information is based on a Schedule 13D filed with the SEC on February 20, 2024. The address for each holder is 595 E. Colorado Blvd., Suite 530, Pasadena, CA 91101.
(4) The information is based on a Schedule 13G filed with the SEC on February 20, 2024. The address for the holder is Rm 1201, 12/F Wing On Centre 111 Connaught Road Central, Hong Kong.
(5) The percentages shown are based on the 4,445,469 shares of our common stock outstanding as of March 15, 2024, plus the number of shares that the named person or group has the right to acquire within 60 days of March 15, 2024. For purposes of computing the percentages of outstanding shares of common stock held by each person, any shares that the person has the right to acquire within 60 days after March 15, 2024 are deemed to be outstanding with respect to such person but are not deemed to be outstanding for the purpose of computing the percentage of ownership of any other person. We also deemed outstanding any shares issued to officers and directors that contain a forfeiture clause.
| 54 |
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTORS INDEPENDENCE
Transactions with Related Persons
A transaction may be a related person transaction if any of our directors, executive officers, owners of more than 5% of our common stock, or their immediate family had a material interest in a transaction in the last fiscal year or in any currently proposed transaction in which the Company was or is to be a participant, and the amount involved exceeds the lesser of $120,000 or 1% of the average of the Company’s total assets at year end for the last two completed fiscal years. The Company engaged in or expects to engage in the following related persons transactions during the period set forth above:
C. Richard Piazza, our Executive Chairman, also served as the President of IGL Pharma Inc., the licensor of the Company’s drug technology, through 2023; and a consultant to IsoTherapeutics Group, LLC, the inventors of the technology. Mr. Piazza received a $500 monthly fee from IGL Pharma and, to our knowledge, holds options to acquire less than 1% of that company. As of January 10, 2024, the Company was informed that Mr. Piazza was no longer serving as President of IGL Pharma.
GSB Holdings, Inc., which holds approximately 14% equity in the Company, had a month-to-month Consulting Agreement with the Company through most of 2023 pursuant to which it received $12,000 and 5,000 shares of common stock per month. GSB Holdings is controlled by David H. Clarke but maintains no equity interest in GSB Holdings, Inc. The Consulting Agreement was canceled by mutual agreement of the parties as of November 30, 2023.
The Company’s Executive Chairman, CEO, General Counsel, and VP-Operations, pursuant to and subject to the terms of their respective employment agreements, and one Director pursuant to and subject to the terms of a separate agreement, are entitled to receive transaction bonuses in the instance any of the Company’s assets are sold or sublicensed or if the Company or QSAM Therapeutics, its subsidiary, is acquired, equal in the aggregate to 6.25% of the consideration received by the Company (the “Transaction Bonuses”). The Transaction Bonuses will apply to the Merger with Telix and will be calculated after payment of fees and expenses including the payment of a license fee to the Company’s licensor. The total value of these Transaction Bonuses as they apply to the Merger is currently estimated to be approximately $1,965,313 in total payments, not including any payments that may be received in the future pursuant to the CVR Agreement.
Policies and Procedures for Related Party Transactions
Our Audit Committee has the primary responsibility for the review, approval and oversight of any “related party transaction,” which is any transaction, arrangement, or relationship (or series of similar transactions, arrangements, or relationships) in which we are, were, or will be a participant and the amount involved exceeds $120,000, and in which the related person has, had, or will have a direct or indirect material interest. In approving or rejecting the proposed transactions, our Audit Committee will take into account all of the relevant facts and circumstances available. No member of the Audit Committee will participate in any review, consideration or approval of any related person transaction with respect to which such member or any of his or her immediate family members is the related person.
Director Independence
While we are not subject to NASDAQ rules, the Board uses the standards set forth by NASDAQ for determination of director independence. Our current board members consist of C. Richard Piazza, Douglas Baum, Charles Link Jr. and Adriann Sax. Two of our currently serving Board members, Dr. Link and Ms. Sax, are independent under director independence standards set forth by NASDAQ. For membership information of our audit and compensation committee, and each of their independence status, please see “Corporate Governance” beginning on page 48 of this annual report.
ITEM 14: PRINCIPAL ACCOUNTANT FEES AND SERVICES
CHANGE IN INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
As reported on our Current Report on Form 8-K filed with the SEC on May 12, 2023, the Audit Committee approved the dismissal of D. Brooks and Associates, CPAs, P.A. (“D. Brooks”) as our independent registered public accounting firm effective as of that date.
D. Brooks’ audit reports on our consolidated financial statements for the fiscal years ended December 31, 2022 and December 31, 2021 did not contain any adverse opinion or disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope, or accounting principles.
During the fiscal years December 31, 2022 and December 31, 2021, and the subsequent interim period from January 1, 2023 through May 12, 2023, there were (i) no disagreements within the meaning of Item 304(a)(1)(iv) of Regulation S-K between us and D. Brooks on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to D. Brooks’ satisfaction, would have caused D. Brooks to make reference to the subject matter of the disagreements in connection with its reports on the Company’s consolidated financial statements for such years and (ii) no “reportable events” within the meaning of Item 304(a)(1)(v) of Regulation S-K.
We previously provided D. Brooks with a copy of the disclosures above and requested that D. Brooks furnish us with a letter addressed to the SEC stating whether it agrees with the statements and, if not, stating the respects in which it does not agree. A copy of D. Brooks’s letter, dated May 12, 2023, was filed as Exhibit 16 with our Current Report on Form 8-K filed with the SEC on May 12, 2023.
On May 12, 2023, the Audit Committee approved the appointment of Assurance Dimensions, Inc. (“Assurance Dimensions”) as our independent registered public accounting firm to perform independent audit services for the fiscal year ending December 31, 2023. During the fiscal years ended December 31, 2022 and December 31, 2021, and the subsequent interim period from January 1, 2023 through May 12, 2023, neither we nor anyone acting on our behalf consulted Assurance Dimensions regarding either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered with respect to our consolidated financial statements, in any case where a written report or oral advice was provided to us by Assurance Dimensions that Assurance Dimensions concluded was an important factor considered by us in reaching a decision as to any accounting, auditing or financial reporting issue; or (ii) any matter that was the subject of a “disagreement” within the meaning of Item 304(a)(1)(iv) of Regulation S-K or a “reportable event” within the meaning of Item 304(a)(1)(v) of Regulation S-K.
PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following is a summary of the fees billed and unbilled to the Company by our independent registered public accounting firm for professional services rendered for 2023 and 2022:
| Fee Category | 2023 | 2022 | ||||||
| Audit Fees | $ | 100,000 | $ | 77,643 | ||||
| Audit-related Fees | - | |||||||
| Tax Fees | $ | - | - | |||||
| All Other Fees | - | - | ||||||
Audit fees - Consists of fees for professional services rendered by and paid to Assurance Dimensions in 2023 and rendered by and paid to D. Brooks and Associates CPAs, P.A. in 2022 for the audit of our annual consolidated financial statements, and the review of interim consolidated financial statements included in our quarterly reports and services that are normally provided by our principal accountants in connection with statutory and regulatory filings or engagements.
Audit-related fees - Consists of fees for assurance and related services by our principal accountants that are reasonably related to the performance of the audit or review of the Company’s consolidated financial statements and are not reported under “Audit fees.”
Tax fees - Consists of fees for professional services rendered by our principal accountants for tax compliance, tax advice and tax planning.
All other fees - Consists of fees for products and services provided by our principal accountants, other than the services reported under “Audit fees,” “Audit-related fees” and “Tax fees” above.
The audit fees for 2023 and 2022 were not reviewed or approved by our Audit Committee.
| 55 |
PART IV
ITEM 15: EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| (a)(1) | Financial Statements. |
| The following financial statements of QSAM Biosciences Inc., together with the report thereon of Assurance Dimensions, an independent registered public accounting firm, are included in this Annual Report on Form 10-K: |
| (a)(2) | Financial Statement Schedules. |
| All schedules have been omitted because they are not required or because the required information is given in the Financial Statements or Notes thereto set forth under Item 8 above. |
| (a)(3) | Exhibits. |
| 56 |
| 101 | The following information from the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, formatted in Inline XBRL (eXtensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Income, (iii) the Consolidated Statements of Comprehensive Income, (iv) the Consolidated Statements of Changes in Shareholders’ Equity, (v) the Consolidated Statements of Cash Flows, and (vi) the Notes to Consolidated Financial Statements. | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
| 57 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| QSAM BIOSCIENCES, INC. | ||
| Date: March 20, 2024 | By: | /s/ Douglas Baum |
| Douglas Baum | ||
| Director and Chief Executive Officer | ||
| Date: March 20, 2024 | By: | /s/ Adam King |
| Adam King | ||
| Chief Financial Officer and Principal Accounting Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Date: March 20, 2024 | By: | /s/ C. Richard Piazza |
| C. Richard Piazza | ||
| Executive Chairman of the Board of Directors | ||
| Date: March 20, 2024 | By: | /s/ Douglas Baum |
| Douglas Baum | ||
| Director and Chief Executive Officer | ||
| Date: March 20, 2024 | By: | /s/ Charles J. Link Jr. |
| Charles J. Link Jr. | ||
| Director | ||
| Date: March 20, 2024 | By: | /s/ Adriann Sax |
| Adriann Sax | ||
| Director |
| 58 |
Report of Registered Independent Public Accounting Firm

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of QSAM Biosciences, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of QSAM Biosciences, Inc. (the Company) as of December 31, 2023, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for year ended December 31, 2023, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023, and the results of its operations and its cash flows for year ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company had a net loss and net cash used in operating activities of $4,393,126 and $2,848,136, respectively, for the year ended December 31, 2023, and an accumulated deficit of approximately $40,158,601, respectively, as of December 31, 2023. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Emphasis of Matter
As disclosed in Note 1, the Company signed a merger agreement with Telix Pharmaceuticals Limited on February 7, 2024, and expects the transaction to close in the first half of 2024. Our opinion is not modified with respect to this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
We did not identify any critical audit matters that need to be communicated.
|
|
| We have served as the Company’s auditor since 2023. | |
| March 20, 2024 |
| F-1 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of QSAM Biosciences, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of QSAM Biosciences, Inc (f/k/a Q2Earth, Inc., and referred to as the “Company”) as of December 31, 2022, and the related consolidated statement of operations, stockholders’ deficit, and cash flows for the year then ended and the related notes to the consolidated financial statements (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has incurred operating losses, has incurred negative cash flows from operations and has an accumulated deficit. These and other factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plan regarding these matters is also described in Note 2 to the consolidated financial statements. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits include performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| We have served as the Company’s auditor from 2019 to 2023. | |
 |
|
| Palm Beach Gardens, FL | |
| March 29, 2023 |

| F-2 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses and other assets | ||||||||
| Other assets | ||||||||
| Deferred offering costs | ||||||||
| TOTAL CURRENT ASSETS | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Accrued payroll and related expenses | ||||||||
| Accrued Series B preferred stock dividends | ||||||||
| Notes payable, net of discount | ||||||||
| Notes payable - related parties | ||||||||
| TOTAL CURRENT LIABILITIES | ||||||||
| Notes payable, net of discount | ||||||||
| Total Liabilities | ||||||||
| Redeemable convertible preferred stock - Series A; $ par value, designated Series A, and shares issued and outstanding (liquidation preference of $ | ||||||||
| Stockholders’ Equity (Deficit) | ||||||||
| Preferred stock, Series B, $ par value; shares authorized, shares issued and outstanding (liquidation preference of $ | ||||||||
| Common stock, $ par value, shares authorized, and shares issued and outstanding as of December 31, 2023 and December 31, 2022, respectively | ||||||||
| Unearned deferred compensation | ( | ) | ( | ) | ||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total Stockholders’ Equity (Deficit) | ( | ) | ||||||
| Total Liabilities & Stockholders’ Equity (Deficit) | $ | $ | ||||||
See notes to the consolidated financial statements.
| F-3 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the years ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| REVENUES | $ | $ | ||||||
| Operating Expenses | ||||||||
| Compensation and related expenses | ||||||||
| Professional Fees | ||||||||
| General and administrative | ||||||||
| Research and development | ||||||||
| Total Operating Expenses | ||||||||
| Loss from Operations | ( | ) | ( | ) | ||||
| Other Income (Expense) from operations | ||||||||
| Financing costs including interest | ( | ) | ( | ) | ||||
| Inducement interest | ( | ) | ||||||
| Gain on conversion of convertible debt | ||||||||
| Total Other Expenses, net | ( | ) | ( | ) | ||||
| Loss from operations before income taxes | ( | ) | ( | ) | ||||
| Income Taxes | ||||||||
| NET LOSS | ( | ) | ( | ) | ||||
| PREFERRED STOCK | ||||||||
| Series A convertible contractual dividends | ( | ) | ( | ) | ||||
| Series B convertible contractual dividends | ( | ) | ( | ) | ||||
| Deemed dividend warrant modification | ( | ) | ( | ) | ||||
| Deemed dividends from Series A and B conversion price reduction | ( | ) | ( | ) | ||||
| NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS | $ | ( | ) | $ | ( | ) | ||
| NET LOSS PER SHARE ATTRIBUTABLE TO COMMON STOCKHOLDERS: BASIC AND DILUTED: | $ | ) | $ | ) | ||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: BASIC AND DILUTED | ||||||||
See notes to the consolidated financial statements.
| F-4 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Preferred Stock | Common Stock | Additional Paid In | Deferred Stock-based | Stock | Accumulated | Total Stockholders’ | ||||||||||||||||||||||||||||||
| Shares | Value | Shares | Value | Capital | Compensation | Subscription | Deficit | Equity (Deficit) | ||||||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | | $ | $ | | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||||
| Common stock issued for services, including a director | - | ( | ) | |||||||||||||||||||||||||||||||||
| Conversion of debentures and accrued expenses | - | |||||||||||||||||||||||||||||||||||
| Incremental value from warrant modifications | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Deemed dividend from Series A conversion price adjustment | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Deemed dividend from Series B conversion price adjustment | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Series B, preferred stock contractual dividends | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Accretion of stock-based compensation to employees and directors | - | |||||||||||||||||||||||||||||||||||
| - | ||||||||||||||||||||||||||||||||||||
| Issuance of common stock for cash | - | |||||||||||||||||||||||||||||||||||
| Conversion of deferred employee compensation | - | |||||||||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2022 | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | ||||||||||||||||||||||
| Common stock issued for services | - | |||||||||||||||||||||||||||||||||||
| Incremental value from warrant modifications | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Deemed dividend from Series A conversion price adjustment | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Deemed dividend from Series B conversion price adjustment | - | - | ( | ) | ||||||||||||||||||||||||||||||||
| Series A, preferred stock contractual dividends | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Series B, preferred stock contractual dividends | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Accretion of stock-based compensation to employees and directors | - | ( | ) | |||||||||||||||||||||||||||||||||
| Issuance of common stock for cash | - | |||||||||||||||||||||||||||||||||||
| Sale of exclusivity option to Telix | - | - | ||||||||||||||||||||||||||||||||||
| Exercise of warrants for cash to common stock | - | |||||||||||||||||||||||||||||||||||
| Inducement for conversion of convertible debt | - | - | ||||||||||||||||||||||||||||||||||
| Conversion of Series A, preferred stock to common stock | - | |||||||||||||||||||||||||||||||||||
| Cashless exercise of warrants to common stock | - | ( | ) | |||||||||||||||||||||||||||||||||
| Conversion of convertible debt to common stock | - | |||||||||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2023 | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | |||||||||||||||||||||||||
See notes to the consolidated financial statements.
| F-5 |
QSAM BIOSCIENCES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the years ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash provided by operations: | ||||||||
| Stock-based compensation for services and warrant modification | ||||||||
| Stock-based compensation to employees and directors | ||||||||
| Amortization of debt issuance costs | ||||||||
| Inducement expense | ||||||||
| Gain on conversion of convertible debt | ( | ) | ||||||
| Changes in operating assets and liabilities | ||||||||
| Decrease (Increase) in prepaid expenses and other current assets | ( | ) | ||||||
| Increase (Decrease) in accounts payable and accrued expenses | ( | ) | ||||||
| Increase (Decrease) accrued payroll and related expenses | ( | ) | ||||||
| Decrease in accrued interest | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Deferred offering costs | ||||||||
| Proceeds from warrant exercise | ||||||||
| Proceeds from exclusivity option sold to Telix | ||||||||
| Proceeds from issuance of common stock | ||||||||
| Net cash provided by financing activities | ||||||||
| NET INCREASE (DECREASE) IN CASH | ( | ) | ||||||
| CASH - Beginning of year | ||||||||
| CASH - End of year | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOW DISCLOSURES: | ||||||||
| Payment of interest in cash | $ | $ | ||||||
| Payment of income taxes | $ | $ | ||||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Accrual of contractual dividends on Series A convertible preferred stock | $ | $ | ||||||
| Accrual of contractual dividends on Series B convertible preferred stock | $ | $ | ||||||
| Incremental value on Series A modification | $ | $ | ||||||
| Deemed dividend on warrant modifications | $ | $ | ||||||
| Deemed dividend on Series A conversion price modifications | $ | $ | ||||||
| Deemed dividend on Series B conversion price modifications | $ | $ | ||||||
| Conversion of debentures and accrued expenses to common stock | $ | $ | ||||||
| Conversion of accrued salary and bonus to common stock | $ | $ | ||||||
| Conversion of convertible debt and accrued interest to common stock | $ | $ | ||||||
| Conversion of Series A preferred stock to common stock | $ | $ | ||||||
| Deferred Compensation for employees and consultants | $ | $ | ||||||
Subscription receivable for proceeds from sale of common stock | $ | $ | ||||||
See notes to the consolidated financial statements.
| F-6 |
QSAM BIOSCIENCES INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
QSAM
Biosciences Inc. (hereinafter the “Company”, “we”, “our”, “us”), incorporated in Delaware
on August 26, 2004, is currently engaged in the business of developing a novel radiopharmaceutical drug candidate for the treatment of
bone cancer. This business line commenced in earnest in the fourth fiscal quarter of 2020 as a result of the separation and transfer
pursuant to an Omnibus Separation Agreement dated November 6, 2020 (the “Separation Agreement”) of the Company’s prior
business in a separate sector (the “Legacy Business”) through an unconsolidated investee entity called Earth Property Holdings
LLC, a Delaware limited liability company (“EPH”). Pursuant to the Separation Agreement, the Company transferred to EPH all
assets and related liabilities in connection with the Legacy Business in return for a forgiveness of debt. The Company sold its entire
equity interest in EPH to a third party in the first quarter of 2021 for $
In April 2020, the Company established QSAM Therapeutics Inc. (“QSAM”) as a wholly-owned subsidiary incorporated in the state of Texas, and through QSAM, executed a Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) with IGL Pharma, Inc. (“IGL”). The License Agreement provides QSAM with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and knowhow with respect to Samaium-153 DOTMP aka CycloSam® (the “Technology”), a clinical stage novel radiopharmaceutical meant to treat different types of bone cancer.
In connection with the transition to the life sciences sector, the Company changed its name to QSAM Biosciences Inc. on September 4, 2020, and subsequently changed its stock symbol to QSAM, to better reflect its business moving forward.
On
March 4, 2022, the
On February 7, 2024, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Telix”), and certain subsidiaries of Telix established for the purpose of completing a reverse triangular merger with a second forward merger (collectively, the “Merger”). The Merger is expected to close in the first half of 2024, at which time all of the Company’s assets and operations will be owned by Telix and the Company will cease to exist as a separate corporate entity. See Note 10 - Subsequent Events.
NOTE 2 – BASIS OF PRESENTATION AND GOING CONCERN
For
the year ended December 31, 2023, the Company used net cash in operating activities for its operations of $
The
Company has supported operations through the issuance of common stock, preferred stock and debt over the last 12 months. This includes
a common stock and warrant offering which closed March 31, 2023, of which a total of $
On November 14, 2023, we signed a non-binding term sheet with Telix with respect to the Merger. Upon execution of that term sheet, Telix paid us $2 million as an Option and Pre-Closing Collaboration Fee, and to assure 60 days of exclusivity as they completed their due diligence and the parties negotiated the definitive Merger Agreement (the “Telix Option Fee”). If the Merger does not close, the Telix Option Fee will be automatically converted to QSAM common stock at a price of $6.70 per share. The Company is accounting for this as additional paid in capital akin to a prepaid warrant.
| F-7 |
Management expects expenses to increase through the end of 2024 as our drug technology continues through clinical trials, and as a result, if the Merger does not close in the first half of 2024, we will need to raise additional capital to support these operations. If the Company is not successful in raising additional capital, it may need to delay clinical trials, reduce overhead, or in the most extreme scenario, shut down operations.
There is no guarantee that the Merger will close, or whether the Company will be able to generate revenue and/or raise capital sufficient to support its continuing operations. The ability of the Company to continue as a going concern is dependent on management’s plans which include implementation of its business model to develop and commercialize its drug candidate, seek strategic partnerships to advance clinical trials and other research endeavors which could provide additional capital to the Company, and continue to raise funds for the Company through equity or debt offerings. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. The consolidated financial statements do not include any adjustments that might result from the outcome of these uncertainties.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of QSAM Biosciences Inc. and its wholly-owned subsidiaries QSAM Therapeutics Inc. and Q2Power Corp. (Q2Power was inactive throughout 2023 and dissolved on January 11, 2024, see Note 10 – Subsequent Events). All significant inter-company transactions and balances have been eliminated in consolidation. References herein to the Company include the Company and its Subsidiaries unless the context otherwise requires.
Cash and Cash Equivalents
The
Company considers cash, short-term deposits, and other investments with original maturities of no more than ninety days when acquired
to be cash and cash equivalents for the purposes of the statement of cash flows. The Company maintains cash balances at one national
financial institution and has experienced no losses with respect to amounts on deposit. Any loss incurred or a lack of access to such
funds above the FDIC limit could have a significant adverse impact on the Company’s financial condition, results of operations
and cash flows. The Company held
Revenue Recognition
The Company recognizes revenue in accordance with ASC Topic 606, “Revenue from Contracts with Customers (“ASC 606”) and all the related amendments.
The core principle of ASC 606 requires that an entity recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the company expects to be entitled in exchange for those goods or services. ASC 606 defines a five-step process to achieve this core principle and, in doing so, it is possible more judgment and estimates may be required within the revenue recognition process than previously required under U.S. GAAP, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation.
The Company had revenue in 2023 and 2022.
The Company applies the fair value method of Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 718, “Share Based Payment”, in accounting for its stock-based compensation with employees and non-employees. This standard states that compensation cost is measured at the grant date based on the fair value of the award and is recognized over the service period, which is usually the vesting period. The Company values stock-based compensation at the market price for the Company’s common stock and other pertinent factors at the grant date.
| F-8 |
The Black-Scholes option pricing valuation method is used to determine fair value of stock options consistent with ASC 718, “Share Based Payment”. Use of this method requires that the Company make assumptions regarding stock volatility, dividend yields, expected term of the awards and risk-free interest rates.
Research and Development
Research
and development costs are expensed as incurred. Research and development costs were $
Fair Value Measurement
The Company has determined the fair value of certain assets and liabilities in accordance with generally accepted accounting principles, which provides a framework for measuring fair value. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques should maximize the use of observable inputs and minimize the use of unobservable inputs.
A fair value hierarchy has been established, which prioritizes the valuation inputs into three broad levels. Level 1 inputs consist of quoted prices in active markets for identical assets or liabilities that the reporting entity has the ability to access at the measurement date. Level 2 inputs are inputs other than quoted prices included within Level 1 that are observable for the related asset or liability. Level 3 inputs are unobservable inputs related to the asset or liability.
Income Taxes
Income taxes are accounted for under the asset and liability method as stipulated by FASB ASC 740, “Income Taxes” (“ASC 740”). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under ASC 740, the effect on deferred tax assets and liabilities or a change in tax rate is recognized in income in the period that includes the enactment date. Deferred tax assets are reduced to estimated amounts to be realized by the use of a valuation allowance. A valuation allowance is applied when in management’s view it is more likely than not (50%) that such deferred tax will not be utilized.
In the event that an uncertain tax position exists in which the Company could incur income taxes, the Company would evaluate whether there is a probability that the uncertain tax position taken would be sustained upon examination by the taxing authorities. Reserves for uncertain tax positions would be recorded if the Company determined it is probable that a position would not be sustained upon examination or if payment would have to be made to a taxing authority and the amount is reasonably estimated.
As of December 31, 2023, the Company does not believe it has any uncertain tax positions that would result in the Company having a liability to the taxing authorities. Interest and penalties related to any unrecognized tax benefits is recognized in the consolidated financial statements as a component of income taxes.
Net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period. Diluted net loss per share is calculated by dividing the net loss attributable to common stockholders by the weighted average number of common shares outstanding during the period plus any potentially dilutive shares related to the issuance of stock options, shares from the issuance of stock warrants, shares issued from the conversion of convertible preferred stock and shares issued for the conversion of convertible debt.
| F-9 |
| Shares from common stock options | ||||
| Shares from Telix option fee conversion | ||||
| Shares from the conversion of Series A Stock inclusive of cumulative dividends | ||||
| Shares from the conversion of Series B Preferred Stock inclusive of dividends (1) | ||||
| (1) |
As
of December 31, 2022, there were the following potentially dilutive securities that were excluded from diluted net loss per share because
their effect would be anti-dilutive (
| Shares from common stock options | ||||
| Shares from common stock warrants | ||||
| Shares from the conversion of convertible notes and accrued interest | ||||
| Shares from the conversion of Series A Stock inclusive of cumulative dividends | ||||
| Shares from the conversion of Series B Preferred Stock inclusive of dividends | ||||
Significant Estimates
U.S. Generally Accepted Accounting Principles (“GAAP”) requires the Company to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements, the reported amounts of revenues and expenses, cash flows and the related footnote disclosures during the period. On an on-going basis, the Company reviews and evaluates its estimates and assumptions, including, but not limited to, those that relate to the fair value of stock-based compensation fair value of convertible bridge notes, and a valuation allowance on deferred tax assets and contingencies. Actual results could differ from these estimates.
Recent Accounting Pronouncements
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt — Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging — Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) to simplify accounting for certain financial instruments. ASU 2020-06 eliminates the current models that require separation of beneficial conversion and cash conversion features from convertible instruments and simplifies the derivative scope exception guidance pertaining to equity classification of contracts in an entity’s own equity. The new standard also introduces additional disclosures for convertible debt and freestanding instruments that are indexed to and settled in an entity’s own equity. ASU 2020-06 amends the diluted earnings per share guidance, including the requirement to use the if-converted method for all convertible instruments. ASU 2020-06 is effective January 1, 2022 for public business entities that are not smaller reporting companies and for all other entities, fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. The standard should be applied on a full or modified retrospective basis, with early adoption permitted beginning on January 1, 2021. Effective January 1, 2021, the Company adopted ASU 2020-06 and noted no material impact to the consolidated financial statements.
| F-10 |
Management does not believe that any other recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on its unaudited financial statements.
Concentration of Risk
The
Company expects cash to be the asset most likely to subject the Company to concentrations of credit risk. The Company’s bank deposits
may at times exceed federally insured limits. The Company’s policy is to maintain its cash with high credit quality financial institutions
to limit its risk of loss exposure. The Company’s cash balance as of December 31, 2023 is in excess of FDIC limits in the amount
of approximately $
The Company is subject to a number of risks similar to those of other companies at a clinical-stage for radiopharmaceutical drug candidates, including dependence on key individuals; the need to develop commercially viable therapeutics; competition from other companies, many of which are larger and better capitalized; intellectual property risks; and the need to obtain adequate additional financing to fund the development of its products. The Company currently depends on third-party suppliers for key materials and services used in its research and development manufacturing process, and is subject to certain risks related to the loss of these third-party suppliers or their inability to supply the Company with adequate materials and services. The Company’s primary Technology is licensed from one party and is subject to general risks related to contractual relationships and the performance of the parties under the contract.
Fair Value of Financial Instruments
In accordance with Accounting Standards Codification (“ASC”) 825, Financial Instruments, disclosures of fair value information about financial instruments are required, whether or not recognized in the balance sheet, for which it is practicable to estimate that value. Cash is carried fair value.
Other financial instruments, including accounts payable, accrued liabilities and short-term debt, are carried at cost, which approximates fair value given their short-term nature.
Deferred Offering Cost
Costs incurred prior to an equity offering are capitalized until the offering occurs. Upon the equity offering, all accumulated costs are charged against proceeds. If the Company determines that the equity offering will not occur, the accumulated costs are charged to operations.
Segment Reporting
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. To date, the Company views its operations and manages its business as one segment.
NOTE 4 – RELATED PARTY TRANSACTIONS
The
Company has a License Agreement with IGL Pharma, Inc. (“IGL”), an entity for which, as of December 31, 2023, the Company’s
Executive Chairman serves as President, holds options to purchase less than a
As
of December 31, 2023 and December 31, 2022, amounts outstanding due IGL for their services amounted to $
| F-11 |
During
the year ended December 31, 2020, the Company received $
During
2022, the Company started accruing compensation for the Board of Directors. Each board member receives an annual retainer of $
During
the third quarter of 2023, the Company raised $
NOTE 5 –CONVERTIBLE PROMISSORY NOTES AND DEBENTURES
Convertible Promissory Notes
In
the fourth quarter of 2021, the Company issued a total of $
In
the fourth quarter of 2022, two note holders converted $
During
the year ended December 31, 2023 and 2022, the Company recorded interest expense related to the amortization of the discount of $
Debentures
On
February 22, 2022, the holder of Original Issue Discount Senior Secured Convertible Debentures (the “Debentures”) issued
by the Company in 2014, converted the full remaining balance of $
NOTE 6 – TEMPORARY EQUITY, PREFERRED STOCK, COMMON STOCK, AND WARRANTS
Option and Pre-Closing Collaboration Fee
On
November 14, 2023, we signed a non-binding term sheet with Telix with respect to the Merger. Upon execution of that term sheet, Telix
paid us $
Series A Redeemable Convertible Preferred Stock (“Series A Stock”)
As
of December 31, 2023 and 2022, the Company had and shares of Series A Stock issued and outstanding, respectively.
The Series A Stock has no voting rights until converted to common stock and has a liquidation preference equal to the aggregate purchase
price of the outstanding shares of Series A Stock at $
| F-12 |
In December 2023, the Company signed blanket Conversion Notice and Reservation of Rights Agreements (the “Conversion Notice”) with both of the Series A Stockholders providing that immediately prior to closing the proposed Merger with Telix, all remaining shares of Series A Stock held by such holders plus all accrued dividends would be automatically converted to common stock. In return for this agreement, the Company agreed that it would not seek to redeem the Series A Stock as of January 1, 2024, and the parties waived all existing defaults. The Conversion Notice is non-revokable but expires upon the sooner of the closing of the Merger or 12 months from the date of signing, and as a result, the new Redemption Date under the Series A Stock was reset to December 15, 2024.
As of February 8, 2024, all shares of Series A Stock plus all accrued dividends had been converted to common stock and the Series A Stock was retired in full (see Note 10 – Subsequent Events).
The
Series A Stock carries a
The
outstanding principal and accrued dividends on the shares of Series A Stock as of December 31, 2023 are convertible into the Company’s
common stock at $
Prior
to the last Conversion Price adjustment, the outstanding shares of Series A Stock as of December 31, 2022 were convertible at $
Management has determined that the Series A Stock is more akin to a debt security than equity primarily because it contains a mandatory 2-year redemption date at the option of the holder (which pursuant to the December 2023 modification signed by both Series A Stockholders, was changed to December 31, 2024 from December 31, 2023), which only occurs if the Series A Stock is not converted to common stock. Therefore, management has presented the Series A Stock outside of permanent equity as mezzanine equity, which resides between liabilities and equity.
| F-13 |
Series B Convertible Preferred Stock (“Series B Stock”)
In
December 2020, the Company filed an amendment to its Articles of Incorporation to authorize the issuance of up to shares of Series
B Stock, par value $ per share, pursuant to a Certificate of Designation (“Series B Designation”). The Series B Stock
provides the holders a
The
Series B Stock was originally convertible into common stock at a ratio of $
In
the period ended March 31, 2023, the anti-dilution protections were triggered as a result of additional common stock issuances and the
warrant conversion repricing, and the conversion ratio was reduced to $
In
the period ended September 30, 2023, the anti-dilution protections were triggered as a result of additional common stock issuances, and
the conversion ratio was reduced to $
As of October 31, 2023, all remaining Series B Stockholders signed agreements with the Company providing that if the Company lists its common shares on Nasdaq or otherwise signs an agreement to be acquired, such shareholders will automatically and without any further action on their part exchange their shares of Series B Stock for common shares at an exchange ratio of $, inclusive of all accrued dividends through September 30, 2023. In the instance of an acquisition of the Company, such exchange into common shares at the reduced price is in lieu of any contracted liquidation preference payments. As a result of the signing of the Merger Agreement with Telix, on February 6, 2024 all shares of Series B Stock plus all accrued dividends were exchanged into shares of common stock (see Note 10 – Subsequent Events).
Common Stock
| For the Years Ended December 31, | ||||||||
| 2023 | 2022 | |||||||
| Adjustment for 40:1 Reverse Stock Split | ||||||||
| Conversion of debentures and accrued interest | ||||||||
| Conversion of accrued salary to management | ||||||||
| Exercise of common stock warrants to common stock | ||||||||
| Stock based compensation to employees and directors | ||||||||
| Cashless exercise of service warrants into common stock | ||||||||
| Conversion of Series A preferred stock to common stock | ||||||||
| Conversion of convertible debt and interest to common stock | ||||||||
| Issuance of common stock for cash | ||||||||
| Stock based compensation for consulting services | ||||||||
| Total common shares issued | ||||||||
| F-14 |
During
the year ended December 31, 2023, the Company issued common stock for cash in private placement offerings as follows: shares of
common stock for $
As
of March 31, 2023, the Company converted outstanding convertible debt of $
During
the year ended December 31, 2023, the Company converted $
During
the year ended December 31, 2023, the Company issued shares of common stock through a cashless exercise of
Additionally, during the year ended December 31, 2023, the Company issued shares to board members and employees for their annual bonus as deferred compensation until vested. The awards are subject to vesting and forfeiture conditions including satisfaction of certain performance-based milestones, as follows:
Further,
the Company issued immediately vested unregistered shares of common stock to its Chief Financial Officer in lieu of deferred cash
compensation of approximately $
For the years ended December 31, 2023 and 2022, the Company recognized stock-based compensation to employees and directors totaling $ and $, respectively, related to common stock that was exchanged on December 6, 2021 from Series E-1 Preferred Stock (that was concurrently retired), which is included in compensation and related expenses on the consolidated statements of operations. As of December 31, 2023, there is no unrecognized compensation remaining from these common shares.
During
the year ended December 31, 2022, $
Warrants
During
the year ended December 31, 2023, the Company issued
| F-15 |
A summary of warrant activity and related information for the year ending December 31, 2023 is as follows:
| Warrants | Weighted Average Exercise Price | Aggregate Intrinsic Value | ||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||
| Issued | ||||||||||||
| Exercised | ( | ) | - | |||||||||
| Terminated in connection with cashless exercise | ( | ) | $ | - | ||||||||
| Expired | ( | ) | - | |||||||||
| Outstanding as of December 31, 2023 | $ | $ | ||||||||||
The
aggregate intrinsic value of the warrants is the difference between the fair market value of the Company’s closing price of its
common stock at each reporting date, less the exercise price multiplied by the number of warrants outstanding, which was $
In 2016 to compensate officers, directors and other key service providers with equity grants, the Board approved the 2016 Omnibus Equity Incentive Plan (“2016 Plan”), which initially allowed for shares of common stock, stock options, stock rights (restricted stock units), or stock appreciation rights to be granted by the Board in its discretion. This authorized amount was increased multiple times by Board resolution, most recently to shares on May 23, 2023. As of December 31, 2023, there are stock options issued, restricted common shares issued, and shares available under the 2016 Plan for future issuance; however, the Board may increase the authorized shares under the 2016 Plan each year to an amount equal to % of the total issued and outstanding common shares of the Company or such other amount in its reasonable discretion.
The Company has two prior equity plans still active: the 2014 Employee Stock Option Plan and the 2014 Founders Stock Option Plan (the “Legacy Plans”), which have a total of and options issued thereunder, respectively, and have and shares available for future issuance, respectively.
The Company did not issue any stock options during the year ended December 31, 2023; however, it did issue shares of restricted stock under the 2016 Plan in May 2023 (see “Common Stock” above).
| Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||||||
| Outstanding as of December 31, 2022 | $ | $ | ||||||||||||||
| Expired (1) | ( | ) | $ | $ | - | |||||||||||
| Granted | $ | - | $ | - | ||||||||||||
| Outstanding as of December 31, 2023 | $ | $ | ||||||||||||||
| (1) |
The
Company recorded $
The aggregate intrinsic value of options is the difference between the fair market value of the Company’s closing price of its common stock at each reporting date, less the exercise price multiplied by the number of options granted, which was $ at December 31, 2023.
| F-16 |
As of December 31, 2023, there was unrecognized stock-based compensation of $ which is expected to be expensed through March 2026 based on the restricted stock vesting requirements.
We estimate the fair value of stock-based awards on the date of grant using the Black-Scholes option pricing model using the fair market value of our common stock on the date of grant and a number of other assumptions. These assumptions include estimates regarding the expected term of the awards, estimates of the stock volatility over a duration that approximates the expected term of the awards, estimates of the risk-free rate, and estimates of expected dividend rates.
NOTE 8 – INCOME TAXES
A
reconciliation of the differences between the effective income tax rates and the statutory federal tax rates for the years ended December
31, 2023 and 2022 (computed by applying the U.S. Federal corporate tax rate of
| 2023 | 2022 | |||||||
| Tax benefit at U.S. statutory rate | $ | ( | ) | $ | ( | ) | ||
| State taxes, net of federal benefit | ||||||||
| Stock based compensation | ||||||||
| Meals & Entertainment | ||||||||
| Gain on extinguishment of liabilities | ( | ) | ||||||
| Other permanent differences | ( | ) | ||||||
| Change in valuation allowance | ||||||||
| $ | $ | |||||||
The tax effect of temporary differences that give rise to significant portions of the deferred tax assets and liabilities for the years ended December 31, 2023 and 2022 consisted of the following:
| 2023 | 2022 | |||||||
| Net operating loss carry-forward | $ | $ | ||||||
| Accrued expenses | ||||||||
| Stock based compensation | ||||||||
| Section 174 R&D expenses | ||||||||
| Charitable contribution | ||||||||
| Net deferred tax assets | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Total net deferred tax asset | $ | $ | ||||||
At
December 31, 2023 and 2022, the Company had net deferred tax assets of $
The Company’s U.S. federal and state income tax returns are generally subject to tax examinations for the tax years ended December 31, 2020 through December 31, 2022. There are currently no pending income tax examinations. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service and state tax authorities to the extent utilized in a future period. The Company’s policy is to record interest and penalties related to income taxes as part of its income tax provision.
The Company’s NOL and tax credit carryovers may be significantly limited under the Internal Revenue Code (“IRC”). NOL and tax credit carryovers are limited under Section 382 when there is a significant “ownership change” as defined in the IRC. During the year ended December 31, 2023 and in prior years, the Company may have experienced such ownership changes, which could impose such limitations.
| F-17 |
The limitations imposed by the IRC would place an annual limitation on the amount of NOL and tax credit carryovers that can be utilized. When the Company completes the necessary studies, the amount of NOL carryovers available may be reduced significantly. However, since the valuation allowance fully reserves for all available carryovers, the effect of the reduction would be offset by a reduction in the valuation allowance.
NOTE 9 – COMMITMENTS AND CONTINGENCIES
Employment Agreements
Pursuant
to the 2022 Amendments, $
The
shares of common stock issued pursuant to the 2022 Amendments are restricted and shall be subject to forfeiture as follows: until such
time that the Company successfully closes $
During the year ended December 31, 2023, the Company issued shares to employees for their annual bonus and two board members for additional board compensation as deferred compensation until vested. The awards are subject to vesting and forfeiture conditions, including satisfaction of certain performance-based milestones, as follows:
| F-18 |
Board of Director Agreements
In
January 2022, Adriann Sax was appointed as a Director to the Board and awarded an annual retainer of $
In
February 2022, Charles J. Link, Jr. was appointed as a Director to the Board and awarded an annual retainer of $
In May 2023, each of Ms. Sax and Dr. Link received shares of restricted common stock as compensation in lieu of cash. .
License Agreement
The
License Agreement for the Technology, as amended, between the Company’s wholly-owned subsidiary QSAM Therapeutics and IGL is
for
In
connection with the License Agreement, QSAM signed a two-year Consulting and Confidentiality Agreement (the “Consulting Agreement”)
with IGL, which provided IGL with payments of $
During
the year ended December 31, 2023, the Company paid $
As
of December 31, 2023, $
| F-19 |
NOTE 10 – SUBSEQUENT EVENTS
Merger Agreement
On February 7, 2024, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Telix”), Cyclone Merger Sub I, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub I”), Cyclone Merger Sub II, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub II”, and collectively with Merger Sub I, the “Merger Subs”) and David H. Clarke, as stockholder representative to the QSAM stockholders (the “QSAM Stockholder Representative”), pursuant to which, on the terms and subject to the conditions set forth in the Merger Agreement, Telix will acquire the Company through the merger of Merger Sub I with and into the Company, with the Company surviving as a direct, wholly-owned subsidiary of Telix (“First Merger”), and as part of the same overall transaction, the Company will merge with and into Merger Sub II, at which time the Company shall cease to exist and Merger Sub II will remain as the surviving corporation (“Second Merger”, collectively with First Merger, the “Merger”). A full description of the Merger Agreement, inclusive of the other agreements included as exhibits thereto, as well as the agreements themselves, have been included in our Form 8-K filed with the SEC on February 8, 2024.
Our board of directors unanimously approved on February 6, 2024, the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement, and QSAM stockholders adopted and approved, by written consent dated February 6, 2024, the Merger Agreement, the Merger and the other transactions contemplated by the Merger Agreement.
Reverse Stock Split, Options and Preferred Stock
In
connection with and as a condition to the Merger, our Board and a majority of our stockholders on February 6, 2024 approved a
reverse stock split of all the issued and outstanding shares of our common stock, in a ratio between
Pursuant to the Merger Agreement, effective as of the date QSAM files an Information Statement describing the Merger to our stockholders, each then-outstanding and unexercised option to purchase shares of our common stock issued pursuant to any stock incentive or equity-related agreement or plan of QSAM (each such option, a “QSAM Option”) will vest in full and become exercisable up to and through the close of regular trading on the seventh business day after the date the Information Statement is filed (such date, the “Last Exercise Date”) in accordance with the terms and conditions of such QSAM Option, and such QSAM Option will terminate for no consideration and be of no further force or effect as of immediately prior to closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date.
As of February 6, 2024, all holders of our Series B Preferred Stock exchanged the remaining outstanding shares plus all accrued dividends for shares of common stock pursuant to their respective Exchange Agreements signed in October 2023. As of February 8, 2024, all holders of our Series A Preferred Stock converted their remaining shares plus all accrued dividends into common stock, which included conversions into shares in 2023 and shares in 2024. As of the date of this filing, no shares of Series A or Series B Preferred Stock remain outstanding. As of March 19, 2024, there are shares of common stock outstanding, not inclusive of stock options that must be exercised or will terminate as described above.
Amendment to the License Agreement
As
a condition to the execution of the Merger Agreement, QSAM and IGL have entered into the Second Amendment to the License Agreement (the
“Second Amendment”), which provides among other terms: (i) modifications to sublicense fees, royalties and other amounts
payable to IGL; (ii) modifications to the definitions of “Commercially Reasonable Efforts,” “Products” and “Patents”
described in the License Agreement; and (iii) an additional payment to IGL of $
Lock-Up Agreement
Concurrently with the execution of the Merger Agreement, all of our officers, directors, and a key employee have agreed to enter into lock-up agreements (the “Lock-Up Agreements”), pursuant to which they accepted certain restrictions on transfers of shares of the Telix Ordinary Shares they would receive in connection with the Transaction until 12 months after the Closing upon the terms set forth in the Lock-Up Agreements.
Dissolution of Q2Power Corp.
On January 11, 2024, the Company dissolved its wholly-owned subsidiary, Q2Power Corp., with the State of Delaware. This company had previously been inactive.
| F-20 |