UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14C INFORMATION
Information Statement Pursuant to Section 14(c)
of the Securities Exchange Act of 1934
Check the appropriate box:
| ☒ | Preliminary Information Statement |
| ☐ | Confidential, for use of the Commission only (as permitted by Rule 14c-5(d)(2)) |
| ☐ | Definitive Information Statement |
QSAM BIOSCIENCES, INC.
(Name of Registrant As Specified In Its Charter)
Payment of Filing Fee (Check the appropriate box):
| ☐ | No fee required. |
| ☒ | Filing Fee computed on table in exhibit per Exchange Act Rules 14c-5(g) and 0-11. |
| ☐ | Fee paid previously with preliminary materials. |
| ☐ | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| 1) | Amount Previously Paid: | |
| 2) | Form, Schedule or Registration Statement No: | |
| 3) | Filing Party: | |
| 4) | Date Filed: |
PRELIMINARY COPY - SUBJECT TO COMPLETION
QSAM BIOSCIENCES, INC.
9442 Capital of Texas Hwy N, Plaza 1, Suite 500
Austin, TX 78759
NOTICE OF ACTION BY WRITTEN CONSENT AND INFORMATION STATEMENT
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY
To the Stockholders of QSAM Biosciences, Inc.:
This notice of action by written consent and the accompanying information statement (this “Information Statement”) is being furnished by the Board of Directors (the “Board”) of QSAM Biosciences, Inc., a Delaware corporation (“QSAM,” the “Company,” “we,” “us” or “our”), to the holders of record at the close of business on [●, 2024] (the “Record Date”) of the outstanding shares of our common stock, par value $0.0001 per share (“Common Stock” or “QSAM Common Stock”), pursuant to Rule 14c-2 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
The purpose of the accompanying Information Statement is to inform the Company’s stockholders that on February 6, 2024, holders of over 60% of the voting power of capital stock of the Company acted by an irrevocable written consent (the “Written Consent”) in lieu of a special meeting of stockholders to approve: (1) the Agreement and Plan of Merger (the “Merger Agreement”) by and among Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Buyer” or “Telix”), Cyclone Merger Sub I, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub I”), Cyclone Merger Sub II, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub II”, and collectively with Merger Sub I, the “Merger Subs”) and David H. Clarke, as stockholder representative to the QSAM stockholders (the “QSAM Stockholder Representative”); and (2) a reverse stock split of outstanding shares of our Common Stock in a range between 1:1000 and 1:2000 (the “Reverse Split”), and to authorize the Board to determine the exact ratio at its discretion and to effectuate and file an amendment to the certificate of incorporation of the Company (the “Certificate of Amendment”) immediately prior to the consummation of the Merger.
Pursuant to the terms of the Merger Agreement and the Reverse Split, the aggregate consideration that QSAM stockholders will be entitled to receive pursuant to the Merger and the Reverse Split will be equal to:
(i) USD $33.1 million, reduced by (a) the amount of certain of QSAM’s unpaid expenses, indebtedness, change-of-control bonuses, and other payables as of the closing of the Merger, (b) a fee equal to 218,496 ordinary shares of Telix (“Telix Ordinary Shares”) payable to QSAM’s licensor, IGL Pharma, Inc. (“IGL”), upon the closing of the Merger, representing $1,655,000 (or 5% of the aggregate closing consideration) divided by the Buyer Stock Price (defined below), and (c) 66,011 ordinary shares of Telix (“Telix Ordinary Shares”), representing $500,000 divided by the Buyer Stock Price (defined below), as a source of recovery for post-closing purchase price adjustments (collectively, the “Closing Consideration”); and
(ii) contingent value rights (“CVRs”) which will represent the right to receive contingent payments of up to USD $90 million in the aggregate, in cash and/or Telix Ordinary Shares, without interest, upon the achievement of certain milestones, at the times and subject to the terms and conditions of the CVR Agreement (as defined below) (such contingent payments, the “Milestone Payments”).
The Closing Consideration will be paid to holders of whole numbers of shares of QSAM Common Stock in the Merger in the form of Telix Ordinary Shares, except in certain specified circumstances, while payments in connection with the Reverse Split will be paid in cash. The number of shares issuable to QSAM stockholders in the Merger shall be determined by reference to a deemed value of Telix Ordinary Shares equal to USD $7.5745 per share (the “Buyer Stock Price”), representing the volume weighted average price at which Telix Ordinary Shares traded on the Australian Securities Exchange over the ten (10) trading-day period ending on February 6, 2024, the business day prior to the date of the Merger Agreement, as converted from Australian dollars to United States dollars at the exchange rate published in the Wall Street Journal as of February 6, 2024, the business day prior to the date of the Merger Agreement.
In connection with and as a condition to the Merger, QSAM will effect the Reverse Split, in which any outstanding fractional shares of QSAM Common Stock (determined after determining the whole number of shares of QSAM Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (i) the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration and (ii) one (1) CVR for each share of QSAM Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares) pursuant to the Reverse Split. For additional details, see “The Merger Agreement – Merger Consideration” on page 44 of the accompanying Information Statement.
The Company’s Board carefully reviewed and considered the terms and conditions of the Merger Agreement and the transactions contemplated thereby, including the Merger and the Reverse Split (the “Transactions”). The Board (i)(A) determined that the Merger Agreement and the Transactions are fair and in the best interests of the Company and its stockholders, (B) approved and declared advisable the Transactions, on the terms and subject to the conditions set forth in the Merger Agreement, and (C) recommended that the stockholders of the Company approve the Transactions, and (ii) directed that the Transactions be submitted to the holders of the Common Stock for their approval.
The approval of the Transactions by the Company’s stockholders via Written Consent was effected in accordance with the Company’s Certificate of Incorporation, as amended (the “Company Charter”), the Amended and Restated Bylaws of the Company (the “Amended and Restated Bylaws”) and the General Corporation Law of the State of Delaware (the “DGCL”). No further approval of the stockholders under the DGCL is required to complete the Transactions. As a result, the Company has not solicited and will not be soliciting your vote for the Transactions and does not intend to call a meeting of stockholders for purposes of voting on the adoption thereof.
Pursuant to Rule 14c-2 of the Exchange Act, the actions contemplated by the Written Consent may not be taken until [●], 2024, which is 20 calendar days following the date we first mail the accompanying Information Statement to our stockholders.
This notice of action by written consent and the accompanying Information Statement shall constitute notice to you from the Company that the Transactions have been approved by the holders of a majority of the voting power of the Common Stock by written consent in lieu of a special meeting in accordance with Section 228(e) of the DGCL.
QSAM IS NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND QSAM A PROXY.
The Information Statement accompanying this letter provides you with more specific information concerning the Transactions. We encourage you to carefully read the accompanying Information Statement, copies of the Merger Agreement including the annexes and the schedules attached thereto, the Certificate of Amendment, and the other appendices attached to the accompanying Information Statement.
THE INFORMATION STATEMENT IS FOR YOUR INFORMATION ONLY. YOU DO NOT NEED TO DO ANYTHING IN RESPONSE TO THE INFORMATION STATEMENT. THIS IS NOT A NOTICE OF A MEETING OF STOCKHOLDERS AND NO STOCKHOLDER MEETING WILL BE HELD TO CONSIDER ANY MATTER DESCRIBED IN THE INFORMATION STATEMENT.
By Order of the Board of Directors, [●], 2024 | |
| /s/ C. Richard Piazza | |
| C. Richard Piazza, Executive Chairman |
QSAM BIOSCIENCES, INC.
9442 Capital of Texas Hwy N, Plaza 1, Suite 500
Austin, TX 78759
INFORMATION STATEMENT
ABOUT THIS INFORMATION STATEMENT
General
This Information Statement is being furnished by QSAM Biosciences, Inc., a Delaware corporation (“we,” “us”, “our”, “QSAM”, or the “Company”), in connection with action taken by an irrevocable written consent (the “Written Consent”) on February 6, 2024, by the holders of a majority of the voting power of the Company’s issued and outstanding capital stock entitled to vote thereon (the “Majority Stockholders”), approving: (1) the Agreement and Plan of Merger (the “Merger Agreement”) by and among Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia (“Buyer” or “Telix”), Cyclone Merger Sub I, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub I”), Cyclone Merger Sub II, Inc., a Delaware corporation and direct wholly-owned subsidiary of Telix (“Merger Sub II”, and collectively with Merger Sub I, the “Merger Subs”) and David H. Clarke, as stockholder representative to the QSAM stockholders (the “QSAM Stockholder Representative”); and (2) a reverse stock split of outstanding shares of our Common Stock in a range between 1:1000 and 1:2000 (the “Reverse Split”), and to authorize the Board to determine the exact ratio at its discretion and to effectuate and file an amendment to the certificate of incorporation of the Company (the “Certificate of Amendment”) immediately prior to the consummation of the Merger.
Pursuant to the Merger Agreement, the Buyer, the Merger Subs and the Company intend to effect a reorganization in which, as steps in a single, integrated transaction, (a) Merger Sub I will merge with and into the Company, Merger Sub I will cease to exist, and the Company will survive as a direct, wholly owned subsidiary of Buyer (the “First Merger”), and (b) as part of the same overall transaction, the Company will merge with and into Merger Sub II, the Company will cease to exist, and Merger Sub II will survive as a direct, wholly owned subsidiary of Buyer (the “Second Merger” and, collectively with the First Merger, as appropriate, the “Merger”). The closing of the First Merger is referred to herein as the “Closing”, the filing of the certificate of merger in connection with the First Merger, the “First Effective Time”, and the filing of certificate of merger in connection with the Second Merger, the “Second Effective Time.”
Vote Required
As the matters set forth in this Information Statement have been duly authorized and approved by the written consent of the holders of at least a majority of the voting power of the Company’s issued and outstanding capital stock entitled to vote thereon, we are not seeking any consent, vote or authorization. Section 228 of the DGCL provides that any action required or permitted to be taken by the stockholders of the Company may be effected by the consent in writing of the holders of outstanding capital stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted.
Holders of the Company’s Common Stock are entitled to one vote per share.
As of the close of business on the date of Written Consent, 4,387,282 shares of Common Stock were issued and outstanding. The Majority Stockholders together represented an aggregate of 2,694,512 shares of Common Stock, constituting over 60% of the voting power of the Company’s issued and outstanding capital stock. Accordingly, the Written Consent has been executed and delivered to the Company by the Majority Stockholders pursuant to Section 228 of the DGCL.
Notice Requirement
The purpose of this notice and the accompanying Information Statement is to (1) inform the Company’s stockholders of the action described above before it takes effect, in accordance with Rule 14c-2 promulgated under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and (2) provide the notice required under Section 228(e) of the Delaware General Corporation Law (the “DGCL”) of the taking of a corporate action by written consent to stockholders of the Company as of the Record Date who have not consented in writing to such action and who would have been entitled to notice of the meeting if the action had been taken at a meeting. This Information Statement serves as the notice required by Section 228(e) of the DGCL. In accordance with Rule 14c-2 under the Exchange Act, the actions described herein will become effective no earlier than the 20th calendar day after the date on which this definitive Information Statement has been provided to the Company’s stockholders. The Information Statement is being mailed on or about [●], 2024, to the Company’s stockholders of record as of the close of business on the Record Date.
Dissenter’s Rights of Appraisal
Stockholders of the Company’s Common Stock are entitled to exercise dissenter rights with respect to the Merger. These rights can be referenced on page 39 of this Information Statement.
Expenses
We will bear all expenses in connection with the distribution of this Information Statement.
Currency
Unless otherwise specified in this Information Statement, all monetary amounts are in U.S. dollars, all references to “$,” “US$,” “USD” and “dollars” mean U.S. dollars, and all references to “A$” and “AUD” mean Australian dollars.
Table of Contents
| -i- |
This summary highlights selected information appearing elsewhere in this Information Statement and is, therefore, qualified in its entirety by the more detailed information appearing elsewhere in this Information Statement. It may not contain all the information that is important to you. The Company urges you to read carefully this entire Information Statement and the other documents to which it refers to understand fully the terms of the Merger. You should pay special attention to “Risk Factors” and “Cautionary Statement Concerning Forward-Looking Statements.”
The Parties
Telix Pharmaceuticals Limited
Telix Pharmaceuticals Limited (“Telix” or “Buyer”), is a public limited company registered under the laws of the Commonwealth of Australia. Telix is a biopharmaceutical company focused on the development and commercialization of diagnostic and therapeutic radiopharmaceuticals and associated medical devices. Telix is headquartered in Melbourne, Australia with international operations in the United States, Europe (Belgium and Switzerland), and Japan. Telix is developing a portfolio of clinical-stage products that aims to address significant unmet medical needs in oncology and rare diseases. Telix’s ordinary shares are listed on the Australian Securities Exchange (ASX: TLX).
Telix’s principal executive offices are located at 55 Flemington Road, North Melbourne, Victoria, 3051, Australia, and its reception telephone number is +61 3-9093-3855. The corporate website address is www.telixpharma.com. Telix’s website and the information contained on, or that can be accessed through the website, is not deemed to be incorporated by reference in, and is not considered part of, this Information Statement.
Merger Sub I
Cyclone Merger Sub I, Inc., a Delaware corporation (referred to as “Merger Sub I”), is a wholly owned subsidiary of the Buyer. Merger Sub I was formed by the Buyer solely in contemplation of the Merger, has not conducted any business and has no assets, liabilities or obligations of any nature other than as set forth in the Merger Agreement.
Merger Sub II
Cyclone Merger Sub II, Inc., a Delaware corporation (referred to as “Merger Sub II”), is a wholly owned subsidiary of the Buyer. Merger Sub II was formed by the Buyer solely in contemplation of the transactions, has not conducted any business and has no assets, liabilities or obligations of any nature other than as set forth in the Merger Agreement.
QSAM
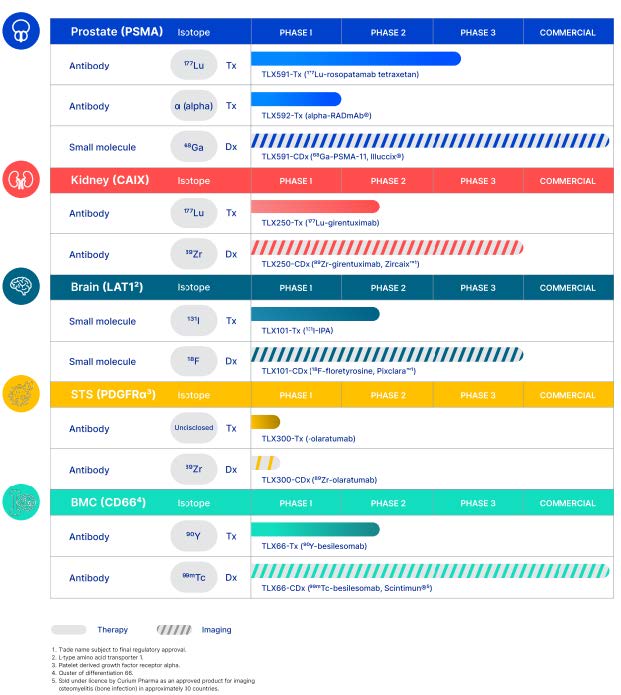
QSAM develops next-generation nuclear medicines for the treatment of cancer. QSAM’s technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “Technology”), a clinical-stage bone targeting radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. Improvements in formulation and manufacturing from a prior FDA-approved drug utilizing the same radioisotope (Quadramet®) has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability.
In August 2021, the Food & Drug Administration (FDA) cleared QSAM’s Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas. QSAM initiated this trial in November 2021 and to date has dosed five patients. Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults; and in February 2022, the FDA granted Rare Pediatric Disease Designation for the same indication.
| 1 |
What is CycloSam®. CycloSam® is a targeted, bone seeking radiopharmaceutical that combines the beta-emitting radioisotope Samarium-153 (153Sm) with a chelating agent, DOTMP (1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetramethylenephosphonic acid). Samarium-153 is acquired from a third-party nuclear reactor and the chelating agent is supplied in the form of kits. Chelating agents are organic compounds capable of linking together metal ions to form complex ring-like structures. This combination forms a stable complex which delivers a radioactive dose to sites of rapid bone mineral turnover such as bone cancers and tumors. CycloSam® has a physical half-life of 46 hours (radiation decreases by half in 46 hours) and emits both medium-energy beta particles that produce the therapeutic effect, and gamma photons that make it possible to take images of the skeleton and locate and characterize the size and nature of tumors. The use of radioisotopes to both diagnose and treat disease is called “theranostics” and is a rapidly growing area of medical discovery.
QSAM’s principal executive offices are located at 9442 Capital of Texas Hwy N, Plaza 1, Suite 500, Austin, Texas 78759, and the telephone number is 512-343-4558. The corporate website address is www.qsambio.com. QSAM’s website and the information contained on, or that can be accessed through the website, is not deemed to be incorporated by reference in, and is not considered part of, this Information Statement.
The Merger
QSAM Board Recommendation and its Reasons for the Merger
The QSAM Board unanimously approved the Merger, adoption of the Merger Agreement, and the transactions and agreements arising out of the Merger.
In the course of reaching its decision to approve the Merger Agreement and the transactions contemplated thereby, the QSAM Board considered a number of factors. For a more complete discussion of these factors, see “The Merger—Rationale for the Merger” beginning on page 29.
Opinion of QSAM’s Financial Advisor
QSAM retained Newbridge Securities Corporation (“Newbridge”) to act as its financial advisor in connection with entering into a Merger Agreement with Telix. Pursuant to its engagement, QSAM’s Board requested that Newbridge render an opinion to the Board as to the fairness, from a financial point of view, to the holders of the outstanding shares of QSAM Common Stock (other than such holders who properly exercise appraisal rights with respect to their common stock), of the Closing Consideration to be paid to the QSAM stockholders pursuant to the terms and subject to the conditions set forth in the Merger Agreement. On February 2, 2024, Newbridge delivered its oral opinion to the Board (subsequently confirmed in its written opinion dated February 7, 2024) to the effect that, based upon and subject to the assumptions, qualifications and limitations stated in its written opinion, as of November 14, 2023 (the date on which QSAM and Telix first announced the signing of the term sheet contemplating the Merger), the Closing Consideration was fair, from a financial point of view, to the QSAM stockholders. The full text of Newbridge’s written opinion to QSAM’s Board, which describes, among other things, the assumptions made, procedures followed, factors considered and limitations on the review undertaken, is attached as Appendix C hereto and is incorporated by reference herein in its entirety. Newbridge provided its opinion, which was addressed to QSAM’s Board, for the information, assistance and use of the Board in connection with its consideration of the Merger Agreement.
For a further discussion of Newbridge’s opinion, see “The Merger—Opinion of QSAM’s Financial Advisor” beginning on page 31, which provides a summary of Newbridge’s opinion and the methodology that Newbridge used to render its opinion that is qualified in its entirety by reference to the full text of the opinion attached hereto as Appendix C.
| 2 |
Interests of QSAM Non-Employee Directors, Key Employees and Key Consultant in the Merger
In considering the recommendation of the QSAM Board with respect to the Merger, QSAM stockholders should be aware that QSAM’s executive officers, C. Richard Piazza, Douglas Baum and Christopher Nelson, and one key employee, Namrata Chand (collectively, the “Key Employees”), as well as two non-employee director members of QSAM’s Board, Charles J. Link and Adriann Sax (collectively, the “Directors”), and QSAM’s CFO, Adam King (the “Key Consultant”), have various interests in the Merger that may be in addition to, or different from, the interests of QSAM stockholders generally. The members of the QSAM Board were aware of these interests and considered them at the time they approved the Merger Agreement and in making their recommendation that QSAM stockholders adopt the Merger Agreement. The Majority Stockholders were also made aware by the Board of these interests and found them to be fair, just, and reasonable and in the best interest of the Company and its stockholders as indicated in the Written Consent. These interests include, but are not limited to:
● The Key Employees may be entitled to receive severance payments and benefits, as provided in their respective employment agreements, as amended, if their employment with the Company terminates without cause or following a material change, as such terms are defined in the applicable employment agreements. Such severance payments and benefits are not payable if the Key Employee is not subject to a termination of employment, such as would be the case if a Key Employee continues employment following the closing of the Merger. In addition, certain Key Employees may agree to forego the severance payments and benefits in part or in their entirety due to lack of financial resources at QSAM and the adverse impact it may have on the Closing Consideration; and
● The Key Employees and one Director, Charles J. Link, may receive transaction bonuses in the aggregate amount of 6.25% of the Closing Consideration (calculated after deduction of the IGL License Fee in the amount of $1,655,000 payable at the closing of the Merger and less any unpaid QSAM expenses that are not being assumed by Telix) in connection with the Merger, pursuant to, with respect to the Key Employees, the terms of their respective employment agreements, as amended, and, with respect to the Director, as provided in an agreement between the Director and the Company. These individuals may also be entitled to receive transaction bonuses in the aggregate of 6.25% of any Milestone Payments, after deduction of the amount of the IGL License Fee, that become payable in accordance with the terms of the Merger Agreement.
● The Directors, Key Employees and Key Consultant have been issued restricted shares of common stock (the “Restricted Shares”) that are subject to performance and time-based vesting and that will accelerate and become fully vested upon the closing of the Merger.
For additional information on the interests of the Directors, Key Employees and Key Consultant in the Merger, see “The Merger—Interests of QSAM Directors, Key Employees and Key Consultant in the Merger” beginning on page 36.
Regulatory Approvals
Telix and QSAM have each agreed to use their reasonable best efforts to take all actions and to do all things necessary, proper or advisable to consummate and make effective the Merger and the other transactions contemplated by the Merger Agreement.
Neither Telix nor QSAM is aware of any material regulatory approvals or actions that are required for completion of the Merger. It is presently contemplated that if any such additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any additional approvals or actions will be obtained.
De-Listing and Deregistration of QSAM Common Stock After the Merger
Following the Merger, QSAM Common Stock currently trading on the OTCQB tier of the OTCMKTS will cease trading and will be deregistered under the Exchange Act as promptly as practicable after the Closing.
Appraisal Rights
QSAM Stockholders and beneficial owners will have the right to demand appraisal of their shares of QSAM Common Stock and obtain payment in cash for the fair value of their shares, only if they perfect their appraisal rights and comply with the applicable provisions of Delaware law. A copy of Section 262 (“Section 262”) of the General Corporation Law of the State of Delaware (the “DGCL”) related to appraisal rights is attached as Appendix D to this Information Statement, and a summary of these provisions can be found under “The Merger—Appraisal and Dissenters Rights” beginning on page 39. It is a condition precedent to the completion of the Merger that the aggregate number of dissenting shares shall not exceed two percent (2%) of the number of outstanding shares of Company capital stock, on a fully-diluted basis, as of the First Effective Time. Due to the complexity of the procedures for exercising the right to seek appraisal, QSAM stockholders and beneficial owners who are considering exercising such rights are encouraged to seek the advice of legal counsel. Failure to strictly comply with Section 262 may result in the loss of the right of appraisal.
| 3 |
Anticipated Accounting Treatment of the Merger
The Merger is expected to be accounted for as an asset acquisition pursuant to IFRS 3–Business Combinations as well as IAS 38-Intangible Assets.
The Merger Agreement
On February 7, 2024, Telix and QSAM entered into the Merger Agreement attached as Appendix A to this Information Statement. QSAM’s Board and the Telix Board of Directors (the “Telix Board”) have both unanimously approved the Merger pursuant to the terms of the Merger Agreement. You are encouraged to read the entire Merger Agreement carefully because it is the principal legal document governing the Merger.
Structure of the Merger (page 43)
Buyer, the Merger Subs and the Company intend to effect a reorganization in which, as steps in a single, integrated transaction, (a) Merger Sub I will merge with and into the Company, Merger Sub I will cease to exist, and the Company will survive as a direct, wholly owned subsidiary of Buyer, and (b) as part of the same overall transaction, the Company will merge with and into Merger Sub II, the Company will cease to exist, and Merger Sub II will survive as a direct, wholly owned subsidiary of Buyer.
The parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”), and that the Merger be a “plan of reorganization” for purposes of Sections 354 and 361 of the Code and within the meaning of Section 1.368-2(g) of the United States Treasury regulations promulgated under the Code.
Merger Consideration (page 44)
Stock, Cash and CVR Consideration:
Pursuant to the terms of the Merger Agreement and the Reverse Split, the aggregate consideration that QSAM stockholders will be entitled to receive pursuant to the Merger and the Reverse Split will be equal to:
(i) USD $33.1 million, reduced by (a) the amount of certain of QSAM’s unpaid expenses, indebtedness, change-of-control bonuses, and other payables as of the closing of the Merger, (b) a fee equal to 5% of the aggregate closing consideration payable to QSAM’s licensor, IGL Pharma, Inc. (“IGL”), upon the closing of the Merger, and (c) 66,011 ordinary shares of Telix (“Telix Ordinary Shares”), representing $500,000 divided by the Buyer Stock Price (defined below), as a source of recovery for post-closing purchase price adjustments (collectively, the “Closing Consideration”); and
| 4 |
(ii) contingent value rights (“CVRs”) which will represent the right to receive contingent payments of up to USD $90 million in the aggregate, in cash and/or Telix Ordinary Shares, without interest, upon the achievement of certain milestones, at the times and subject to the terms and conditions of the CVR Agreement (as defined below).
The Closing Consideration will be paid to holders of whole numbers of shares of QSAM Common Stock in the Merger in the form of Telix Ordinary Shares, except in certain specified circumstances, while payments in connection with the Reverse Split will be paid in cash. The number of shares issuable to QSAM stockholders in the Merger shall be determined by reference to a deemed value of Telix Ordinary Shares equal to USD $7.5745 per share (the “Buyer Stock Price”), representing the volume weighted average price at which Telix Ordinary Shares traded on the Australian Securities Exchange over the ten (10) trading-day period ending on February 6, 2024, the business day prior to the date of the Merger Agreement, as converted from Australian dollars to United States dollars at the exchange rate published in the Wall Street Journal as of February 6, 2024, the business day prior to the date of the Merger Agreement. The Buyer Stock Price is a negotiated, agreed, fixed value, and the trading price of Telix’s Ordinary Shares could be, at the time of the Merger, higher or lower than the Buyer Stock Price.
Subject to the foregoing and the other risks and uncertainties set forth below under Forward-Looking Statements, QSAM management currently estimates the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that will be outstanding prior to the Reverse Split will be approximately $6.63, which would equate to approximately 0.876 Telix Ordinary Shares for each whole share of QSAM Common Stock (the “Exchange Ratio”) prior to giving effect to the Reverse Split. This estimate is based on QSAM’s estimate of its expenses through the closing of the Merger in excess of certain expenses, in an amount equal to $500,000 in the aggregate, that Telix has agreed to assume, as well as QSAM’s estimate of the total shares of QSAM Common Stock outstanding immediately prior to the Reverse Split and the closing of the Merger.
As described above, the amount of cash and/or shares of Telix Ordinary Shares payable with respect to each outstanding share of QSAM Common Stock is subject to adjustment prior to closing based on, among other things, (i) the amount of QSAM’s indebtedness, unpaid expenses, change-of-control, and similar payments, in each case in connection with the Merger, as well as (ii) the fully diluted number of shares of QSAM common stock issued and outstanding as of the date of closing of the Merger, which could increase if certain stock options are exercised or other reasons. As a result, the actual amount of Closing Consideration could be lower on the date of Closing than was previously estimated as of the date of the Merger Agreement and as of the date of this Information Statement. For illustration purposes only, if indebtedness, expenses or other payables at closing is $500,000 greater than the current estimate of $3.62 million, then the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.52, which would equate to an Exchange Ratio of 0.861 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. Similarly, if the outstanding shares of QSAM Common Stock are greater by 100,000 shares at the time of Closing due to exercise of stock options, the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.49, which would equate to an Exchange Ratio of 0.856 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. These two examples of possible changes to the Closing Consideration payable to QSAM shareholders are shown in the table below:
| Assumptions | Estimated Value per QSAM Share | Estimated Exchange Ratio | ||||||
| Current estimates of total QSAM shares outstanding and total closing indebtedness | $ | 6.63 | 0.876 | |||||
| Closing indebtedness $500,000 greater than current estimate | $ | 6.52 | 0.861 | |||||
| Total QSAM shares outstanding 100,000 greater than current estimate | $ | 6.49 | 0.856 | |||||
| 5 |
In connection with and as a condition to the Merger, QSAM will effect a reverse stock split of all the issued and outstanding shares of QSAM Common Stock, in a ratio between 1:1000 and 1:2000 (the “Reverse Split”) (as determined by the QSAM Board prior to closing), in which any outstanding fractional shares of QSAM Common Stock (determined after determining the whole number of shares of QSAM Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (i) the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration and (ii) one (1) CVR for each share of QSAM Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares) pursuant to the Reverse Split.
In connection with the Reverse Split, all per share values and exchange ratios set forth above would be proportionately increased. For instance, in the case of a 1:1000 Reverse Split, where every 1,000 shares will consolidate into one share, the currently estimated value per share of QSAM Common Stock would be $6.63 X 1,000 = $6,630, and the estimated Exchange Ratio would be 0.876 X 1,000 = 876.
All QSAM stockholders will receive one Contingent Value Rights (“CVR”) certificate for every share they held prior to the Reverse Split. The non-transferrable CVRs provide their holder with a right to receive contingent payments from the Buyer upon achievement of certain clinical, regulatory and commercial milestones described below, up to a total aggregate value of $90 million (the “Earnout Consideration”). There is no guarantee that the Buyer will achieve any of the milestones in the timeframe required, and the Earnout Consideration may never be paid. See “The Contingent Value Rights Agreement” beginning on page 56 below.
The Telix Ordinary Shares issued in the Merger or pursuant to the CVR Agreement will not be registered under the Securities Act of 1933, as amended (the “Securities Act”) and will be issued pursuant to an exemption to the registration requirements thereunder. The Telix Ordinary Shares will be subject to escrow and held on the issuer sponsored subregister and subject to a holding lock for any required holding period under Rule 144 of the Securities Act. If Telix determines that a valid exemption to the registration requirements under the Securities Act would not be available with respect to the issuance of any Telix Ordinary Shares, it may, with the approval of the QSAM Stockholder Representative (not to be unreasonably withheld, conditioned or delayed) elect to pay such QSAM stockholders exclusively in cash in lieu of Telix Ordinary Shares.
Treatment of Options (page 36)
Pursuant to Merger Agreement, effective as of the date QSAM files the definitive Information Statement, each then-outstanding and unexercised option to purchase shares of QSAM Common Stock issued pursuant to any stock incentive or equity-related agreement or plan of QSAM (each such option, a “QSAM Option”) will vest in full and become exercisable up to and through the close of regular trading on the seventh business day after the date the definitive Information Statement is filed (such date, the “Last Exercise Date”) in accordance with the terms and conditions of such QSAM Option, and such QSAM Option will terminate for no consideration and be of no further force or effect as of immediately prior to closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date. According to the terms of the grants made by the Company, all QSAM Options were fully vested as of March 31, 2024 and will remain exercisable until the Last Exercise Date.
| 6 |
Conditions to Completion of the Merger (page 51)
The obligations of each of the parties to consummate the Merger are subject to the satisfaction (or waiver by each of Telix and QSAM if permissible under applicable law) prior to the First Effective Time, of certain conditions, including:
| ● | the continued accuracy of the parties’ representations and warranties contained in the Merger Agreement subject to certain specified materiality standards; | |
| ● | compliance with covenants contained in the Merger Agreement in all material respects; | |
| ● | the absence of any law or order of any governmental authority of competent jurisdiction that enjoins, prohibits or makes illegal the consummation of the Merger; | |
| ● | the Reverse Split shall have been effected; | |
| ● | there shall be no more than 27 Company stockholders that are not “accredited investors”; | |
| ● | exercise of dissenters’ rights by no more than two percent (2%) of the Common Stock outstanding on the First Effective Date; | |
| ● | the absence of any material adverse effect with respect to QSAM as further described in “The Merger Agreement—Definition of ‘Material Adverse Effect’” beginning on page 48 and “The Merger Agreement—Conditions to Completion of the Merger” beginning on page 51. |
QSAM cannot be certain when, or if, the conditions to the Merger will be satisfied or waived, or that the Merger will be completed on the terms and conditions as provided in the Merger Agreement or at all.
Termination of the Merger Agreement (page 53)
The Merger Agreement may be terminated at any time prior to the First Effective Time under the following circumstances:
| ● | by mutual written consent of the Buyer and QSAM; | |
| ● | by either the Buyer or QSAM if the Merger has not been consummated on or before August 7, 2024, (subject to extension as set forth in the Merger Agreement); | |
| ● | by either the Buyer or QSAM if the consummation of any of the transactions contemplated hereby is permanently enjoined, prohibited or otherwise restrained by the terms of a final, non-appealable order or judgment of a court of competent jurisdiction; | |
| ● | by QSAM if either the Buyer and/or Merger Sub breaches or otherwise fails to perform any of their respective representations or covenants that would cause the failure of any of the related closing conditions to be satisfied; and | |
| ● | by the Buyer if QSAM breaches or otherwise violates any of its representations or covenants that would cause the failure of any of the related closing conditions to be satisfied. |
Fees and Expenses (page 55)
Generally, all fees and expenses incurred in connection with the Merger Agreement, the CVR Agreement and the transactions contemplated by the Merger Agreement will be paid by the party incurring such expenses, whether or not the Merger is consummated. See “The Merger Agreement—Fees and Expenses” beginning on page 55. Additionally, Telix has agreed to pay or assume specified items of indebtedness and transaction expenses of the Company in an amount not to exceed $500,000.
| 7 |
The Contingent Value Rights Agreement
At or prior to the First Effective Time, Telix and QSAM will enter into a CVR Agreement, substantially in the form attached as an exhibit to the Merger Agreement, with a rights agent designated by Telix (the “Rights Agent”) governing the terms of the CVRs that the holders of QSAM Common Stock as of the effective time of the Reverse Split and the Merger will be entitled to receive in connection with the Reverse Split and the Merger, respectively (the “CVR Agreement”). The right to the contingent consideration as evidenced by the CVR Agreement is a contractual right only and will not be transferable, except in the limited circumstances specified in the CVR Agreement.
Pursuant to the CVR Agreement, each CVR will entitle the holder thereof to receive such CVR’s ratable allocation (based on the total number of CVRs outstanding) of the Earnout Consideration from Telix upon the achievement of certain milestones (the “Milestones”), if achieved, by the date that is the ten-year anniversary of the closing of the Merger (“Milestone Period”). The Milestone Payments, if any become payable, will be made in Telix Ordinary Shares and/or cash, upon the terms and subject to the conditions set forth in the CVR Agreement. Telix has agreed to use commercially reasonable efforts, on terms specified in the CVR Agreement, to, among other things, develop an acquired product in the United States, France, Germany, Italy, Spain, Japan, United Kingdom, Australia, Canada, Brazil or China (each a “Major Market Country”) and to commercialize at least one acquired product in the Major Market Countries after receipt of the applicable regulatory approval. For more details, see “The Contingent Voting Rights Agreement” on page 56.
| Milestone | Milestone Payment | |
| Successful Completion of a Pivotal Clinical Trial with respect to any acquired product | USD $10 million | |
| First Commercial Sale in a Major Market Country for any Indication | USD $20 million | |
| First Commercial Sale in a Major Market Country for the second Indication | USD $10 million | |
| Cumulative worldwide Net Sales for any and all acquired products of USD $500 million | USD $50 million |
The amount of each Milestone Payment is subject to reduction in the event any amount is set-off pursuant to Telix’s indemnification rights under the Merger Agreement, including the amount of any additional fees owed pursuant to the IGL License Fee and any transaction bonuses to officers, directors or employees of QSAM payable in connection with any such milestone payment, or in the event the QSAM Stockholder Representative is entitled to reimbursement from or indemnification by the QSAM stockholders pursuant to the Merger Agreement.
If and when a Milestone Payment becomes due pursuant to the terms of the CVR Agreement, Telix will pay the Rights Agent the aggregate amount of such Milestone Payment, who will then distribute the Milestone Payment among the holders of CVRs.
Reverse Stock Split (page 60)
On February 6, 2024, the Board approved and recommended that its stockholders approve, and on February 6, 2024, the Majority Stockholders took action by written consent to approve an amendment to the Certificate of Incorporation, as amended (the “Company Charter”) to effect a reverse stock split of outstanding shares of the Company’s Common Stock at a ratio in the range of 1:1000 and 1:2000, such ratio to be determined by the Board at a later date at its sole discretion, but prior to the Closing, if at all, and only in connection with the Merger. The Board may choose not to undertake the Reverse Split at all if it finds it to be unnecessary to complete the Merger.
| 8 |
QSAM stockholders will receive Telix Ordinary Shares in the Merger. The Telix Ordinary Shares will be issued pursuant to an exemption from registration of shares under Section 4(a)(2) or Regulation D of the Securities Act to “accredited investors” as that term is defined thereunder. Regulation D provides that there be no more than 35 non-accredited investors in an offering utilizing the safe harbor exemption in connection with a private placement. Accordingly, the Merger Agreement contemplates that there be no more than 27 non-accredited investors at the time of Closing, who may receive Telix Ordinary Shares. However, the Buyer may, with the approval of the QSAM Stockholder Representative, elect to pay a non-accredited investor in lieu of Telix Ordinary Shares, cash in an amount equal to the shareholder’s pro rata share of the Closing Consideration. The Reverse Split will be conducted in order to assure that the issuance of Telix Ordinary Shares complies with these U.S. securities regulations.
Pursuant to the Reverse Split, any outstanding fractional shares of QSAM Common Stock (determined after determining the whole number of shares of QSAM Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (i) the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration and (ii) one (1) CVR for each share of QSAM Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares) pursuant to the Reverse Split.
Our Board authorized, and the Majority Stockholders have approved the Reverse Split, which may be effected only in connection with the Merger immediately preceding the First Effective Date.
Risk Factors (page 12)
You should consider all the information contained in or incorporated by reference into this Information Statement. In particular, you should consider the factors described under “Risk Factors” beginning on page 12. Some of the risks related to QSAM, Telix and the Merger are summarized below:
| ● | Substantial doubt exists as to QSAM’s ability to continue as a going concern if the Merger does not occur. Unless the Merger occurs or QSAM is able to raise additional capital during the second quarter of 2024 to continue to finance QSAM’s operations, QSAM’s long-term business plan may not be accomplished, and QSAM may be forced to cease, restructure, reduce, or delay operations. QSAM’s efforts to raise additional funds could be affected by negative conditions in the capital markets, which in recent months have been especially challenging, and there are numerous companies in the pharmaceutical and biotech sectors seeking additional capital from many of the same sources, which may also limit the amount of capital, if any, available to QSAM. | |
| ● | The number of Telix Ordinary Shares that QSAM stockholders will receive as stock consideration is based on a fixed aggregate purchase price and fixed Buyer Stock Price, and will not be adjusted in the event of any change in the price of either Telix Ordinary Shares or QSAM Common Stock. Because the market price of Telix Ordinary Shares will fluctuate, QSAM stockholders cannot be certain of the value of the stock consideration that they will receive at the time of or following Closing. | |
| ● | QSAM stockholders will have a substantially smaller ownership and voting interest in Telix upon completion of the Merger, compared to their ownership and voting interest in QSAM prior to the Merger. | |
| ● | Your Telix Ordinary Shares will be “restricted securities” and will not be available for sale for at least a period of one year under Rule 144 of the U.S. Securities Act unless Telix chooses to become a reporting company under U.S. securities law, in which case, your Telix Ordinary Shares will not be available for sale for at least a period of six (6) months from closing of the Merger, provided that Telix has been a reporting company for at least 90 days prior to the date of sale. | |
| ● | The market price of Telix Ordinary Shares may decline as a result of or following the completion of the Merger. | |
| ● | QSAM stockholders may not receive any payments under the CVRs, and even if they do receive payments the timing and the exact amount of such payments (in the instance, for example, of an off-set from an indemnity claim) is uncertain, which makes it difficult to value the CVR. |
| 9 |
COMPARATIVE PER SHARE MARKET PRICE AND DIVIDEND INFORMATION
Market Prices
Telix Ordinary Shares are listed on the Australian Stock Exchange (ASX) under the symbol “TLX.” QSAM Common Stock is traded on the OTCQB under the symbol “QSAM.” The following table sets forth the Buyer Stock Price, i.e. the price utilized to determine the number of Telix Ordinary Shares issuable to QSAM stockholders, as of February 6, 2024 (one day prior to the date of execution of the Merger Agreement) and the closing price of QSAM Common Stock on the same date, and the closing price per share of Telix Ordinary Shares and QSAM Common Stock on [●], 2024, the most recent practicable trading day prior to the date of this Information Statement for which this information was available. Any over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions. The table also shows the implied value of the stock consideration for each share of QSAM Common Stock as of the same dates. This implied value was calculated by multiplying the closing price of a Telix Ordinary Share on the relevant date by the estimated Exchange Ratio. All values in the following section are in United States dollars; the conversion price from Australian dollars to United States dollars is at the exchange rate published in the Wall Street Journal as of the relevant date.
Telix Ordinary Shares (ASX) | QSAM Common Stock (OTCQB) | Implied Per Share Value of | ||||||||||
| February 6, 2024 | $ | 7.57 | $ | 5.10 | $ | 6.63 | ||||||
| [●], 2024 | $ | [● | ] | $ | [● | ] | $ | [● | ] | |||
The market prices of Telix Ordinary Shares and QSAM Common Stock have fluctuated since the date of execution of the Merger Agreement and will continue to fluctuate from the date of this Information Statement to the date the Merger is completed. No assurance can be given concerning the market prices of Telix Ordinary Shares and shares of QSAM Common Stock before completion of the Merger or shares of Telix Ordinary Shares after completion of the Merger. Except as mentioned elsewhere in this Information Statement, the Closing Consideration will not be adjusted prior to Closing due to fluctuations in the market prices of QSAM Common Stock or Telix Ordinary Shares. As such, fluctuations in the market price of Telix Ordinary Shares between the date of execution of the Merger Agreement and Closing may change the implied total stock consideration and the per share value of the stock consideration at Closing. For example, prior to the Closing Date, every USD $1.00 increase or decrease in the value of Telix Ordinary Share relative to the Buyer Stock Price will result in an adjustment of USD $0.876 in the implied per share value of the stock consideration presented in the table above based on the currently estimated Exchange Ratio, which as discussed above, may change prior to the closing of the Merger. After the Closing, QSAM stockholders are cautioned that the market value of the Telix Ordinary Shares may vary significantly from its market value as of the date of execution of the Merger Agreement and the date of this Information Statement. Additionally, since fractional shares of QSAM Common Stock resulting from the Reverse Split will be exchanged for cash equal to such fractional share’s pro rata share of the Closing Consideration, and such value is not affected by fluctuations in the stock price of Telix Ordinary Shares, it is possible that the cash received for fractional shares may be higher or lower than the value of the stock consideration received by QSAM stockholders. See “Risk Factors—Risks Related to the Merger” beginning on page 12.
Following the Merger, there will be no further market for shares of QSAM Common Stock and QSAM anticipates that its stock will be delisted from OTCMKTS and deregistered under the Exchange Act. As a result, following the Merger and such deregistration, QSAM would no longer file periodic reports with the SEC.
Dividends
QSAM has never declared or paid any cash dividends on shares of QSAM Common Stock. Under the terms of the Merger Agreement, during the period before completion of the Merger, QSAM is not permitted to declare, set aside, make or pay any dividend or other distribution, other than dividends or distributions by wholly-owned subsidiaries of QSAM to QSAM or another wholly-owned subsidiary of QSAM.
After completion of the Merger, any former QSAM stockholder who holds shares of Telix Ordinary Shares into which shares of QSAM Common Stock have been converted in connection with the Merger will receive whatever dividends are declared and paid on Telix Ordinary Shares. Notwithstanding the foregoing, any determination to pay cash dividends subsequent to the Merger will be at the discretion of the Telix Board and will depend upon a number of factors, including Telix’s results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors the Telix Board deems relevant. There can be no assurance that any future dividends will be declared or paid by Telix or as to the amount or timing of those dividends, if any.
Telix has never declared or paid any cash dividends on shares of Telix Ordinary Shares. Telix anticipates that it will retain its future earnings, if any, to finance the development of Telix’s business and does not anticipate paying cash dividends in the foreseeable future.
| 10 |
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
Statements contained in this Information Statement and the documents incorporated by reference herein that are not strictly historical may be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act and Section 21E of the Exchange Act and involve a number of risks and uncertainties. There are a number of important factors that could cause actual events to differ materially from those suggested or indicated by such forward-looking statements, many of which are outside of the control of Telix and QSAM, and you should not place undue reliance on any such forward-looking statements. These factors include risks and uncertainties related to, among other things:
| ● | the uncertain value of the Closing Consideration that QSAM stockholders will receive in the Merger; | |
| ● | the inability to close the Merger in a timely manner; | |
| ● | the inability of the parties to complete the Merger due to the failure to satisfy other conditions of Closing; | |
| ● | the failure of the Merger to close for any other reason; | |
| ● | the contractual restrictions imposed by the Merger Agreement; | |
| ● | the possibility that the integration of QSAM’s business and operations with those of Telix may be more difficult and/or take longer than anticipated, may be more costly than anticipated and may have unanticipated adverse results relating to Telix’s existing businesses; | |
| ● | diversion of management’s attention from ongoing business concerns; | |
| ● | restrictions in the Merger Agreement that may discourage other companies from trying to acquire QSAM; | |
| ● | the effect of any litigation relating to the Merger; | |
| ● | risks related to CVRs, including the difficulty of valuing the CVRs and the wide variety of factors affecting the value of CVRs, transfer restrictions on CVRs, and the uncertain tax treatment of CVRs; | |
| ● | the potential changes in the relative values of Telix and QSAM subsequent to the delivery of the fairness opinion related to the Merger; | |
| ● | potential termination of the Merger by either party upon failure of the Merger to timely close; | |
| ● | the effect of the Merger on Telix’s stock price; | |
| ● | other factors that may affect future results of the combined company described in the section titled “Risk Factors” beginning on page 12 and in QSAM’s filings with the SEC that are available on the SEC’s web site located at www.sec.gov, including the sections entitled “Risk Factors” in QSAM’s Annual Report on Form 10-K for the fiscal year ended 2023; and | |
| ● | the risks set forth into this Information Statement, including the risks set forth in the section titled “Risk Factors” beginning on page 12. |
The forward-looking statements made herein speak only as of the date hereof and QSAM or any of its affiliates assumes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events and developments or otherwise, except as required by law.
| 11 |
In addition to the other information included in, or incorporated by reference into, this Information Statement, including the matters addressed in the section titled “Cautionary Statement Concerning Forward-Looking Statements” beginning on page 11 and QSAM’s Annual Report on Form 10-K for the year ended December 31, 2023 as filed with the SEC, you should carefully consider the following risk factors. This summary of risks is not exhaustive. New risks may emerge from time to time and it is not possible to predict all risk factors, nor can QSAM or Telix assess the impact of all factors on the Merger and the combined company following the Merger or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in or implied by any forward-looking statements. Please also see “Where You Can Find More Information” beginning on page 129.
Risks Related to the Merger
The number of shares of Telix Ordinary Shares that QSAM stockholders will receive as stock consideration is based on a fixed aggregate purchase price and will not be adjusted in the event of any change in the price of either Telix Ordinary Shares or QSAM Common Stock. Because the market price of Telix Ordinary Shares will fluctuate, QSAM stockholders cannot be certain of the value of the stock consideration that they will receive at the Closing in the Merger.
At the First Effective Time, each share of QSAM Common Stock (other than shares held by the Buyer, Telix, Merger Subs, QSAM or their respective direct or indirect wholly owned subsidiaries) issued and outstanding immediately prior to the First Effective Time, will be automatically entitled to receive stock or cash consideration in connection with the Merger or Reverse Split and one CVR for every share held pre-Reverse Split, which CVR will represent the right to receive the Earnout Consideration upon the achievement of certain milestones, if and to the extent achieved. See “The Merger Agreement—Merger Consideration” beginning on page 44. The formula to calculate the number of Telix Ordinary Shares is based on the Buyer Stock Price. This price was fixed as of the date of the Merger Agreement and will not change based on fluctuation in the price of Telix Ordinary Shares between the date of Merger Agreement and Closing. As a result, the actual market value of Telix Ordinary Shares may be lower than the Buyer Stock Price at the time of Closing. You should consider that if the market price of Telix Ordinary Shares declines between the date the Merger Agreement and the Closing, including for any of the reasons described below, QSAM stockholders will receive overall less value in stock consideration than expected. Further, the Telix Ordinary Shares are restricted securities and may not be sold immediately at Closing. Accordingly, there is no certainty that the shares will be trading at a price equal to or higher than the Buyer Stock Price at the time of QSAM stockholders are able to sell their Telix Ordinary Shares. In other words, if the value of Telix Ordinary Shares drops on or after the date of Closing, QSAM stockholders may not realize the expected gains.
Stock price changes may result from a variety of factors (many of which are beyond the control of Telix and QSAM), including the following:
| ● | market reaction to the announcement of the Merger and Telix’s prospects following the Closing; | |
| ● | changes in the respective businesses, operations, assets, liabilities, financial positions and prospects of Telix and QSAM or in market assessments thereof; | |
| ● | changes in the operating performance of Telix, QSAM or similar companies; | |
| ● | changes in market valuations of similar companies; |
| 12 |
| ● | interest rates, general market and economic conditions; | |
| ● | federal, state and local legislation, governmental regulation and legal developments relevant to the businesses that Telix and QSAM operate; | |
| ● | changes that affect Telix’s and QSAM’s industry, the U.S. or global economy, or capital, financial or securities markets generally; and | |
| ● | other factors beyond the control of either Telix or QSAM, including those described or referred to elsewhere in this “Risk Factors” section. |
Total Closing Consideration may be lower if indebtedness or number of QSAM shares outstanding is higher.
The amount of cash and/or shares of Telix Ordinary Shares payable with respect to each outstanding share of QSAM Common Stock as of the date hereof is subject to adjustment prior to closing based on, among other things, (i) the amount of indebtedness, unpaid expenses, change-of-control, and similar payments, in each case in connection with the Merger, as well as (ii) the fully diluted number of shares of QSAM common stock issued and outstanding as of the date of closing of the Merger, which could increase if certain stock options are exercised or other reasons. As a result, the amount of Closing Consideration payable with respect to each outstanding share of QSAM Common Stock could be lower on the date of Closing than was previously estimated as of the date of the Merger Agreement and as of the date of this Information Statement. For illustration purposes only, if indebtedness, expenses or other payables at closing is $500,000 greater than the current estimate of $3.62 million, then the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.52, which would equate to 0.861 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. Similarly, if the outstanding shares of QSAM Common Stock are greater by 100,000 shares at the time of Closing due to exercise of stock options, the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.49, which would equate to 0.856 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split.
Your Telix Ordinary Shares will be “restricted securities” and will not be available for sale for at least a period of one year under Rule 144 of the U.S. Securities Act unless Telix chooses to become a reporting company under U.S. securities law, which is not guaranteed.
As indicated elsewhere in this Information Statement, Telix Ordinary Shares are being issued to QSAM stockholders pursuant to a private placement exemption from registration set forth in section 4(a)(2) and Regulation D of the Securities Act. Shares issued in private placements are considered “restricted securities” as that term is defined under Rule 144 of the Securities Act and may not be sold except pursuant to registration or an exemption from registration. However, a shareholder may sell “restricted securities” utilizing the safe harbor conditions of Rule 144. Typically, under Rule 144, a person that is not an affiliate of the issuer (Telix being the issuer) at the time of, or at any time during the three months preceding, a sale and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six (6) months, may sell shares subject only to the availability of current public information about the issuer, and any such person who has beneficially owned restricted shares of issuer’s common stock for at least one year may sell shares without restriction; provided that the issuer is a public reporting company under the Exchange Act. Since Telix is not a public reporting company under the Exchange Act in the United States, the holding period for its restricted securities is currently one year. While Telix has publicly disclosed its intention of becoming a reporting company in the United States, there is no guarantee that will happen within the six months following the closing of the Merger, or at all. As such, while the Telix Ordinary Shares have a market in Australia, due to their restricted nature under the United States securities laws, they are not available for sale and there will be no liquidity to QSAM stockholders until the shares become eligible for sale or alternatively are registered for sale. We cannot guarantee that the stock price of Telix Ordinary Shares will be higher than the value attributable to them at the time of Closing, and if the price declines, QSAM stockholders may not realize the expected gains.
| 13 |
The market price of Telix Ordinary Shares may decline as a result of or following the completion of the Merger.
The market price of Telix Ordinary Shares may decline as a result of the completion of the Merger for a number of reasons, including if the Merger is viewed as an unsuitable investment by financial and industry analysts or by Telix shareholders, or if the effect of the Merger on Telix’s financial results is not consistent with market expectations. In addition, if the Merger is consummated, Telix stockholders, including the former QSAM stockholders, will own interests in a company operating an expanded business with a different mix of assets, risks and liabilities. Current stockholders of Telix and former QSAM stockholders may not wish to continue to invest in Telix, or for other reasons may wish to dispose of some or all of their shares of Telix Ordinary Shares. Further, Telix’s share price may decline independent of the Merger due to adverse changes in its business operations, product regulatory approval or commercialization of Telix’s products, or market conditions. Further, while QSAM’s stockholders cannot sell their Telix Ordinary Shares for an extended period post-Closing due to lock-up and restrictions under Rule 144, if, following the consummation of the Merger, there is selling pressure on Telix Ordinary Shares that exceeds demand at the market price, the price of Telix Ordinary Shares could decline.
The consummation of the Merger is subject to a number of conditions, and if these conditions are not satisfied or waived on a timely basis, the Merger Agreement may be terminated and the Merger may not be completed.
The Merger is subject to certain closing conditions set forth in the Merger Agreement, including: (i) the Reverse Split is effected; (ii) no more than two (2%) of the stockholders have exercised their dissenters’ rights in connection with the Merger; (iii) no more than 27 company stockholders are not “accredited investors”; and (iv) the absence of any law or order of any governmental authority of competent jurisdiction that enjoins, prohibits or makes illegal the consummation of the Merger. In addition, each of Telix’s and QSAM’s obligations to complete the Merger is subject to certain other conditions, such as (a) the accuracy of the representations and warranties of the other party, subject to the standards set forth in the Merger Agreement; (b) compliance by the other party with its covenants in all material respects; and (c) the absence of a material adverse effect on QSAM. See “The Merger Agreement—Conditions to Completion of the Merger” beginning on page 51. The failure to satisfy all of the required conditions could delay the completion of the Merger by a significant period of time or prevent it from occurring. Any delay in completing the Merger could cause the parties to not realize some or all of the benefits that are expected to be achieved if the Merger is successfully completed within the expected timeframe. There can be no assurance that the conditions to closing of the Merger will be satisfied or waived or that the Merger will be completed.
Failure to complete the Merger would adversely affect the stock price, future business and financial results of QSAM.
There can be no assurance that the conditions to the Closing will be satisfied or waived or that the Merger will be completed. If the Merger is not completed, the ongoing business of QSAM would be adversely affected and QSAM will be subject to a variety of risks and possible consequences associated with the failure to complete the Merger, including the following:
| ● | upon termination of the Merger Agreement, the $2 million option and collaboration fee paid by Telix to the Company upon execution of the term sheet for Telix’s acquisition of QSAM will convert into shares of QSAM Common Stock at a price of $6.70 per share, which could have a dilutive effect; | |
| ● | QSAM will incur certain significant transaction costs, including legal, accounting, financial advisor, filing, printing and mailing fees, regardless of whether the Merger closes; |
| 14 |
| ● | under the Merger Agreement, QSAM is subject to certain restrictions on the conduct of its business prior to the Closing, which may adversely affect its ability to execute certain of its business strategies; | |
| ● | QSAM may lose key employees during the period in which QSAM and Telix are pursuing the Merger, which may adversely affect QSAM in the future if it is not able to hire and retain qualified personnel to replace departing employees; and | |
| ● | the proposed Merger, whether or not it closes, will divert the attention of certain management and other key employees of QSAM from ongoing business activities, including the pursuit of other opportunities that could be beneficial to QSAM as an independent company. |
If the Merger is not completed, these risks could materially affect the business and financial results of QSAM and its stock price, including to the extent that the current market price of QSAM Common Stock is positively affected by a market assumption that the Merger will be completed.
QSAM will need additional capital to meet its current obligations and continue to operate its business if the Merger is not completed in a timely fashion or at all.
QSAM’s management has indicated that if QSAM had adequate funding in a timely manner, it may be able to complete its current Phase 1 safety trial of approximately 17 patients in 2024. Management has estimated that an additional $3 million to $4 million will be required to complete this phase of its study. The next step in QSAM’s clinical trial program, if cleared by the FDA, is expected to be a study designed to show both safety and efficacy in the treatment of bone tumors utilizing a multi-dose regimen of CycloSam®. Management estimates that an additional $12 million to $14 million will be required to complete the first portion of this critical phase of the study over the next 24 to 30 months.
Advancement of current and future plans for our technology requires significant additional funding, which would most likely result in the issuance of more common stock, preferred stock or debt in subsequent raises. There is no guaranty that we would be successful in raising such funding on terms acceptable to our shareholders, if at all, and if we were not successful, we may be required to slow down or cease our clinical trials, or in a worst-case scenario, shut down the Company. Our independent registered public accounting firm has issued a going concern opinion for the year ended December 31, 2023. This means that our auditors believe there is substantial doubt that we can continue as an on-going business for the next 12 months. Management expects expenses to increase in 2024 as our drug technology advances through clinical trials, and as a result, we will need to raise significant additional capital to support these operations. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company. Any funds raised will likely result in material dilution to our current shareholders (in addition to dilution caused by conversion of the Option Payment (defined below in this Information Statement) to shares of Common Stock) or be in the form of debt which poses other risks of default. Our current cash or cash equivalents can enable us to fund our operations through the second quarter of 2024. If we are not successful in completing this Merger prior to June 30, 2024, or raising additional capital, or providing other options to support operations and our trials, we may need to delay clinical trials, reduce overhead, or in the most extreme scenario, shut down operations.
While the Merger is pending, QSAM will be subject to business uncertainties and certain contractual restrictions that could adversely affect the business and operations of QSAM.
In connection with the pending Merger, some operators, managers, suppliers, vendors or other third parties of QSAM may react unfavorably, delay or defer decisions concerning their business relationships or transactions with QSAM, which could adversely affect the clinical stages of QSAM, regardless of whether the Merger is completed. In addition, due to certain restrictions in the Merger Agreement on the conduct of business prior to completing the Merger, QSAM may be unable (without Telix’s prior written consent), during the pendency of the Merger, to pursue strategic transactions, undertake significant capital projects, undertake certain financing transactions and otherwise pursue other actions, even if such actions would prove beneficial and may cause QSAM to forego certain opportunities it might otherwise pursue. In addition, the pendency of the Merger may make it more difficult for QSAM to effectively retain and incentivize key personnel and may cause distractions from QSAM’s strategy and day-to-day operations for its current employees and management.
| 15 |
QSAM will incur substantial transaction fees and Merger-related costs in connection with the Merger.
QSAM expects to incur non-recurring transaction fees, which include legal and advisory fees and substantial Merger-related costs associated with completing the Merger. These fees will be payable by QSAM even if the Merger does not close, and therefore, will impose a significant financial burden on QSAM which would materially impair its ability to continue operations in the instance the Merger is terminated. Further, QSAM will continue to bear the costs and expenses of its operations prior to the Closing, and any delays in the anticipated closing date will result in additional indebtedness and/or payables existing at Closing which QSAM may not have the available cash resources to pay. In this instance, Telix would have the right to reduce the Closing Consideration payable to the QSAM stockholders in the amount of any additional indebtedness that it is required to pay or assume.
QSAM stockholders will have a substantially smaller ownership and voting interest in Telix upon completion of the Merger compared to their ownership and voting interest in QSAM prior to the Merger.
Upon completion of the Merger, each QSAM stockholder at the First Effective Time will become a Telix stockholder with a percentage ownership of Telix that is substantially smaller than the QSAM stockholder’s current percentage ownership of QSAM. Upon completion of the Merger, based on the number of shares of Telix Ordinary Shares and QSAM Common Stock outstanding on March 31, 2024, the latest practicable date prior to the filing of this Information Statement, it is estimated that continuing Telix stockholders will own greater than 98% of the issued and outstanding ordinary shares of Telix, and former QSAM stockholders will collectively own less than 2% of the issued and outstanding ordinary shares of Telix. Accordingly, the former QSAM stockholders will exercise significantly less influence over Telix after the Merger relative to their influence over QSAM prior to the Merger, and thus will have a less significant impact on the approval or rejection of future Telix proposals submitted to a Telix stockholder vote.
Litigation against QSAM, Telix or the members of their respective boards could prevent or delay the completion of the Merger or result in the payment of damages following completion of the Merger.
It is a condition to the Merger that no temporary restraining order, preliminary or permanent injunction or other order preventing the consummation of the Merger Agreement or the transactions contemplated thereby shall have been issued by any court of competent jurisdiction or other governmental authority of competent jurisdiction and remain in effect. It is possible that Telix or QSAM stockholders may file lawsuits challenging the Merger or the other transactions contemplated by the Merger Agreement, which may name Telix, members of the Telix Board, QSAM and/or members of the QSAM Board as defendants. The outcome of such lawsuits cannot be assured, including the amount of costs associated with defending these claims or any other liabilities that may be incurred in connection with the litigation of these claims. If plaintiffs are successful in obtaining an injunction prohibiting the parties from completing the Merger on the agreed-upon terms, such an injunction may delay the consummation of the Merger in the expected timeframe, or may prevent the Merger from being consummated at all. Whether or not any plaintiff’s claim is successful, this type of litigation can result in significant costs and divert management’s attention and resources from the Closing and ongoing business activities, which could adversely affect the operation of Telix’s and QSAM’s businesses.
| 16 |
Directors, Key Employees and Key Consultant of QSAM have interests in the Merger that are different from, or in addition to, the interests of other QSAM stockholders.
Directors, Key Employees and a Key Consultant of QSAM have interests in the Merger that may be different from, or in addition to, the interests of other QSAM stockholders, generally. These interests include, among others: severance payments; transaction bonuses; the acceleration of vesting of Restricted Shares; and rights to ongoing indemnification and insurance coverage by the surviving company for acts or omissions occurring prior to the Merger. The QSAM Board was aware of and considered those interests, among other matters, in reaching its decision to approve and adopt the Merger Agreement, approve the Merger and recommend the approval of the Merger Agreement to QSAM stockholders. These interests, among other factors, may have influenced the directors and executive officers of QSAM to support or approve the Merger. See “The Merger—Interests of QSAM Directors and Officers in the Merger” beginning on page 36.
QSAM stockholders may not receive any payments under the CVRs, which makes it difficult to value the CVRs.
Under the Merger Agreement, holders of QSAM Common Stock have the right to receive one CVR for each pre-Reverse Split share of QSAM Common Stock held by such person. Each CVR will entitle its holder to receive one or more contingent payments in cash or Telix Ordinary Shares or a combination of both from the Earnout Consideration upon the achievement of certain milestones, if achieved. See “Agreements Related to the Merger—The Contingent Value Rights Agreement” beginning on page 56. Therefore, QSAM stockholders’ right to receive any future payment with respect to the CVRs will be contingent upon whether the milestones are achieved.
Telix is obligated to use commercially reasonable efforts to satisfy the milestone events on the terms specific in the CVR Agreement. A variety of factors can affect the ability, timing and success of these milestones. If any milestones are not achieved within the tenth anniversary of the date of the CVR Agreement (the deadline period), any unachieved milestone payments will not be made under the CVRs and the CVRs will expire. Accordingly, the value, if any, of the CVRs is highly speculative and uncertain, and the CVRs may ultimately have no value at all.
The CVRs are nontransferable.
The CVRs are nontransferable, meaning that they may not be sold, assigned, transferred, pledged, encumbered or in any other manner transferred or disposed of either in whole or in part, other than in certain limited circumstances. The CVRs will not be registered as securities and they will not be listed or traded on any stock exchange in the United States or elsewhere. Therefore, the CVRs are not liquid and QSAM stockholders will not be permitted to sell or transfer them, except in certain limited circumstances.
The Merger may not qualify as a “reorganization” under Section 368(a) of the Code or may be taxable under Section 367(a) of the Code.
As discussed in “Material U.S. Federal Income Tax Considerations” beginning on page 119 of this Information Statement, the Merger is intended to qualify as a “reorganization” within the meaning of Section 368(a) of the Code and not to be subject to Section 367(a)(1) of the Code. Subject to the qualifications, exceptions, assumptions and limitations contained in “Material U.S. Federal Income Tax Considerations,” if the Merger qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, is not subject to Section 367(a)(1) of the Code, and is treated as a “closed transaction” rather than an “open transaction” for U.S. federal income tax purposes, then, generally, gain will be recognized by a U.S. Holder (as defined in “Material U.S. Federal Income Tax Considerations”) equal to the lesser of (i) the fair market value of the CVRs received in connection with the Merger, and (ii) the difference, if any, between (x) the sum of the fair market values of the Telix Ordinary Shares and the CVRs received in connection with the Merger, and (y) such holder’s adjusted tax basis in the shares of Common Stock surrendered in the Merger. The tax treatment of the receipt of the CVRs and payments thereunder is uncertain, and the alternative treatments are described in “Material U.S. Federal Income Tax Considerations” beginning on page 119 of this Information Statement.
| 17 |
Subject to the qualifications, exceptions, assumptions and limitations contained in “Material U.S. Federal Income Tax Considerations” beginning on page 119 of this Information Statement, if the Merger is treated as a closed transaction but does not qualify as a “reorganization” under Section 368(a) of the Code, then, for U.S. federal income tax purposes, a U.S. Holder would recognize gain or loss in an amount equal to the difference, if any, between (i) the sum of the fair market values of the Telix Ordinary Shares received at Closing and the CVRs, and (ii) such U.S. Holder’s adjusted tax basis in the shares of Common Stock surrendered. If the Merger is treated as a closed transaction and is subject to Section 367(a)(1) of the Code, then, a U.S. Holder would recognize gain, but not loss, in an amount equal to the difference, if any, between (i) the sum of the fair market values of the Telix Ordinary Shares received at Closing and the CVRs, and (ii) such U.S. Holder’s adjusted tax basis in the shares of Common Stock surrendered.
Each QSAM Stockholder is urged to read the discussion in the section entitled “Material U.S. Federal Income Tax Considerations” beginning on page 119 of this Information Statement and to consult its tax advisor to determine the particular U.S. federal, state or local or non-U.S. income or other tax consequences to it of the Merger.
The U.S. federal income tax treatment of the CVRs is uncertain.
There is no legal authority directly addressing the U.S. federal income tax treatment of the CVRs or the treatment of payments that may be received pursuant to the CVRs. Accordingly, the amount, timing and character of any gain or loss with respect to the CVRs are uncertain. For a more detailed summary of the material U.S. federal income tax consequences of the Merger, see “Material U.S. Federal Income Tax Considerations” beginning on page 119 and “Material Federal Income Tax Consequences of the Reverse Stock Split” beginning on page 62.
The fairness opinion obtained from Newbridge, the financial advisor to the QSAM Board, will not reflect subsequent developments between the signing of the Merger Agreement and the Closing.
In connection with the proposed Merger, the QSAM Board received an opinion on February 7, 2024, from Newbridge as to the fairness, from a financial point of view, and as of such date, of the Stock Consideration to be paid to the holders (other than holders of cancelled or dissenting shares) of QSAM Common Stock, which opinion was based on and subject to various assumptions, procedures, considerations, limitations and qualifications, more fully described in the section titled “The Merger—Opinion of QSAM’s Financial Advisor” beginning on page 31. The opinion does not reflect developments that may occur or may have occurred after the date of the opinion, including changes in the market prices of Telix Ordinary Shares and QSAM Common Stock, changes to the operations and prospects of Telix or QSAM, changes in general market and economic conditions or regulatory or other factors. Any such changes, or other factors on which the opinions are based, may materially alter or affect the relative values of Telix or QSAM.
If the Merger is not consummated by August 7, 2024, either QSAM or Telix may terminate the Merger Agreement.
Either QSAM or Telix may terminate the Merger Agreement if the Merger has not been consummated by August 7, 2024, six months from the date of signing the Merger Agreement. However, this termination right will not be available to a party if that party failed to fulfill its obligations under the Merger Agreement and that failure was the principal cause of, or directly resulted in, the failure to consummate the Merger on time. See “The Merger Agreement—Termination of the Merger Agreement” beginning on page 53. In the event the Merger Agreement is terminated by either party due to the failure of the Merger to close by August 7, 2024, QSAM will have incurred significant costs and will have diverted significant management focus and resources from other strategic opportunities and ongoing business activities without realizing the anticipated benefits of the Merger.
| 18 |
Risks Related to the Combined Company Following the Merger
Following the Merger, Telix may be unable to integrate the QSAM business successfully or realize the anticipated synergies and related benefits of the Merger.
Telix and QSAM entered into the Merger Agreement with the expectation that the Merger will result in various benefits and synergies. However, the Merger involves the combination of two companies that currently operate as independent public companies. Telix may be unable to successfully operate QSAM’s business or integrate it into its own operations as a combined company.
After the Closing, Telix will be required to devote significant management attention and resources to integrating the portfolio and operations of QSAM. Potential difficulties that Telix may encounter in the integration process include the following:
| ● | the inability to combine the businesses of Telix and QSAM in a manner that permits Telix to achieve the cost savings or other synergies anticipated as a result of the Merger or to achieve such cost savings or other anticipated synergies in a timely manner, which could result in Telix not realizing some anticipated benefits of the Merger in the time frame currently anticipated, or at all; | |
| ● | the inability to realize the anticipated value from various QSAM assets; | |
| ● | the inability to coordinate and integrate research and development teams across technologies and products to enhance product development; | |
| ● | the inability to coordinate distribution and marketing efforts; and | |
| ● | potential unknown liabilities and unforeseen increased expenses, delays or unfavorable conditions in connection with the Closing and the subsequent integration. |
It is possible that the integration process could result in the distraction of Telix’s management, the disruption of Telix’s ongoing business or inconsistencies in Telix’s operations, services, standards, controls, procedures and policies, any of which could adversely affect the ability of Telix to maintain relationships with third parties and employees or to achieve the anticipated benefits of the Merger, or could otherwise adversely affect the business and financial results of Telix.
Telix Ordinary Shares to be received by QSAM stockholders in the Merger will have rights different from the shares of QSAM Common Stock.
After the First Effective Time, QSAM stockholders who receive Telix Ordinary Shares in connection with the Merger will no longer be stockholders of QSAM but instead will hold shares of Telix. The two corporations operate in separate legal and geographical jurisdictions – QSAM is a Delaware corporation whereas Telix is an Australian corporation. As stockholders of Telix, former QSAM stockholders will have different rights under the terms of Telix’s governance documents, and those rights may be, or may be perceived to be, less favorable than their current rights as QSAM stockholders. See “Comparison of Rights of Holders of Telix Ordinary Shares and QSAM Common Stock” beginning on page 111.
There can be no assurance that Telix will not be a passive foreign investment company for any taxable year, which could result in adverse U.S. federal income tax consequences to QSAM Stockholders who receive Telix Ordinary Shares in the Merger.
In general, a corporation organized outside the U.S. will be classified for U.S. federal tax purposes as a passive foreign investment company, or PFIC, for any taxable year in which either (i) 75% or more of its gross income consists of “passive income,” or (ii) 50% or more of the value of its assets (generally determined on an average quarterly basis) consists of assets that produce, or are held for the production of, passive income. For purposes of the above calculations, a foreign corporation that owns (or is treated as owning) at least 25% by value of the shares of another corporation is treated as if it held its proportionate share of the assets of that other corporation and received directly its proportionate share of the income derived by that other corporation. “Passive income” generally includes dividends, interest, rents, royalties and certain gains. Cash is a passive asset for these purposes.
| 19 |
Based on the expected nature and amount of Telix’s estimated gross income, the anticipated nature and estimated average value of its gross assets, the anticipated cash needs of its group’s operations and the nature and extent of the active businesses conducted by its “25% or greater” owned subsidiaries, Telix does not expect that it will be classified as a PFIC in the current taxable year or for foreseeable future. However, Telix’s PFIC status for any taxable year can be determined only after the end of such year and will depend on the composition of its income and assets and the value of its assets from time to time (which may be determined, in part, by reference to the market price of the Telix Ordinary Shares, which could be volatile). Furthermore, the composition of Telix’s income and assets for the current and future taxable years will be affected by how, and how quickly, Telix spends the cash it has on hand. Accordingly, there can be no assurance that Telix will not be a PFIC for its current or any future taxable year. If Telix is a PFIC for any taxable year during which a U.S. person is treated as owning Telix Ordinary Shares, such U.S. person generally would be subject to adverse U.S. federal income tax consequences, possibly including increased tax liability on disposition gains and “excess distributions,” and additional reporting requirements. See “Material U.S. Federal Income Tax Considerations—Passive Foreign Investment Company Considerations” beginning on page 126.
Risks Relating to Telix’s Business
Telix has a history of significant net losses, may increase operating expenses in the future, and may not maintain profitability in future periods.
Until 2023, Telix incurred significant operating losses. Telix’s operating profit was A$15.8 million and net operating cash inflow was A$23.9 million for the year ended December 31, 2023. As of December 31, 2023, Telix had an accumulated deficit of A$263.7 million. Although Telix launched Illuccix® in April 2022 and has recognized revenue from its sales, Telix cannot be certain that it will sustain profitability or positive cash flows from operations in future periods.
Telix has invested most of its resources in developing technology and product candidates, building intellectual property portfolio, developing supply chains, conducting business planning, raising capital and providing general and administrative support for these operations. Telix expects to continue to incur significant expenses as it continues to commercialize Illuccix® in the United States, Australia, New Zealand, and Canada and engage in activities to prepare for the potential approval and commercialization of other product candidates. Because of the numerous risks and uncertainties associated with pharmaceutical product development and commercialization, Telix is unable to accurately predict the timing or amount of our revenue and expenses or if it will be able to maintain profitability. Telix cannot be certain that revenue from sales of Illuccix® alone, in the currently approved indications, will be sufficient for Telix to remain profitable in future periods. Telix may not generate revenues that are significant or large enough to sustain or increase profitability on an annual basis. Telix’s failure to remain profitable would decrease the value of the company and could impair its ability to raise capital, maintain research and development and commercialization efforts, expand business and/or continue operations.
Telix may not be able to effectively integrate the businesses that it has acquired and/or may acquire in the future.
Telix’s ability to realize the anticipated benefits of acquisitions it has completed and/or may complete in the future will depend on its ability to integrate those businesses with its own. The combination of multiple independent businesses is a complex, costly and time-consuming process and there can be no assurance that Telix will be able to successfully integrate businesses into its business, or if such integration is successfully accomplished, that such integration will not be costlier or take longer than presently contemplated. If Telix cannot successfully integrate and manage the businesses within a reasonable time, Telix may not be able to realize the potential and anticipated benefits of such acquisitions, which could have a material adverse effect on its business, financial position, and results of operations. Telix faces numerous risks relating to the integrated of acquired businesses, including:
| ● | the inability to integrate effectively the operations, products, technologies and personnel of the acquired companies (some of which are in diverse geographic regions) and achieve expected synergies; |
| 20 |
| ● | the potential disruption of existing business and diversion of management’s attention from day-to-day operations; | |
| ● | the inability to maintain uniform standards, controls, procedures and policies; | |
| ● | the need or obligation to divest portions of the acquired companies to satisfy regulatory requirements; | |
| ● | the potential failure to identify material problems and liabilities during due diligence review of acquisition targets; | |
| ● | the potential failure to obtain sufficient indemnification rights to fully offset possible liabilities associated with acquired businesses; and | |
| ● | the challenges associated with operating in new product segments and/or geographic regions. |
Telix may need to raise additional capital to achieve its business objectives if it is unable to fund its operations with its cash flows from the sale of its products. If Telix is unable to raise capital when needed or on acceptable terms, Telix would be forced to delay, reduce or eliminate its research and development programs and/or commercialization efforts.
Discovering, developing and commercializing products involve time-consuming, expensive and uncertain processes that take years to complete. Telix has used substantial funds to develop Illuccix® and expects its operating expenses to continue to increase as it continues to commercialize Illuccix® or any future approved products, conduct further research and development of its product candidates, seek approval and prepare for commercialization of TLX250-CDx, seek approval of TLX101-CDx and continue to conduct clinical trials for its other product candidates. Furthermore, Telix will continue to incur additional costs associated with operating as a public company, hiring additional personnel and expanding its geographical reach. Although currently Illuccix® is commercially available in four jurisdictions, Telix cannot be certain that its revenue from product sales of Illuccix® will be sufficient for it to remain profitable on an annual basis. Accordingly, Telix may need to continue to rely on additional financing to achieve its business objectives.
As of December 31, 2023, Telix had A$123.2 million in cash and cash equivalents. The amount and timing of its future capital requirements will depend on many factors, including, but not limited to:
| ● | the scope, progress, results, timing and costs of its current and planned development efforts and regulatory review of its product candidates; | |
| ● | the amount and timing of revenues from sales of Illuccix®, or any product candidate that it develops or acquires; | |
| ● | the cost of, and its ability to expand and maintain, the commercial infrastructure required to support the commercialization of Illuccix® and any other product for which Telix receives regulatory approval, including medical affairs, manufacturing, marketing and distribution functions; | |
| ● | Telix’s ability to establish and maintain collaboration, partnership, licensing, marketing, distribution or other arrangements on favorable terms and the level and timing of success of these arrangements; | |
| ● | the extent to which Telix acquires or in-licenses other products, product candidates and technologies; and | |
| ● | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing its intellectual property rights and defending intellectual property-related claims. |
In addition, the terms of any financing may adversely affect the holdings or the rights of Telix’s shareholders. If Telix raises additional funds by issuing equity securities, dilution to its existing shareholders will result. In addition, as a condition to providing additional funding to Telix, future investors may demand, and may be granted, rights superior to those of existing shareholders. Moreover, any debt financing, if available, may involve restrictive covenants that could limit Telix’s flexibility in conducting future business activities and, in the event of insolvency, would be paid before holders of equity securities received any distribution of corporate assets. Telix’s ability to satisfy and meet any future debt service obligations will depend upon our future performance, which will be subject to financial, business and other factors affecting its operations, many of which are beyond its control.
Even if Telix believes it has sufficient funds for its current or future operating plans, Telix may seek additional capital due to favorable market conditions or strategic considerations. Any future fundraising efforts could divert Telix’s management’s attention away from its day-to-day activities. Further, adequate additional financing may not be available to it on acceptable terms, or at all. In addition, raising funds in the current economic environment may present additional challenges. For example, any sustained disruption in the capital markets from adverse macroeconomic conditions, such as the disruption and uncertainty caused by rising inflation, increasing interest rates and slower economic growth or recession, could negatively impact Telix’s ability to raise capital and it cannot predict the extent or duration of such macro-economic disruptions. If adequate funds are not available to Telix on a timely basis or on attractive terms, Telix may be required to delay, reduce or eliminate its research and development programs or any current or future commercialization efforts for one or more of its products or product candidates, any of which could have a material adverse effect on its business, operating results and prospects.
| 21 |
Relevant Historical Background of QSAM
Commencement of Operations to Develop CycloSam®
The Company was incorporated on August 26, 2004 under the name Telecomm Sales Network, Inc. The Company engaged in successive legacy businesses, including listing on the OTCQB in 2015 under the name Q2Power Technologies, Inc., before we commenced our current business of developing CycloSam® in April 2020, when we established QSAM Therapeutics Inc. (“QSAM Therapeutics”) as a wholly-owned subsidiary incorporated in the state of Texas, and through QSAM Therapeutics, executed a Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) for Samaium-153 DOTMP aka CycloSam® (the “Technology”) with IGL. The License Agreement, as amended, provides the Company, through QSAM Therapeutics with exclusive, worldwide and sub-licensable rights to all of IGL’s patents, product data and know-how with respect to the Technology, a clinical stage novel radiopharmaceutical meant to treat different types of bone cancer. In connection with the commencement of our current business of developing CycloSam®, we changed our name to QSAM Biosciences, Inc., and we divested our legacy assets and terminated our legacy business.
As with all early-stage biotech companies, the advancement of the Company’s Technology has and will require significant capital expenditures. The drug development process is long and expensive, requiring capital for pre-clinical studies, human clinical trials, intellectual property protection, and other critical activities including general operations and overhead. Since the beginning of 2021, the Company has raised approximately $7.3 million in four main financing rounds of preferred stock, debt, and common stock, as follows:
Series B Round. The Company completed its first capital raise to advance the Technology in January 2021 with the closing of its Series B Preferred Stock offering (the “Series B Round”). In the Series B Round, the Company issued a total of 2,500 Series B Preferred shares at a price of $1,000 per share (“Series B Stock”), raising an aggregate amount of $2.5 million, inclusive of $156,000 in debt conversion. The Series B Round was led by Checkmate Capital Group, LLC, a California based investment firm focused on biotechnology and other technology investments.
In July and August 2021, 15 holders of 991 shares of Series B Stock converted their Series B Stock plus all accrued dividends into 163,134 shares of Company common stock. In October 2023, all remaining Series B stockholders, representing 1,509 shares of Series B Stock, signed agreements with the Company providing that if the Company lists its common stock on Nasdaq or otherwise signs an agreement to be acquired or merged with another company, such shareholders will automatically and without any further action on their part exchange their shares of Series B Stock into common stock at an exchange ratio of $3.00, inclusive of all accrued dividends. As a result of the signing of the Merger Agreement, the total principal and dividend amount of the Series B Stock was exchanged into 658,968 shares of common stock, and all Series B shares were retired.
In connection with this Series B Round closing, we issued in 2021 approximately 150,000 common stock warrants, which were originally exercisable prior to July 8, 2021 at an exercise price of $14.00 per share, and later modified by our Board to expire on October 15, 2021 and be exercisable at $10.00 per share. As of October 15, 2021, seven holders of the Series B warrants exercised those warrants and received a total of 46,786 common shares for total consideration to the Company of $467,858.
| 22 |
Convertible Note Round. In the fourth quarter of 2021, we entered into convertible note purchase agreements with eight accredited investors, pursuant to which we issued an aggregate of $605,000 of 6% annual interest, unsecured convertible notes (the “Convertible Notes”). The Convertible Notes were due to mature on December 31, 2023, and were originally convertible into shares of common stock of the Company at a conversion price of $8.00 per share. On March 31, 2023, our Board approved a reduction of the conversion price from $8.00 to $3.50 as an inducement to immediate conversion. All outstanding Convertible Notes were converted into 164,446 shares of common stock. As of the date of signing the Merger Agreement, we had no Convertible Notes outstanding.
Common Stock and Warrant Unit Round. On March 31, 2023, we closed a total of $2.86 million in a common stock and warrant unit offering, which consisted of 381,500 shares of common stock at $4.50 and the issuance and exercise of 381,500 two-year common stock warrants (the “Warrants”). The Warrants originally had an exercise price of $6.00 per share, but on March 31, 2023, our Board approved a reduction of the price to $3.00 as an inducement to immediate exercise of the Warrants. All Warrant holders exercised at this reduced price, which raised an additional $1.14 million for the Company, inclusive of $342,669 in subscription receivables which were received in April 2023.
Common Stock Round. In the third quarter of 2023, the Company issued 176,470 shares of common stock to three accredited investors, one of whom is a family member of our Executive Chairman, for a total of $600,000 in funding. In December 2023, a fourth investor purchased 50,000 shares of common stock for $250,000, which funds were received in early January 2024.
Many of the investors in our first Series B Round were also investors in the Convertible Note Round, the Common Stock and Warrant Unit Round and the latest Common Stock Round. As such, these investors have provided significant capital resources to the Company over the last three years to assist the Company in its development efforts. After the closing of this last round, many of these investors informed the Company’s management that their desire and ability to continue to fund the Company was limited and, if any future investments would be considered, they would be conditioned upon the uplisting of the Company’s common stock to Nasdaq to provide greater liquidity for their investments.
Attempted Underwritten Offering and NASDAQ Uplisting. In December 2021, the Company filed a registration statement on Form S-1 with the SEC to raise up to $20 million through a common stock offering underwritten by an investment bank based in New York. Concurrently, the Company submitted an application with the Nasdaq Stock Market LLC to list its common shares on the Nasdaq Capital Market. Due to difficult market conditions, especially for micro-cap, early-stage biotech companies at this time, in May 2022, the Company chose to terminate this offering.
Strategy to Advance Technology Development
We are actively advancing the development of our Technology through clinical trials. If we had adequate funding in a timely manner, QSAM management believes that we can complete the current Phase 1 safety trial of approximately 17 patients in 2024. We have estimated that an additional $3 million to $4 million will be required to complete this phase of our study. The next step that we envision in our clinical trial program, if cleared by the FDA, is expected to be a study designed to show both safety and efficacy in the treatment of bone tumors utilizing a multi-dose regimen of CycloSam®. Management estimates that an additional $12 million to $14 million will be required to complete the first portion of this critical phase of our study over the next 24 to 30 months.
Beyond our current focus of studying CycloSam® in the treatment of metastatic bone cancer, management has indicated its desire to advance other potential indications for our Technology, including the treatment of pediatric osteosarcoma and Ewing’s sarcoma, and the use of CycloSam® to treat the debilitating pain from cancer that has metastasized to the bone. Such trials would require initiating separate protocols and/or studies for these important indications.
| 23 |
Advancement of our Technology requires significant funding, which has in the past and would most likely in the future result in the issuance of more common stock, preferred stock or debt in subsequent raises. There is no guaranty that we would be successful in raising such funding on terms acceptable to our shareholders, if at all, and if we were not successful, we may be required to slow down or cease our clinical trials, or in a worst-case scenario, shut down the Company. Our independent registered public accounting firm had issued a going concern opinion for each of the years ended December 31, 2021, 2022 and 2023. This means that our auditors believed there was substantial doubt that we could continue as an on-going business for the next 12 months.
Against the backdrop of these financial viability concerns, the Company’s prior funding rounds, the then active Common Stock and Warrant Unit Round, and the need to raise additional capital in the future to continue to advance the Technology, the Company’s Board of Directors (“Board”) tasked management in the fall of 2022 with presenting options to properly fund the Company and its operations through the completion of Phase 2 clinical trials and/or the partnership of the Company with a larger pharmaceutical concern with substantially greater capital assets, including the potential of a co-funded, co-development agreement. As noted above, management estimated that an additional $3 to $4 million would be needed to complete Phase 1 of the ongoing trials, and an additional $12 to $14 million for the first stage of Phase 2 trials, not inclusive of general overhead and public company expenses. Such Phase 2 budget was estimated to be sufficient to advance the trial far enough along to demonstrate meaningful Phase 2 data, but not fully complete that stage of the trial. In its deliberations, the Board also considered the Company’s stock price and market capitalization, and the current state of the economy and the capital markets including the widespread difficulty small, early-stage biotech companies were having in attracting capital. Given the reluctancy of the Company’s prior investors to provide additional capital in future private offerings, the Board considered the option of conducting additional PIPE transactions while trading on the OTCQB to be highly uncertain. Further, if funds were available to the Company through a PIPE, the Board believed that such terms would be highly dilutive to prior investors and current shareholders.
In early spring 2023, after multiple Board meetings to discuss the findings of management, the Board concluded that there were two viable paths forward for the Company: (1) seek a Nasdaq uplisting with a concurrent underwritten offering with an investment bank, or (2) seek a partnership or co-development agreement with a larger pharmaceutical concern that could off-set or assume the expenses of our clinical trials. Both these paths had considerable risks and uncertainties, and therefore, the Board decided to pursue both in parallel.
Investment Banking – Nasdaq Uplisting Efforts
In March 2023, after the closing of its Common Stock and Warrant Unit Round, management began contacting investment banks to inquire about the possibility of completing a Nasdaq uplisting with a concurrent underwritten offering during the following six to 12 months. Several of these banks were under consideration or involved with the Company’s 2021/2022 attempt to list on Nasdaq; others were recommendations of consultants and shareholders or were firms otherwise known to members of management.
Between March and May 2023, management spoke with over 12 investment banks which management believed would be interested in engaging with the Company to pursue this type of transaction. Of these, six of the banks informed the Company that (1) market conditions were not conducive to Nasdaq uplisting transactions and/or micro-cap, early-stage biotech public transactions, (2) they were doubtful whether they could successfully raise the required funds for the Company to uplist to Nasdaq, and/or (3) they were not interested in pursuing such a transaction with QSAM at that time. Management pursued further discussions with the remaining six banks that expressed cautious optimism that an uplisting transaction could be completed.
| 24 |
In late April 2023, the Company received unsigned, draft Letters of Engagement (“LOE”) from three investment banks to raise capital in a firm underwriting to be completed concurrently with a Nasdaq uplisting (Bank #1, Bank #2, and Bank #3). In May 2023, the Company received a similar unsigned LOE with a fourth investment bank (Bank #4); and in July 2023 another draft LOE from Bank #5. The final LOE was received in September 2023 from Bank #6, and was separately considered by the Company’s Board, as discussed below.
Banks #1, #2 and #3 were all similar in size, reputation, and track record for the types of transactions that the Company was considering. The terms provided in the three LOE’s were reasonably similar in fees, expense caps, terms of engagement and follow-on funding rights (such as rights of first refusal and “tail” fees, “Follow-On Rights”). With respect to Bank #4, while the proposed terms of their engagement were in line with the other three banks, this bank had been established recently as a growth division of foreign brokerage operation, and management had limited data to determine their ability to close an underwritten Nasdaq uplisting transaction. Similarly, Bank #5, which provided its draft LOE in July, had not engaged in a Nasdaq uplisting transaction in several years and, as a result, they lacked an ascertainable track record for the Board to consider. However, the terms of Bank #5’s proposal were more beneficial to the Company with respect to fees and Follow-On Rights.
In early June through late August 2023, management continued its discussions with the five banks from which we had received draft LOE’s. In July, Bank #2 informed the Company that it would not be able to proceed with an underwritten offering as they felt market conditions were not improving, and in August, the Company decided not to proceed with Bank #4 because of their failure to provide information to allow the Board to assess their ability to close an uplisting transaction.
In our analysis of the relative strengths and weaknesses of Banks #1, #3 and #5, the Board considered the following items:
| 1) | Terms of engagement provided in the LOEs, including but not limited to, fees and Follow-On Rights, especially in the instance the Company were to be acquired after or prior to the proposed Nasdaq listing; | |
| 2) | Reputation, track record and recently closed transactions for each of the banks, including the types of investors they historically attracted to fund their transactions; | |
| 3) | Amount of funds each of the banks believed they could raise, the amount of funds that management would be required to contribute to the transaction through investors brought by management, and whether the sum of these amounts would be sufficient to reach minimum shareholder equity requirements for listing on the Nasdaq Capital Market; and | |
| 4) | Anticipated terms of the funding round, including pricing, warrant coverage and necessity for a reverse split of our common stock. |
In its deliberations the Board also sought the recommendations on a confidential basis of certain of the Company’s larger investors and financial consultants, as well as its outside securities counsel and regulatory experts.
Management estimated that the Company would need to raise at least $8 million to have the required minimum shareholder equity to qualify for the Nasdaq Capital Market for a potential end of 2023 closing. This funding was only a portion of the capital that management forecasted would be needed to advance the Technology through Phase 2 trials; however, all four banks at this time were concerned with the potential for success of larger capital raises, especially on structure terms that were not highly dilutive to current shareholders. Additionally, management had concerns about the Company’s stock price, which was trading near the $4.00 range at that time on the OTCQB. To qualify for a Nasdaq uplisting, the Company would need to have a minimum $4.00 bid price. Several of the banks warned that another reverse split of our common stock may be needed to qualify for Nasdaq.
| 25 |
Due to these concerns and recommendations from advisors that market conditions could possibly improve in the fourth quarter 2023 or first quarter 2024, the Board decided to wait until after the Labor Day holiday at the earliest to make any decision on engaging an investment bank. Furthermore, generally around this time, two other investment banking options had arisen, and more importantly, our discussions with Telix were becoming more visible with respect to a potential a strategic partnership or acquisition (see “Discussions with Telix” below).
In August 2023, management began discussions with Bank #6. This group had certain advantages over the other three still in consideration. Firstly, Bank #6 had a dedicated and large retail investor basis through a partner group with a strong track record of successful fund raises in the $10 million to $15 million range. Secondly, their LOE contained no Follow-On Rights, including no right of first refusal or “tail” fee rights. Thirdly, Bank #6 was recommended by several parties that were consulting with the Company.
Also in late summer 2023, management began discussions with a separate division of Bank #1 – a wealth management / merchant banking group with a solid reputation for funding private biotech transactions. As part of these discussions, the merchant banking group engaged in preliminary technical and scientific due diligence with the Company, including holding two fairly detailed and lengthy diligence calls with the group’s scientific advisor. By mid-August 2023, we were informed by Bank #1, and specifically their merchant banking group, that they wanted to pursue a funding transaction; however, public underwriting was not their area of expertise, and therefore, they would need to work with their capital markets team to figure out a structure for engagement. We were informed that this would take a few weeks.
In September 2023 after the Labor Day holiday, the Board had narrowed down its choices of potential banks to Bank #1 and Bank #6. Bank #3 had expressed confidence in closing a $10 million underwriting, but our Board was concerned with the structure of that proposed transaction and the short-term investment strategies of investors that would likely lead the round. Bank #5 had been wavering over the prior month on their ability to complete a funding round large enough to meet Nasdaq minimum shareholder equity requirements, and as a result, the Board terminated further discussions.
By late September, management heard back from Bank #1 regarding the terms it would require to lead a $15 to $20 million financing through its merchant banking division. The fees and structure proposed were not in line with market terms, and the Board felt that they put the Company at risk. Further, they would not be able to commence a funding transaction until Q3 2024, which also put the Company at risk and in need of completing another private offering prior to the Nasdaq uplisting. As a result, by October 2023, only Bank #6 remained a viable option; however, this group had not yet committed as to timing for our contemplated offering.
Activities to Seek Partnership or Co-Development
Through mostly the efforts and contacts of our Executive Chairman, the Company had conducted several discussions with larger pharmaceutical companies over the previous three years. The nature of almost all these conversations was informative in substance, namely making sure potential future partners knew about CycloSam® and were keeping track of our clinical progress. It is common in the biotech industry for large pharmaceutical companies to monitor the progress of smaller, early-stage companies like QSAM. Typically, but not always, partnership or acquisition transactions are not pursued in earnest until the smaller entity has demonstrated sufficient clinical data to mark a “de-risking” of the technology. This often happens during or after Phase 2 trials, which the Company had not yet achieved, and as noted above, required additional funding to pursue.
In the spring of 2023, when the Board determined it to be in the best interest of the shareholders to pursue in parallel both a Nasdaq uplisting and potential strategic partnerships, our Executive Chairman redoubled his efforts in attracting the attention of larger firms in the sector. As part of these efforts, certain members of management attended the June 4-6, 2023 American Society of Clinical Oncology (ASCO) Conference in Chicago, and then all members of our management attended the June 24-27, 2023 Society of Nuclear Medicine and Medical Imaging (SNMMI) Conference, also in Chicago.
| 26 |
During the summer of 2023, the Company was in contact with six potential strategic partners in addition to Telix. These companies were:
| (1) | A large multi-national diversified pharmaceutical company listed on a major US stock exchange (Company #1). Our contact there was the Global Head of Business Development. | |
| (2) | A large multi-national diversified radiopharmaceutical company listed on a major US stock exchange (Company #2). Our contact there was the Chairman. | |
| (3) | A large multi-national nuclear medicine company (Company #3). Our contact was a senior technology scout. | |
| (4) | A large multi-national nuclear medicine company (Company #4). Our contact there was a Board of Directors member. | |
| (5) | A large multi-national radiopharmaceutical company (Company #5). Our contact there was the Global Director of Business Development. | |
| (6) | A mid-sized nuclear medicine company (Company #6). Our contact there was the CEO. |
The elements of the Company’s contact with these firms included telephone calls, emails and delivery of technology presentations, and meetings at the ASCO and/or SNMMI conferences. The feedback that the Company received from five of the six potential partners was that they were interested in the Company’s Technology but they would require Phase 2 data to proceed with more meaningful partnership discussions. The sixth company did not express an interest in pursuing indications in bone cancer.
Discussions with Telix
On May 5, 2023, our VP-Operations was invited by IsoTherapeutics Group, a company closely affiliated with our Licensor and who provides certain advisory and contracted manufacturing services for the Company, to meet with representatives of Telix at IsoTherapeutics’ office in Angleton, TX. IsoTherapeutics had been engaged by Telix for several years to perform research, development and manufacturing services for Telix. Among the Telix attendees at this initial meeting were its CEO; SVP Global Manufacturing, Supply Chain and Logistics; VP of Business Development; and Director of R&D. At this meeting the parties discussed the Company’s Technology, general clinical strategy, the ongoing CycloSam® trials and early data therefrom. Telix provided no ascertainable initial feedback from this first meeting.
On May 12, 2023, our VP Operations emailed Telix’s CEO to follow up on certain clinical items that arose during the initial meeting, including sending a corporate presentation which included early data from our current trial focused on metastatic bone disease, and our near-term strategic plans. Telix’s CEO responded to this email stating his interest in having a more strategic discussion accompanied by an invitation to meet at ASCO (June 2-6, 2023) in Chicago with him and Telix’s Chief Commercial Officer.
The Company’s CEO, General Counsel and VP-Operations met with Telix in Chicago on June 6, 2023. This was meant to be an introductory visit so that Telix could meet other key members of our management team. At this meeting, Telix’s CEO expressed his openness in holding strategic discussions around a possible partnership or an acquisition. The parties agreed to meet at the SNMMI conference later that month to further this conversation.
On June 8, 2023, the parties signed a mutual Confidential Disclosure Agreement.
| 27 |
On June 25, the Company’s Executive Chairman, CEO, General Counsel and VP-Operations met with Telix’s CEO at the SNMMI Conference in Chicago. Telix’s Chief Commercial Officer was also in attendance. At this meeting, the parties discussed in more detail what a possible strategic transaction may look like, including the benefits and downsides of a partnership structure versus an outright acquisition. The meeting ended with Telix’s CEO saying he would provide in the following two weeks general written terms of how he suggests a transaction could be structured.
On July 6, we received an initial draft of a term sheet from Telix. It consisted of a stock for stock acquisition with a commercial sales royalty-based earnout structure. Our Executive Chairman called a Board meeting for the following week to discuss the terms and next steps.
On July 13, we held a Board meeting to discuss the terms provided by Telix. The Board tasked the Company’s General Counsel to develop a financial model and provide relevant market and deal comparables to analyze Telix’s offer and provide the foundation for a counter proposal.
On July 21, the Board convened twice to review modeling and comparables provided by the General Counsel, and to review and finalize a draft counter proposal to Telix. Generally, the Board believed the upfront purchase price that had been offered was too low based upon risk-adjusted net present value analysis of the Company’s technology and market potential, as well as our analysis of comparable market transactions; and also, the offer was based on a premium to market that did not account for our fully diluted capitalization. Further, the Board felt that the earnout potential was too uncertain and undefinable. The Board determined that a clinical and commercial milestone-based earnout structure was more in line with market structures and would potentially provide a better outcome for our stockholders. Finally, from a structural standpoint, our General Counsel noted that a stock-for-stock transaction would be difficult to consummate, and that if the Board wanted to pursue an acquisition of QSAM by Telix, a merger structure would be more appropriate.
On July 22, we provided Telix with our initial counter proposal to their July 6 term sheet, which included the structural and financial term changes discussed in the July 21 Board meetings, including materially higher up-front consideration and defined milestones for future earnout payments.
Telix responded to our counter proposal on July 31, indicating that the parties were very far apart in valuation and other terms, but the CEO would entertain another discussion to understand our position. This was accomplished via a video call held on August 8. In addition to the full management team, Dr. Charles Link, our Director, participated in this meeting.
During the August 8 video conference, the Company led by its General Counsel, presented its analysis of the valuation of CycloSam®, inclusive of a risk adjusted net present value model, market comps and other factors for Telix’s consideration. Telix’s CEO questioned certain aspects of the Company’s assumptions in its modeling, and provided his own thoughts on the value of the Technology, including the costs, time and risks to bring it to market, market size and possible commercial pricing. The call ended with Telix’s CEO saying he would respond formally to our counter proposal within the next few days.
Telix responded to our counter proposal on August 13. In this correspondence, Telix provided materially higher upfront consideration, an earnout structure based on clinical, regulatory and commercial milestones, and a $2 million option and pre-closing collaboration fee that would be paid upon the signing of a formal term sheet. Telix’s CEO also mentioned that several important diligence items related to the supply chain and other technical items needed to be resolved before we could proceed any further. The response ended with a statement indicating that if we could not meet his terms, maybe the parties should discuss a license or partnership arrangement instead of acquisition, or otherwise, terminate discussions.
Over the following two weeks, the Company’s management worked to answer Telix’s diligence concerns, and the parties exchanged several emails on those technical and scientific topics. Management kept the Board informed during this period with several individual calls and a Board meeting held on August 24. On September 7, Telix’s CEO sent an email to the Company’s General Counsel saying that Telix was still “struggling” with several diligence items. Our Executive Chairman suggested that the parties meet in Indianapolis at Telix’s US headquarters when the CEO was there. This meeting took place on September 13.
| 28 |
The Company’s Executive Chairman and General Counsel attended the meeting in Indianapolis on September 13. Telix’s CEO was present in person at that meeting, and he had arranged for a video conference of approximately eight other key members of his scientific, marketing, sales and communications team. The Company’s CEO and VP-Operations attended the video conference. During that meeting, Telix’s team posed many questions on the diligence items they had previously flagged and on which the Company had provided materials in response. The meeting was followed up with a dinner with Telix’s CEO, and the Company’s Executive Chairman and General Counsel.
On September 20, upon the return to Australia of Telix’s CEO, the parties had a follow-up video call. On this call, Telix’s CEO mentioned that the Company had answered most of Telix’s initial diligence questions and that he would respond within the week with their decision on next steps. The Company held a Board meeting on September 22 to update the Directors on the current status of the transaction.
On September 24, Telix’s CEO sent the Company’s General Counsel an email saying that they would like to proceed towards completing the acquisition term sheet.
On September 27 and September 29, the Board met to discuss business and legal items in the term sheet and provide management with direction on changes needed in that document. On October 2, the Company sent a revised version of the term sheet back to Telix containing the business and legal terms that had been discussed and agreed in principle over the last month. Several emails were exchanged over the following week, and the Company’s Board met again on October 6 to discuss additional items in the term sheet. By October 10, the parties had appeared to reach agreement on the remaining material business terms.
Between October 10 and November 12, the Company’s General Counsel and outside securities counsel worked with Telix’s General Counsel and outside US securities counsel to get the term sheet ready to be executed. There were several complicated securities law issues dealing with the issuance of Australian securities to US holders that needed to be resolved with Australian counsel, as well as other tax and structural issues that the parties wanted to resolve in the term sheet prior to Telix paying the Company the option fee.
On October 25, the Company was informed that Telix’s Board minutes had been published and that the Telix Board had met on October 18 and approved the transaction in principle subject to final agreements.
On November 14, the Company’s Board approved by means of unanimous consent the term sheet. Later that day the parties signed the term sheet providing the material business, legal and structural terms of the proposed merger. The term sheet signed on November 14 provided the basis for the Merger Agreement that was signed on February 7, 2024; and the terms of the Merger Agreement mirrored closely the terms provided in that initial term sheet.
Rationale for the Merger with Telix
In making its decision to proceed with the proposed acquisition of the Company by Telix, the Company’s Board considered many factors, including but not limited to the following:
| 1) | Throughout the summer and fall 2023, financial market conditions had not improved materially and small, early-staged biotech companies generally were having a very difficult time attracting capital. Capital that was being invested in these types of companies was highly dilutive and highly structured with warrants and other mechanisms that would be harmful to the economic interests of current shareholders. | |
| 2) | By September, the investment banking offers to raise capital concurrent with a Nasdaq uplisting were highly structured, expensive and still uncertain in their chances of success. The Board believed that there was a reasonable certainty of significant dilution to our current shareholders if we pursued this path, and there was no certainty that we would be able to raise sufficient funding to achieve important value infection points. |
| 29 |
| 3) | Discussions with other potential strategic partners were progressing, however, the Board reasonably believed based on feedback from these other parties that no material agreement would be reached until the Company had completed Phase 2 of its clinical trials. | |
| 4) | The Board believed that the acquisition terms provided by Telix were fair to the Company’s shareholders, especially in light of the other options available to the Company. More specifically, the Board considered: |
| ○ | the premium being offered to QSAM’s then current and historical market price, and the total upfront consideration; | |
| ○ | the nature of the upfront consideration being equity in Telix, and the possibility for long-term value appreciation, and the required restrictions on trading those Telix shares relative to the Company’s limited market liquidity; | |
| ○ | the amount and structure of future contingent milestone payments and the probability of achieving those milestones to trigger the earnout payments; and | |
| ○ | the fact that the $2 million option and pre-closing collaboration fee would support the Company’s operations through a potential closing of the Merger without the need to raise additional capital and dilute our current shareholders; or if additional capital were required, such funding would likely be small and limited in potential dilution. |
| 5) | Telix presented many strengths and synergies that the Board considered important to the future of CycloSam®’s commercialization, therefore, increasing the chance of the Company’s shareholders receiving future contingent milestone payments, including: |
| ○ | An impressive leadership team, headed by a dynamic CEO; | |
| ○ | A strong cash position with growing revenue and a relatively low cost of capital; | |
| ○ | Multiple commercial stage drugs and a deep pipeline with two more drugs possibly coming to market soon, subject to regulatory approvals; | |
| ○ | Clinical and developmental expertise to lead CycloSam® through the clinical and regulatory process more effectively than the Company could at this time; and | |
| ○ | A commercial and logistics infrastructure, especially in the United States, necessary to get CycloSam® to market. |
In addition to its own internal discussion with management, the Board also weighed the opinions of certain key advisors, legal counsel and other key stakeholders. The general consensus of these advisors was that the proposed transaction with Telix presented a good potential outcome for the Company’s shareholders with the possibility of considerable upside, and it gave CycloSam® the best chance of ultimate commercialization where it could improve the lives of many people living with bone cancer.
| 30 |
Opinion of QSAM’s Financial Advisor
On November 30, 2024, QSAM retained Newbridge Securities Corporation (“Newbridge”) to act as its financial advisor in connection with providing a fairness opinion on the Merger Agreement with Telix. Newbridge, as part of its investment banking business, is continually engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, negotiated underwritings, secondary distributions of listed and unlisted securities, private placements and valuations for corporate and other purposes. QSAM selected Newbridge to act as its financial advisor in connection with the fairness opinion on the basis of Newbridge’s experience in similar transactions and its reputation in the investment community.
On February 2, 2024, at a meeting of the Board held to evaluate entering into a Merger Agreement with Telix, Newbridge delivered to the Board an oral opinion, which was confirmed by delivery of a written opinion, dated February 7, 2024, to the effect that, as of the date of November 14, 2023, the opinion and based on and subject to various assumptions and limitations described in its written opinion, that the Initial Consideration (i.e., the $33.1 million defined in the Merger Agreement as payable to QSAM stockholders upon Closing, plus the $2 million option fee and the assumption of $500,000 in closing payables) to be paid by Telix is fair, from a financial point of view, to QSAM’s common stockholders (the “Opinion”). Newbridge used the date of November 14, 2023, because that is the date that QSAM and Telix signed and made public a term sheet which contains substantially the same terms as in the definitive Merger Agreement.
The full text of Newbridge’s written opinion to QSAM’s board of directors, which describes, among other things, the assumptions made, procedures followed, factors considered and limitations on the review undertaken, is attached as Appendix C hereto and is incorporated by reference herein in its entirety. The following summary of Newbridge’s opinion is qualified in its entirety by reference to the full text of the opinion. Newbridge delivered its opinion to the Board for the benefit and use of the Board (in its capacity as such) in connection with and for purposes of its evaluation of entering into a Merger Agreement with Telix. Newbridge’s opinion also does not address the relative merits of entering in a Merger Agreement with Telix as compared to any alternative business strategies or transactions that might exist for QSAM, or the underlying business decision of QSAM whether to proceed with those business strategies or transactions.
In connection with rendering its opinion, Newbridge, among other things:
| ● | Considered its assessment of general economic, market and financial conditions as well as its experience in connection with similar transactions, and business and securities valuations generally; | |
| ● | Reviewed various drafts of the Merger Agreement; | |
| ● | Reviewed QSAM’s publicly available last eight fiscal quarters of historical financial results, (Q4-2021 - Q3-2023) as well as certain publicly available information concerning the trading of, and the trading market for, the ordinary shares of QSAM since January 2022; | |
| ● | Reviewed publicly available financial information of QSAM filed with the U.S. Securities & Exchange Commission, including its Form 10-Ks and 10-Qs, and certain reports on material events filed on Forms 8-K between January 1, 2022, through February 1, 2024; | |
| ● | Reviewed publicly available financial information of Telix filed with the ASX, including its release of new corporate presentations and new announcements between January 1, 2022, through February 1, 2024; | |
| ● | Conducted discussions with QSAM’s management team to better understand QSAM’s recent business history, and near-term financials; |
| 31 |
| ● | Performed a Public Company Comparable Analysis of similar companies to QSAM, that included variables such as companies trading on the OTC Markets, in the Biotechnology or Pharmaceutical sector, and had early-stage clinical drug assets focused on treating cancer, to derivate equity values; and | |
| ● | Performed a Premiums Paid Transaction Analysis that reviewed the variance between purchase price paid and the market capitalization 1-day prior to the public announcement of the executed Term Sheet describing the potential Merger (November 13, 2023, the “Pre-Announcement Date”) for public companies that met certain criteria. |
Newbridge’s analysis was focused on the “Initial Consideration” (defined above), and not the future value to shareholders that may be gained through the Contingent Value Rights. Newbridge determined that these are deferred payments that may not occur, and therefore not included in its calculations.
In conducting its review and arriving at its opinion, Newbridge did not independently verify any of the foregoing information and Newbridge assumed and relied upon such information being accurate and complete in all material respects, and Newbridge further relied upon the assurances of management of QSAM that they are not aware of any facts that would make any of the information reviewed by Newbridge inaccurate, incomplete or misleading in any material respect. In addition, Newbridge has not assumed any responsibility for any independent valuation or appraisal of the assets or liabilities, including any ongoing litigation and administrative investigations, if any, of QSAM, nor has Newbridge been furnished with any such valuation or appraisal. In addition, Newbridge has not assumed any obligation to conduct, nor has it conducted, any physical inspection of the properties or facilities of QSAM.
The issuance of Newbridge’s opinion was approved by an authorized internal committee of Newbridge. Newbridge’s opinion is necessarily based on economic, market and other conditions as they exist and can be evaluated on, and the information made available to it on, the date thereof. Newbridge expressed no opinion as to the underlying valuation, future performance or long-term viability of QSAM or Telix and its successors. Further, Newbridge expressed no opinion as to the prices at which shares of Common Stock of QSAM or Telix will trade at any time. It should be understood that, although subsequent developments may affect Newbridge’s opinion, Newbridge does not have any obligation to update, revise or reaffirm its opinion and has expressly disclaimed any responsibility to do so.
Summary of Financial Analyses
The following represents a brief summary of the material financial analyses reviewed by the Board and performed by Newbridge in connection with its opinion. The financial analyses summarized below include information presented in tabular format. In order to fully understand the financial analyses performed by Newbridge, the tables must be read together with the text of each summary. The tables alone do not constitute a complete description of the financial analyses performed by Newbridge. Considering the data set forth in the tables below without considering the full narrative description of the financial analyses, including the methodologies and assumptions underlying the analyses, could create a misleading or incomplete view of the financial analyses performed by Newbridge.
The preparation of analyses and a fairness opinion is a complex analytic process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances. Therefore, this summary does not purport to be a complete description of the analyses performed by Newbridge or of its presentation provided to QSAM’s board of directors on February 2, 2024.
The order in which these analyses are presented below, and the results of those analyses, should not be taken as an indication of the relative importance or weight given to these analyses by Newbridge or QSAM’s board of directors. Except as otherwise noted, the following quantitative information, to the extent it is based on market data, is based on market data as it existed on or before November 14, 2023, and is not necessarily indicative of current market conditions. All analyses conducted by Newbridge were going-concern analyses and Newbridge expressed no opinion regarding the liquidation value of any entity.
| 32 |
Comparable Public Company Analysis
Newbridge analyzed the current market valuations of comparable publicly listed companies (“peers”). Obtaining a meaningful valuation result from this method depends on ensuring a good level of comparability between QSAM and its peers.
To calculate the implied equity value of QSAM, Newbridge obtained the average equity value of six comparable public companies determined by Newbridge to be similar to QSAM. The comparable public companies were selected using the following criteria: (i) listed on the OTC Markets, (ii) operate in the biotech and pharmaceuticals sector, (iii) lead clinical drug candidate is in the “oncology” space, (iv) lead clinical drug candidate is early-stage (between Phase I and Phase II), and (v) were still in the “pre-commercialization” stage, with less than $1 million in revenue and either zero or negative EBITDA in its last fiscal year. The average equity value using this analysis was $21.6 million.
The tables below summarize certain observed historical financial performance and equity values of the selected comparable public companies, which were sourced from S&P Capital IQ data as of February 1, 2024, as well as qualitative research performed by Newbridge on QSAM’s business model and lead clinical drug candidate.
Industry (Early-Stage Oncology Biotech) 11/13/2023
| Balance Sheet | Income Statement | Other | ||||||||||||||||||||||||||
| Stock | Equity | Total Enterprise | Total Revenue | EBITDA | Industry | Industry | Therapeutic | |||||||||||||||||||||
| Company Name | Symbol | Price | Value | Value | LTM | LTM | Sector | Sub-Sector | Focus | |||||||||||||||||||
| Provectus Biopharmaceuticals, Inc. | OTCPK:PVCT | $ | 0.14 | $ | 58.7 | $ | 61.5 | $ | 0.6 | $ | (3.0 | ) | Healthcare | Pharmaceuticals | Cancer / Oncology | |||||||||||||
| Nascent Biotech, Inc. | OTCPK:NBIO | $ | 0.16 | $ | 25.8 | $ | 25.1 | $ | 0.0 | $ | 0.0 | Healthcare | Biotechnology | Cancer / Oncology | ||||||||||||||
| Zenith Capital Corp. | OTCPK:ZHCL.F | $ | 0.13 | $ | 19.8 | $ | 25.9 | $ | 0.0 | $ | (13.3 | ) | Healthcare | Biotechnology | Cancer / Oncology | |||||||||||||
| Inhibitor Therapeutics, Inc. | OTCPK:INTI | $ | 0.00 | $ | 17.4 | $ | 12.4 | $ | 0.0 | $ | 0.0 | Healthcare | Biotechnology | Cancer / Oncology | ||||||||||||||
| Curative Biotechnology, Inc. | OTCPK:CUBT | $ | 0.01 | $ | 7.0 | $ | 25.9 | $ | 0.0 | $ | (4.3 | ) | Healthcare | Biotechnology | Cancer / Oncology | |||||||||||||
| Oncotelic Therapeutics, Inc. | OTCPK:OTLC | $ | 0.03 | $ | 13.2 | $ | 24.8 | $ | 0.1 | $ | (0.9 | ) | Healthcare | Biotechnology | Cancer / Oncology | |||||||||||||
| Ayala Pharmaceuticals, Inc. | OTCPK:ADXS | $ | 0.90 | $ | 9.7 | $ | 9.7 | $ | 0.0 | $ | 0.0 | Healthcare | Biotechnology | Cancer / Oncology | ||||||||||||||
| ($ in millions, except per share data) | Average | $ | 21.6 | |||||||||||||||||||||||||
Comparable Public Company Analysis OTC Early-Stage Oncology
| Balance Sheet | Other | |||||||||
| Equity | Regulatory Status | Medical Indication + Lead Drug Candidate | | ||||||||
| Company Name | Symbol | Value | (Lead Program) | Additional detail | ||||||
| Provectus Biopharmaceuticals, Inc. | OTC:PVCT | $ | 58.7 | Phase I (Underway) | Treatment of stage III and IV melanoma and different types of liver cancers PV-10: Cancer immunotherapy potentially agnostic to tumor type | |||||
| Nascent Biotech, Inc. | OTC:NBIO | $ | 25.8 | Phase I (Completed) | Develops monoclonal antibodies for the treatment of various forms of cancer Pritumumab: Fully natural human IgG antibody | |||||
| Zenith Capital Corp. | OTC:ZHCLF | $ | 19.8 | Phase II (Underway) | Development of bromodomain inhibitors for the treatment of cancer ZEN-3694: Treatment of metastatic castration resistant prostate cancer | |||||
| Inhibitor Therapeutics, Inc. | OTC:INTI | $ | 17.4 | Phase II (Underway) | Commercializes therapeutics for patients with certain cancers SUBA-Itraconazole: Oral formulation of the drug itraconazole | |||||
| Curative Biotechnology, Inc. | OTC:CUBT | $ | 7.0 | Phase I (Underway) | Develops a pipeline focusing on Neuroncology CUBT906: Monoclonal antibody inhibiting tumor growth and migration of the tumor. | |||||
| Oncotelic Therapeutics, Inc. | OTC:OTLC | $ | 13.2 | Phase II (Completed) | Developing drugs for the treatment of orphan oncology indications OT-101: treatment of solid tumors with focus on brain cancer. | |||||
| Ayala Pharmaceuticals, Inc. | OTC:ADXS | $ | 9.7 | Phase II (Underway) | Developing small molecule therapeutics for rare tumors and aggressive cancers. AL 102: Oral gamma secretase inhibitor for desmoid tumors. | |||||
| Average | $ | 21.6 | ||||||||
Source: S&P Capital IQ (as of February 1st, 2024) and company SEC filings
| 33 |
The comparable public company analysis uses data from comparable guideline companies to develop a measure of current value for QSAM. The theory underlying the comparable public companies’ valuation is that companies in the same industry with similar operating characteristics should have certain valuation benchmarks in common. The goal of the analysis is to develop a premise for relative value, which when coupled with other valuation approaches, presents a foundation for determining an approximate equity value for QSAM.
Premiums Paid Transaction Analysis
Newbridge performed a Premiums Paid Transaction analysis that reviewed the variance between purchase price paid and the market capitalization as of the Pre-Announcement Date for public companies that met certain criteria. Newbridge analyzed the last year (since January 2023) of transaction data in the Pharmaceutical / Biotechnology sectors to find similar transactions where the public targets were being acquired.
The criteria used for the selected transactions were those in which the targets and scenarios most resembled QSAM included: (i) targets with businesses within the “Pharmaceutical / Biotechnology” sector, (ii) companies that trade on a Senior U.S. Exchange (the NYSE American / NASDAQ / NYSE), (iii) where an outside party is crossing the threshold of owning >51% of the company, and (iv) the transaction was not considered a reverse merger.
Newbridge obtained the average “Premium Paid” for this peer group (+48.9%) and compared it to the premium being paid for QSAM (on a fully-diluted basis) on the Pre-Announcement Date (+57.6%). The Premium Paid for QSAM is higher than the average Premium Paid for this peer group.
Newbridge also reviewed the trading history of QSAM to compare where it was valued in the public markets at the time of the deal announcement versus the rest of calendar year 2023. The fully-diluted market value of QSAM at market close on November 13, 2023, was approximately $22.6M, and the trading volume weighted average fully-diluted market capitalization of the company throughout 2023 until the deal announcement was approximately $20.9M. The equity value of QSAM at the time of announcement was approximately 8.2% higher than where it had been trading all year.
| Announced Date | Target/Issuer | Industry Classifications | Buyers/Investors | Target Stock Premium - 1 Day Prior (%) | ||||||
| 05/03/2023 | Quince Therapeutics, Inc. (NasdaqGS:QNCX) | Biotechnology | Biomed Industries, Inc. | 64.1 | % | |||||
| 05/26/2023 | Taro Pharmaceutical Industries Ltd. (NYSE:TARO) | Pharmaceuticals | Sun Pharmaceutical Industries Limited (NSEI:SUNPHARMA) | 48.4 | % | |||||
| 09/21/2023 | Applied Molecular Transport Inc. (NasdaqCM:AMTI) | Biotechnology | Cyclo Therapeutics, Inc. (NasdaqCM:CYTH) | 33.9 | % | |||||
| 10/03/2023 | POINT Biopharma Global Inc. (NasdaqCM:PNT) | Biotechnology | Eli Lilly and Company (NYSE:LLY) | 82.7 | % | |||||
| 10/05/2023 | Orchard Therapeutics plc (NasdaqCM:ORTX) | Biotechnology | Kyowa Kirin Co., Ltd. (TSE:4151) | 96.6 | % | |||||
| 10/08/2023 | Mirati Therapeutics, Inc. (NasdaqGS:MRTX) | Biotechnology | Bristol-Myers Squibb Company (NYSE:BMY) | -3.7 | % | |||||
| 10/13/2023 | Rain Oncology Inc. (NasdaqGS:RAIN) | Pharmaceuticals | Concentra Biosciences, LLC | 28.0 | % | |||||
| 10/18/2023 | Freeline Therapeutics Holdings plc (NasdaqCM:FRLN) | Biotechnology | Syncona Investment Management Limited | 45.3 | % | |||||
| 11/24/2023 | Theseus Pharmaceuticals, Inc. (NasdaqGS:THRX) | Pharmaceuticals | Concentra Biosciences, LLC | 17.4 | % | |||||
| 11/30/2023 | ImmunoGen, Inc. (NasdaqGS:IMGN) | Biotechnology | AbbVie Inc. (NYSE:ABBV) | 79.0 | % | |||||
| 12/06/2023 | Cerevel Therapeutics Holdings, Inc. (NasdaqCM:CERE) | Biotechnology | AbbVie Inc. (NYSE:ABBV) | 26.4 | % | |||||
| 12/12/2023 | Icosavax, Inc. (NasdaqGS:ICVX) | Biotechnology | AstraZeneca PLC (LSE:AZN) | 80.7 | % | |||||
| 12/13/2023 | Rain Oncology Inc. (NasdaqGS:RAIN) | Pharmaceuticals | Pathos AI, Inc. | 3.6 | % | |||||
| 12/22/2023 | Karuna Therapeutics, Inc. (NasdaqGM:KRTX) | Biotechnology | Bristol-Myers Squibb Company (NYSE:BMY) | 53.4 | % | |||||
| 12/26/2023 | RayzeBio, Inc. (NasdaqGM:RYZB) | Biotechnology | Bristol-Myers Squibb Company (NYSE:BMY) | 94.0 | % | |||||
| 12/26/2023 | Reneo Pharmaceuticals, Inc. (NasdaqGM:RPHM) | Biotechnology | Concentra Biosciences, LLC | 32.4 | % | |||||
| Average | 48.9 | % | ||||||||
Source: S&P Capital IQ (as of February 1st, 2024)
| 34 |
Miscellaneous
The discussion set forth above is a summary of the material financial analyses presented by Newbridge to QSAM’s board of directors in connection with its opinion and is not a comprehensive description of all analyses undertaken by Newbridge in connection with its opinion. The preparation of a financial opinion is a complex analytical process involving various determinations as to the most appropriate and relevant methods of financial analysis and the application of those methods to the particular circumstances and, therefore, a financial opinion is not readily susceptible to partial analysis or summary description. Newbridge believes that its analyses summarized above must be considered as a whole. Newbridge further believes that selecting portions of its analyses and the factors considered, or focusing on information presented in tabular format, without considering all analyses and factors or the narrative description of the analyses, could create a misleading or incomplete view of the processes underlying Newbridge’s analyses and opinion. The fact that any specific analysis has been referred to in the summary above is not meant to indicate that such analysis was given greater weight than any other analysis referred to in the summary.
The estimates of the future performance of QSAM and Telix in or underlying Newbridge’s analyses are not necessarily indicative of actual values or actual future results, which may be significantly more or less favorable than those estimates or those suggested by Newbridge’s analyses. The analyses do not purport to be appraisals or to reflect the prices at which a company might actually be sold or the prices at which any securities have traded or may trade at any time in the future. Accordingly, the estimates used in, and the ranges of valuations resulting from, any particular analysis described above are inherently subject to substantial uncertainty and should not be taken to be Newbridge’s view of the actual values of the Common Stock.
Newbridge, as part of its investment banking business, is regularly engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, related party transactions, going private transactions, negotiated underwritings, secondary distributions of listed and unlisted securities, debt restructurings, private placements, and valuations for corporate and other purposes. Newbridge in the future may provide financial advisory or investment banking services to QSAM, Telix or their respective affiliates and may receive compensation for the rendering of these services. During the past two years, (i) QSAM has not engaged Newbridge to provide, and Newbridge has not provided, investment banking, financial advisory or other financial services to Telix unrelated to entering into a Merger Agreement for which QSAM has paid or expects to pay fees to Newbridge, and (ii) neither Newbridge nor any of its affiliates have provided investment banking services or other financial services to Telix or its affiliates unrelated to entering into a Merger Agreement for which Newbridge and its affiliates have received, or may receive, compensation.
Conclusion
The implied equity value of QSAM derived from the Comparable Public Company Analysis that Newbridge used showed a value of approximately $21.6M. The Initial Consideration to be received by QSAM’s shareholders of $35.6M (inclusive of both the Purchase price of $33.1 million, contributions to expenses of $500,000 and the $2 million option and collaboration fee paid to QSAM upon signing the term sheet) is higher than Newbridge’s estimates of the implied equity value of QSAM.
The Premium Paid for QSAM of +57.6% is higher than the Premium Paid for a comparable transaction peer group of +48.9%.
Based upon and subject to the forgoing, it is Newbridge’s Opinion that, as of the date of November 14th, 2023, the Initial Consideration to be paid by Telix is fair, from a financial point of view, to QSAM’s common stockholders.
The type and amount of consideration payable in the Merger Agreement was determined through negotiations between QSAM and Telix, and was approved by the board of directors of QSAM and Telix. As described above, Newbridge’s opinion and analyses were only one of many factors considered by QSAM’s board of directors in its evaluation of whether to enter the Merger Agreement and should not be viewed as determinative of the views of QSAM’s board of directors or management with respect to the entry into a Merger Agreement.
Fees and Expenses
As compensation for Newbridge’s services in connection with the rendering of its opinion to QSAM’s board of directors, QSAM agreed to pay Newbridge a fee of $70,000. $10,000 of the fee was paid as an upfront retainer and the remaining $60,000 was paid upon delivery of the opinion. No portion of the Newbridge fee is refundable or contingent upon the conclusion reached in the opinion. QSAM has also agreed to indemnify Newbridge against certain liabilities and other items that may arise out of QSAM’s engagement of Newbridge. The QSAM board of directors did not limit Newbridge in any way in the investigations it made or the procedures it followed in rendering its opinion.
| 35 |
Interests of QSAM Directors, Key Employees and Key Consultant in the Merger
The Directors, Key Employees and Key Consultant have various interests in the Merger described in this section that may be in addition to, or different from, the interests of QSAM stockholders, generally. The names and positions of each such Director, Key Employee and Key Consultant are set forth below.
| Name | Positions Held | Age | Date
of Election or Designation |
Date
of Termination or Resignation | ||||
| C. Richard Piazza | Executive Chairman | 76 | November 2020 | * | ||||
| Douglas R. Baum | Chief Executive Officer & Director | 57 | January 2020 | * | ||||
| Adam King | Chief Financial Officer | 39 | December 2021 | * | ||||
| Christopher Nelson | General Counsel and Executive VP | 54 | July 2014 | * | ||||
| Namrata Chand | VP-Operations | 39 | January 2020 | * | ||||
| Charles J. Link, Jr. | Director | 64 | February 2021 | * | ||||
| Adriann Sax | Director | 63 | January 2022 | * |
Common Stock Ownership
QSAM’s directors, officers and executive officers will receive the same consideration for any shares of QSAM Common Stock that they hold on the same terms and conditions as other QSAM stockholders. As of March 31, 2024, the latest practicable date prior to the filing of this Information Statement, QSAM’s directors, officers and executive officers beneficially owned, in the aggregate, approximately 26% of the shares of QSAM Common Stock. See “Security Ownership of Certain Beneficial Owners and Management” on page 66 for the QSAM Common Stock ownership of each QSAM director, officer and executive officer.
Treatment of Outstanding Stock Options
Pursuant to Merger Agreement, effective as of the date QSAM files this definitive Information Statement, each QSAM Option will vest in full and become exercisable up to and through the close of regular trading on the Last Exercise Date in accordance with the terms and conditions of such QSAM Option, and such QSAM Option will terminate for no consideration and be of no further force or effect as of immediately prior to closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date. According to the terms of the grants made by the Company, all QSAM Options were fully vested as of March 31, 2024 and will remain exercisable until the Last Exercise Date.
| 36 |
The following table sets forth, for each of the Directors, Key Employees and Key Consultant, the number of QSAM Options held by such individual as of March 31, 2024, the latest practicable date prior to the filing of this Information Statement, along with the weighted average exercise price of such outstanding stock options. All such QSAM Options are fully vested. All QSAM Options have exercise prices that are higher than the current trading price of QSAM on OTCQB as well as the estimated Closing Consideration being paid by Telix per share of QSAM Common Stock.
| Officers and Directors | QSAM Stock Options | Avg. Exercise Price | ||||||
| C. Richard Piazza | 15,625 | $ | 10.88 | |||||
| Douglas Baum | 15,825 | $ | 11.00 | |||||
| Christopher Nelson | 15,690 | $ | 11.01 | |||||
| Adam King | 10,500 | $ | 10.00 | |||||
| Namrata Chand | 27,875 | $ | 8.66 | |||||
| Adriann Sax | 25,000 | $ | 9.50 | |||||
| Charles J. Link | 26,375 | $ | 8.33 | |||||
| Total | 138,890 | |||||||
Severance Benefits
Each Key Employee has entered into an employment agreement with QSAM pursuant to which the Key Employee is entitled to severance benefits upon a qualifying termination of employment. More specifically, if the employment of such Key Employee is terminated without cause or following a material change, as such terms are defined in the applicable employment agreements, the Key Employee will receive his/her salary through the date of termination and for an additional period following termination ranging from 12 to 24 months, as provided in the respective employment agreement. Such Key Employee is also entitled to receive a pro-rated portion of any annual bonus that would have been earned for the year in which the termination is effective (or, if greater, a lump sum payment of 50% of the full target bonus). In addition, all stock options and restricted stock held by such Key Employee vest immediately upon such termination, and any healthcare benefits continue for 18 months (or 12 months in the case of Namrata Chand) following such termination.
Assuming that each Key Employee experiences a termination that results in severance payments and benefits becoming payable at the closing of the Merger, each Key Employee would be entitled to receive the following amount of severance payments and benefits under the employment agreements described above:
| Key Employee | Severance Term | Total Base Salary Severance Payment | Bonus Subject to Severance | Other Benefits Subject to Severance | ||||||
| C. Richard Piazza, Executive Chairman | 24 months | $ | 600,000 | None | None | |||||
| Douglas Baum, CEO | 24 Months | $ | 600,000 | None | None | |||||
| Christopher Nelson, General Counsel and EVP | 18 Months | $ | 337,500 | None | None | |||||
| Namrata Chand, VP-Operations | 12 Months | $ | 225,000 | None | None | |||||
To the extent a Key Employee’s employment is not terminated and the Key Employee continues employment following the closing of the Merger, severance payment and benefits would not be payable pursuant to the applicable employment agreement. In addition, certain Key Employees may agree to forego all severance payments and benefits, in part or in their entirety, due to lack of financial resources at QSAM and the adverse impact it may have on the Closing Consideration.
| 37 |
Transaction Bonuses Upon Change in Control
The Key Employees and one Director, Charles Link, are entitled to receive a transaction bonus in connection with the Merger pursuant to, in the case of the Key Employees, the terms of their employment agreements, as amended, and, in the case of the Director, under an agreement entered into between the Director and the Company. Such transaction bonuses are intended to equal in the aggregate 6.25% of the Closing Consideration (calculated after deduction of the IGL License Fee in the amount of $1,655,000 payable at the closing of the Merger and less any unpaid QSAM expenses that are not being assumed by Telix) of approximately $1,965,313, as currently estimated, in total payments. This estimate assumes that there will be no unpaid QSAM expenses at the closing of the Merger that are not being assumed by Telix. The transaction bonus shall be paid in the form of Telix Ordinary Shares, less appropriate tax withholdings. The value of the transaction bonus attributable to these individuals with respect to the Closing Consideration, assuming there will be no unpaid QSAM expenses at the closing of the Merger that are not being assumed by Telix, is estimated as follows:
Key Employee or Director | Transaction Bonus % | Estimated Approximately Transaction Bonus ($) | ||||||
| C. Richard Piazza, Executive Chairman | 1.75 | % | $ | 550,287 | ||||
| Douglas Baum, CEO | 1.75 | % | $ | 550,287 | ||||
| Christopher Nelson, General Counsel and EVP | 1.0 | % | $ | 315,450 | ||||
| Namrata Chand, VP-Operations | 1.0 | % | $ | 315,450 | ||||
| Charles Link, Director | 0.75 | % | $ | 235,837 | ||||
These individuals may also be entitled to receive transaction bonuses, determined based on their transaction bonus percentages listed above, with respect to any Milestone Payments, after deduction of the amount of the IGL License Fee payable in connection with such Milestone Payments. The estimated value of the potential transaction bonus attributable to these individuals with respect to the possible Milestone Payments, calculated as a percentage of the Milestone Payments, assuming aggregate Milestone Payments of $90,000,000 (and after deducting the $4,500,000 in the aggregate of stock and/or cash that would be payable to IGL in connection with such Milestone Payments as the IGL License Fee) is as follows:
| Key Employee or Director | Transaction Bonus % | Estimated Approximately Transaction Bonus ($) | ||||||
| C. Richard Piazza, Executive Chairman | 1.75 | % | $ | 1,496,250 | ||||
| Douglas Baum, CEO | 1.75 | % | $ | 1,496,250 | ||||
| Christopher Nelson, General Counsel and EVP | 1.0 | % | $ | 855,000 | ||||
| Namrata Chand, VP-Operations | 1.0 | % | $ | 855,000 | ||||
| Charles Link, Director | 0.75 | % | $ | 641,250 | ||||
The actual amount of the aggregate transaction bonuses payable in connection with the Milestone Payments may be lower than the amounts estimated above in the event not all of the Milestones are achieved or there are any deductions to the Milestone Payments pursuant to the terms of the CVR Agreement.
Acceleration of Restricted Shares
The Directors, Key Employees and Key Consultant have been issued Restricted Shares that are subject to performance and time-based vesting and that will accelerate and become fully vested upon the closing of the Merger. The following table sets forth the total number of Restricted Shares that will be subject to accelerated vesting upon the closing of the Merger for each individual:
| Named Executives and Directors | Restricted Shares Subject to Acceleration | |
| C. Richard Piazza (Exec Chair) | 72,267 | |
| Douglas Baum (CEO, Dir) | 72,267 | |
| Christopher Nelson (GC) | 46,365 | |
| Namrata Chand (VP) | 36,365 | |
| Adam King (CFO) | 20,910 | |
| Charles Link | 10,455 | |
| Adriann Sax | 10,455 |
New Employment or Consulting Arrangements
None of the Key Employees have entered into an ongoing employment agreement with Telix as of the date hereof concerning terms and conditions of employment following closing of the Merger. While it is anticipated that some Key Employees will be offered employment agreements with Telix after the date of closing, and others will receive consulting agreements, none of these agreements or terms have been finalized.
Indemnification; Directors’ and Officers’ Insurance
QSAM’s Amended and Restated Bylaws provide for indemnification of QSAM’s directors and executive officers for certain types of expenses, including attorneys’ fees, judgments, fines, and settlement amounts incurred by persons in any action or proceeding, including any action by QSAM or in QSAM’s right, arising out of their services as a director or executive officer. Further, pursuant to the Merger Agreement, the Company shall obtain prior to the Closing a prepaid, non-cancelable six-year “tail” policy containing terms not less favorable than the terms of the Company’s current directors’ and officers’ liability insurance coverage with respect to matters existing or occurring at or prior to the First Effective Time.
Golden Parachute Compensation
In accordance with Item 402(t) of Regulation S-K, the table below presents the estimated amounts of compensation that each named executive officer (“Named Executive Officer”) could receive that are based on or otherwise relate to the Merger. This compensation is referred to as “golden parachute” compensation by the applicable SEC disclosure rules and in this section.
The amounts set forth below on based on the following assumptions:
| ● | the Merger is consummated on March 31, 2024, the latest practicable date prior to the filing of this Information Statement; | |
| ● | the relevant price per share of QSAM Common Stock is $6.63, the average of the closing market price over the first five business days following the public announcement of the Merger; and | |
| ● | the Transaction Bonuses are paid at the First Effective Time. |
The amounts indicated below are estimates of amounts that would be payable to the named executive officers and are based on multiple assumptions that may or may not actually occur. The actual amounts, if any, to be received by a named executive officer may differ in material respects from the estimated amounts below. All dollar amounts have been rounded to the nearest whole number.
| Name | Cash(1) | Equity(2)(3) | Perquisites/ Benefits | Other(4) | Total Compensation | |||||||||||||||
| ($) | ($) | ($) | ($) | ($) | ||||||||||||||||
| C. Richard Piazza | - | $ | 335,633 | - | $ | 2,046,537 | $ | 2,382,170 | ||||||||||||
| Douglas Baum | - | $ | 335,663 | - | $ | 2,046,537 | $ | 2,382,170 | ||||||||||||
| Christopher Nelson | - | $ | 221,269 | - | $ | 1,170,450 | $ | 1,390,718 | ||||||||||||
(1) Represents cash severance payments that would be payable in connection with a termination of employment that results in severance payments under the Named Executive Officers’ employment agreements. The Named Executive Officers are not entitled to severance payments as a result of the consummation of the Merger.
(2) The amounts in this column reflect the aggregate dollar value of Restricted Stock subject to acceleration pursuant to the terms of QSAM’s 2016 Omnibus Equity Incentive Plan as well as amendments to the Named Executive Officers’ employment agreements (each, a “single trigger” benefit).
(3) The amounts in this column do not ascribe any value to QSAM Options held by the Named Executive Officers because all QSAM Options are fully vested and have exercise prices that are higher than the current trading price of QSAM on OTCQB as well as the estimated Closing Consideration being paid by Telix per share of QSAM Common Stock.
(4) The amounts in this column reflect the transaction bonuses payable in the form of Telix Ordinary Shares, assuming that the transaction results in net proceeds of $31,445,000 at the closing of the Merger, and all $90,000,000 of the contingent future Milestone Payments are made by Telix.
| 38 |
Regulatory Approvals
The Buyer and QSAM have each agreed to use their reasonable best efforts to take all actions and to do all things necessary, proper or advisable to consummate and make effective the Merger and the other transactions contemplated by the Merger Agreement.
The parties’ respective obligations to complete the Merger are conditioned, among other matters, upon the absence of any law, decree, injunction or other legal restraint, prohibition or binding order of any governmental authority that restrains, prohibits or otherwise makes the Merger illegal. Neither the Buyer nor QSAM is aware of any material regulatory approvals or actions that are required for completion of the Merger except for compliance with the federal and state securities laws in connection with the issuance of Telix Ordinary Shares to the stockholders of QSAM. It is presently contemplated that if any such additional regulatory approvals or actions are required, those approvals or actions will be sought. There can be no assurance, however, that any additional approvals or actions will be obtained.
De-Listing and Deregistration of QSAM Common Stock after the Merger
Pursuant to the Merger Agreement, when the Merger is completed, the QSAM Common Stock currently listed on the OTCMKTS will be delisted on OTCMKTS subject to the procedure set forth by the Financial Industry Regulatory Authority and will be deregistered under the Exchange Act as promptly as practicable after the First Effective Time.
QSAM Stockholder Representative
Company Stockholder Representative is a representative designated by the parties to act on behalf of the pre-Reverse Split Company stockholders and the Company stockholders, as the exclusive agent and attorney-in-fact for and on behalf of such persons, for certain limited purposes, as specified in the Merger Agreement. The initial Company Stockholder Representative is David H. Clarke.
Mr. Clarke, through entities that he has sole voting authority, is the largest beneficial shareholder of QSAM Common Stock. He presently runs his family real estate development and investment company, GSB Holdings, Inc. From June 2010 until September 2014, Mr. Clarke was Chairman and Chief Executive officer and a controlling shareholder of Hong Kong Exchange listed United Pacific Industries Limited, engaged in diversified manufacturing. Prior that time, he was Vice Chairman of the company. From June 1995 until September 2006, Mr. Clarke served as Chairman and Chief Executive Officer of NYSE listed Jacuzzi Brands, Inc. Jacuzzi Brands was sold to Apollo Management in October 2006. Prior to his appointment at Jacuzzi Brands, Mr. Clarke was with London and New York listed Hanson PLC for 25 years. In later years, he was a director, Vice Chairman of Hanson and CEO of Hanson Industries, Inc., the United States arm of Hanson PLC. Mr. Clarke has served as a director of a number of listed companies including Newmont Mining Corporation, International Protein Corporation, Fiduciary Trust Company International and Omega Protein, Inc. He was chairman of the Fiduciary Trust Audit Committee for over 10 years.
Appraisal and Dissenters Rights
Under Delaware law, QSAM stockholders and beneficial owners are entitled to appraisal rights in connection with the Merger, provided that they meet all of the conditions set forth in Section 262. Pursuant to Section 262, QSAM stockholders and beneficial owners who do not vote in favor of the Merger and who comply with the applicable requirements of Section 262 will have the right to seek appraisal of the fair value of such shares as determined by the Delaware Chancery Court if the Merger is completed. It is possible that the fair value as determined by the Delaware Chancery Court may be more or less than, or the same as, the Closing Consideration. QSAM stockholders and beneficial owners should note that investment banking opinions as to the fairness from a financial point of view of the consideration payable in a sale transaction, such as the Merger, are not opinions as to, and do not in any manner address, fair value under the DGCL.
QSAM stockholders and beneficial owners electing to exercise appraisal rights must comply with the strict procedures set forth in Section 262 in order to exercise and perfect their rights. ANY STOCKHOLDER OR BENEFICIAL OWNER WISHING TO PRESERVE THEIR RIGHTS TO APPRAISAL MUST MAKE A DEMAND FOR APPRAISAL AS DESCRIBED BELOW.
The following is intended as a brief summary of the material provisions of Section 262 required to be followed by dissenting QSAM stockholders or beneficial owners wishing to demand and perfect their appraisal rights. This summary, however, is not a complete statement of all applicable requirements and is subject to and qualified in its entirety by reference to Section 262, the full text of which appears in Appendix D to this Information Statement.
Under Section 262, QSAM is required to notify each stockholder who is entitled to appraisal rights that appraisal rights will be available and include in the notice a copy of Section 262 of the DGCL.
THIS INFORMATION STATEMENT CONSTITUTES QSAM’S NOTICE TO ITS STOCKHOLDERS AND BENEFICIAL OWNERS OF THE AVAILABILITY OF APPRAISAL RIGHTS IN CONNECTION WITH THE MERGER UNDER SECTION 262 AND A COPY OF SECTION 262 IS ATTACHED TO THIS INFORMATION STATEMENT AS APPENDIX D.
If you wish to consider exercising your appraisal rights, you should carefully review the text of Section 262 set forth in Appendix D to this Information Statement and consult your legal advisor. If you fail to timely and properly comply with the requirements of Section 262, your appraisal rights may be lost. To exercise appraisal rights with respect to your shares of QSAM Common Stock:
| ● | you must not vote in favor of approval of the Merger; | |
| ● | you must deliver to the Company a written demand for appraisal within 20 days from date of this definitive Information Statement. This demand will be sufficient if it reasonably informs us of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares; | |
| ● | you must continuously hold the shares from the date of making the demand through the effective time of the Merger; | |
| ● | you (or any other stockholder that has properly demanded appraisal rights and is otherwise entitled to appraisal rights) must file a petition in the Delaware Court of Chancery requesting a determination of the fair value of the shares within 120 days after the effective time of the Merger. The Company (or its successor) is under no obligation to file any such petition in the Delaware Court of Chancery and has no intention of doing so. Accordingly, it is the obligation of the QSAM stockholders to initiate all necessary action to perfect their appraisal rights in respect of shares of QSAM Common Stock within the time prescribed in Section 262 of the DGCL; and | |
| ● | otherwise comply with the procedures set forth in Section 262. |
A demand for appraisal must be executed by or on behalf of the QSAM stockholder of record or beneficial owner and must state that such person intends thereby to demand appraisal of his, her or its shares of QSAM Common Stock issued and outstanding immediately prior to the First Effective Time in connection with the proposed Merger.
Failure to strictly follow the procedures set forth in Section 262 may result in the loss, termination or waiver of appraisal rights. QSAM stockholders or beneficial owners who vote in favor of the adoption and approval of the Merger Agreement will not have a right to have the fair market value of their shares of QSAM Common Stock determined. However, failure to vote in favor of the Merger Agreement is not sufficient to perfect appraisal rights. If you desire to exercise your appraisal rights, you must also submit to QSAM a written demand for appraisal of the QSAM Common Stock held by you.
| 39 |
Written Demand and Notice
Any person wishing to exercise his, her or its appraisal rights must make a written demand for appraisal to QSAM within 20 days from the date of this [definitive] Information Statement. The demand notice shall be sufficient if it reasonably informs QSAM of your identity and that you wish to seek appraisal with respect to your shares of QSAM Common Stock. In addition, in the case of a demand for appraisal made by a beneficial owner of QSAM Common Stock, the demand must also reasonably identify the holder of record of the shares for which the demand is made, be accompanied by documentary evidence of the beneficial owner’s ownership of QSAM Common Stock (such as a brokerage or securities account statement containing such information or a letter from the broker or other record holder of such shares confirming such information) and a statement that such documentary evidence is a true and accurate copy of what it purports to be, and provide an address at which such beneficial owner consents to receive notices given by QSAM and to be set forth on the verified list required by Section 262. All demands should be delivered to:
QSAM Biosciences Inc.
9442 Capital of Texas Hwy N, Plaza 1,
Suite 500 Austin, TX 78759
Attention: Christopher Nelson, General Counsel
With a copy emailed to: cnelson@qsambio.com
The surviving corporation, within ten days after the First Effective Time, will notify each person who has complied with Section 262 that the Merger has become effective.
Judicial Appraisal
Within 120 days after the First Effective Time, the surviving corporation or any person who is entitled to appraisal rights under Section 262 may file a petition with the Delaware Court of Chancery (the “Court of Chancery”) demanding a determination of the value of the shares of QSAM Common Stock. If no petition is filed by either the surviving corporation or any dissenting stockholder or beneficial owner within the 120-day period, the rights of all dissenting persons to appraisal will cease. Persons seeking to exercise appraisal rights should not assume that the surviving corporation will file a petition with respect to the appraisal of the fair value of their shares or that the surviving corporation will initiate any negotiations with respect to the fair value of those shares. The surviving corporation will be under no obligation to take any action in this regard and has no present intention to do so. Accordingly, it is the obligation of persons who wish to seek appraisal of their shares of QSAM Common Stock to initiate all necessary action with respect to the perfection of their appraisal rights within the time periods and in the manner prescribed in Section 262. Failure to file the petition on a timely basis will cause the person’s right to an appraisal to cease.
Upon the filing of the petition described above by any such stockholder or beneficial owner of shares of QSAM Common Stock, service of a copy thereof must be made upon QSAM. The surviving corporation will then be obligated within 20 days to file with the Delaware Register in Chancery (the “Register in Chancery”) a duly verified list, referred to as the “Verified List”, containing the names and addresses of all persons that have demanded payment for their shares and with whom agreements as to the value of their shares have not been reached. Upon the filing of any such petition, the Court of Chancery may order that notice of the time and place fixed for the hearing on the petition be mailed to QSAM and all of the QSAM stockholders or beneficial owners shown on the Verified List. The costs of these notices are borne by QSAM.
After notice to such persons as required by the Court of Chancery, the Court of Chancery is empowered to conduct a hearing on the petition to determine those persons that have complied with Section 262 and that have become entitled to appraisal rights thereunder. At the hearing on such petition, the Court of Chancery shall determine which QSAM stockholders or beneficial owners are entitled to an appraisal of their shares and may require the QSAM stockholders or beneficial owners who have demanded appraisal to submit their certificates to the Register in Chancery so an appropriate legend can be placed on them. Failure to comply with this requirement may result in the dismissal of the appraisal proceedings with respect to you. If immediately before the Merger the shares of QSAM Common Stock are listed on a national securities exchange, the Court of Chancery will dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal rights unless (i) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal or (ii) the value of the merger consideration for such total number of shares exceeds $1 million.
| 40 |
After the Court of Chancery determines which QSAM stockholders or beneficial owners are entitled to appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding, the Court of Chancery shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or expectation of the Merger, together with a fair rate of interest to be paid, if any, upon the amount determined to be the fair value (or, in certain circumstances described below, on the difference between the amount determined to be the fair value and the amount paid by the surviving corporation in the Merger to each person entitled to appraisal prior to the entry of judgment in the appraisal proceeding). Unless the Court of Chancery, in its discretion, determines otherwise for good cause shown, interest from the First Effective Time through the date of payment of the judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the First Effective Time and the date of payment of the judgment. At any time before the entry of judgment in the proceedings, the surviving corporation may pay to each stockholder or beneficial owner entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (i) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court of Chancery, and (ii) interest theretofore accrued, unless paid at that time.
In determining fair value, the Court of Chancery is to take into account all relevant factors. In Weinberger v. UOP, Inc., et al., the Delaware Supreme Court discussed the considerations that could be considered in determining fair value in an appraisal proceeding, stating that “proof of value by any techniques or methods which are generally considered acceptable in the financial community and otherwise admissible in court” should be considered and that “[f]air price obviously requires consideration of all relevant factors involving the value of a company.” The Delaware Supreme Court stated that in making this determination of fair value, the court must consider “market value, asset value, dividends, earnings prospects, the nature of the enterprise and any other facts which were known or which could be ascertained as of the date of merger which throw any light on future prospects of the merged corporation.” The Delaware Supreme Court construed Section 262 to mean that “elements of future value, including the nature of the enterprise, which are known or susceptible of proof as of the date of the merger and not the product of speculation, may be considered.” However, Section 262 provides that fair value is to be determined “exclusive of any element of value arising from the accomplishment or expectation of the merger.” In Cede & Co. v. Technicolor, Inc., the Delaware Supreme Court stated that such exclusion is a “narrow exclusion [that] does not encompass known elements of value,” but which rather applies only to the speculative elements of value arising from such accomplishment or expectation. In Weinberger, the Supreme Court of Delaware also stated that “elements of future value, including the nature of the enterprise, which are known or susceptible of proof as of the date of the merger and not the product of speculation, may be considered.”
QSAM stockholders or beneficial owners who consider seeking appraisal should consider that the fair value of their shares as determined under Section 262 could be more than, the same as, or less than, the Closing Consideration without the exercise of appraisal rights. No representation is made as to the outcome of the appraisal of fair value as determined by the Delaware Court of Chancery. Delaware courts have decided that the statutory appraisal remedy, depending on factual circumstances, may or may not be a dissenter’s exclusive remedy. The Court of Chancery may determine the costs of the appraisal proceeding (which do not include attorneys’ or experts’ fees) and assess it against the parties as the Court of Chancery deems equitable. Upon application of a dissenting person, the Court of Chancery may order that all or a portion of the expenses incurred by any dissenting QSAM stockholder or beneficial owner in connection with the appraisal proceeding, including, without limitation, reasonable attorneys’ fees and the fees and expenses of experts, be charged pro rata against the value of all shares of QSAM Common Stock entitled to appraisal. In the absence of a court determination or assessment, each party will bear its own expenses.
Any QSAM stockholder or beneficial owner who has demanded appraisal in compliance with Section 262 will not, after the First Effective Time, be entitled to vote such stock for any purpose or receive payment of dividends or other distributions, if any, on the QSAM Common Stock, except for dividends or distributions, if any, payable to QSAM stockholders of record at a date before the Merger.
| 41 |
Request for Appraisal Information
If you timely submit a written demand for appraisal of your shares of QSAM Common Stock and otherwise properly perfect your appraisal rights, you may, upon written request mailed to QSAM within 120 days after the First Effective Time, receive a written statement identifying (1) the aggregate number of shares of QSAM Common Stock which were not voted in favor of the adoption and approval of the Merger and with respect to which QSAM has received written demands for appraisal and (2) the aggregate number of QSAM stockholders or beneficial owners holding or owning such shares. QSAM will mail this statement to you within ten days after receiving your written request.
Withdrawal
Even if you submit a written demand for appraisal of your shares of QSAM Common Stock and otherwise properly perfect your appraisal rights, you may withdraw your demand at any time after the First Effective Time except that any such attempt to withdraw made more than 60 days after the First Effective Time will require the written approval of QSAM and, once a petition for appraisal is filed, the appraisal proceeding may not be dismissed as to any person absent court approval. The foregoing, however, will not affect the right of any person who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such person’s demand for appraisal and to accept the terms offered under the Merger Agreement within 60 days after the First Effective Time. If you withdraw your demand, you will be deemed to have accepted the terms of the Merger Agreement, which are summarized in this Information Statement and which is attached in its entirety as Appendix A.
In view of the complexity of Section 262, QSAM stockholders who may wish to dissent from the Merger and seek apprise rights should consult their legal advisors.
Tax Considerations
If you elect to exercise your appraisal rights, the payment in cash of the fair value of your shares of QSAM Common Stock will be a taxable transaction to you.
QSAM stockholders and beneficial owners considering exercising appraisal rights should consult with their tax advisors with regard to the tax consequences of such actions.
The foregoing summary is not intended to be a complete statement of the procedures for exercising appraisal rights under Section 262 and is qualified in its entirety by reference to the full text of Section 262, a copy of which is attached as Appendix D to this Information Statement. QSAM urges any person wishing to exercise appraisal rights, if any, to read this summary and Section 262 carefully, and to consult legal counsel before attempting to exercise appraisal rights. Failure to comply strictly with all of the procedures set forth in Section 262 may result in the loss of your statutory appraisal rights, if any.
Anticipated Accounting Treatment of the Merger
The Merger is expected to be accounted for as an asset acquisition pursuant to IFRS 3–Business Combinations as well as IAS 38-Intangible Assets.
| 42 |
The following is a summary of the material terms of the Merger Agreement. This summary and the descriptions of the Merger Agreement and Merger included elsewhere in this Information Statement are qualified in their entirety by reference to the complete text of the Merger Agreement, a copy of which is attached to this Information Statement as Appendix A and is incorporated by reference into this Information Statement. Capitalized terms in this section that are not defined have the meaning defined in the Merger Agreement. This summary does not purport to be complete and may not contain all of the information about the Merger Agreement that is important to you. The rights and obligations of the parties are governed by the express terms and conditions of the Merger Agreement and not by the following summary or any other information contained in this Information Statement. You are encouraged to read the Merger Agreement carefully and in its entirety before making any decisions regarding the Merger Agreement and the Merger.
This summary and the Merger Agreement attached to this Information Statement as Appendix A are included in this Information Statement to provide you with information regarding the terms and conditions of the Merger Agreement, and not to provide any other factual information about QSAM or Telix or their respective subsidiaries or businesses. Factual disclosures about QSAM and Telix contained in this Information Statement or in the public reports of QSAM and Telix filed with the SEC and/or the ASX may supplement, update or modify the factual disclosures about QSAM and Telix contained in the Merger Agreement. The Merger Agreement contains representations, warranties and covenants by QSAM, on the one hand, and by Telix and Merger Subs, on the other hand. Such representations, warranties and covenants are qualified and subject to important limitations agreed to by QSAM, Merger Subs and Telix. In particular, in your review of the representations and warranties contained in the Merger Agreement and described in this summary and elsewhere in this Information Statement, it is important to bear in mind that the representations and warranties were negotiated with the principal purpose of establishing circumstances in which a party to the Merger Agreement may have the right not to consummate the Merger if the representations and warranties of the other party prove to be untrue due to a change in circumstance or otherwise, and allocating risk between the parties to the Merger Agreement, rather than establishing matters as facts. The representations and warranties also may be subject to a contractual standard of materiality different from that generally applicable to stockholders and reports and documents filed with the SEC, and certain of the representations and warranties were qualified by the matters contained in the confidential disclosure schedule that QSAM, Merger Subs and Telix each delivered in connection with the Merger Agreement in addition to certain documents filed with the SEC. Moreover, information concerning the subject matter of the representations and warranties, which do not purport to be accurate as of the date of this Information Statement, may have changed since the date of the Merger Agreement. The representations and warranties in the Merger Agreement will not survive the completion of the Merger.
For the foregoing reasons, the representations and warranties contained in the Merger Agreement or any descriptions of those provisions contained in this Information Statement should not be read alone or relied upon as characterizations of the actual state of facts or condition of QSAM or Telix or any of their respective subsidiaries or businesses. Instead, such provisions or descriptions should be read only in conjunction with the other information provided elsewhere in this Information Statement or incorporated by reference into this Information Statement. See “Where You Can Find More Information” beginning on page 129.
Structure of the Merger
Telix, the Merger Subs and QSAM intend to effect a reorganization in which, as steps in a single, integrated transaction, (a) Merger Sub I will merge with and into QSAM, Merger Sub I will cease to exist, and QSAM will survive as a direct, wholly owned subsidiary of Telix (the “First Merger”), and (b) as part of the same overall transaction, QSAM will merge with and into Merger Sub II, QSAM will cease to exist, and Merger Sub II will survive as a direct, wholly owned subsidiary of Telix (the “Second Merger” and, collectively or ad seriatim with the First Merger, as appropriate, the “Merger”).
| 43 |
Merger Consideration
Pursuant to the terms of the Merger Agreement and the Reverse Split (as defined below), the aggregate consideration that QSAM stockholders will be entitled to receive pursuant to the Merger and the Reverse Split will be equal to:
(i) USD $33.1 million, reduced by (a) the amount of certain of QSAM’s unpaid expenses, indebtedness, change-of-control bonuses, and other payables as of the closing of the Merger, (b) a fee equal to 5% of the aggregate closing consideration payable to QSAM’s licensor, IGL Pharma, Inc., upon the closing of the Merger (the “Closing IGL License Fee”), and (c) 66,011 ordinary shares of Telix (“Telix Ordinary Shares”), representing $500,000 divided by the Buyer Stock Price (defined below), as a source of recovery for post-closing purchase price adjustments (collectively, the “Closing Consideration”); and
(ii) contingent value rights (“CVRs”) which will represent the right to receive contingent payments of up to USD $90 million in the aggregate, in cash and/or Telix Ordinary Shares, without interest, upon the achievement of certain milestones, at the times and subject to the terms and conditions of the CVR Agreement (as defined below).
The Closing Consideration will be paid to holders of whole numbers of shares of common stock of QSAM (“QSAM Common Stock”) in the Merger in the form of Telix Ordinary Shares, except in certain specified circumstances, while payments in connection with the Reverse Split will be paid in cash. The number of shares issuable to QSAM stockholders in the Merger shall be determined by reference to a deemed value of Telix Ordinary Shares equal to USD $7.5745 per share (the “Buyer Stock Price”), reflecting the volume weighted average price at which Telix Ordinary Shares traded on the Australian Securities Exchange over the ten (10) trading-day period ending on February 6, 2024, the business day prior to the date of the Merger Agreement, as converted from Australian dollars to United States dollars at the exchange rate published in the Wall Street Journal as of February 6, 2024, the business day prior to the date of the Merger Agreement. The number of Telix Ordinary Shares to be issued in the Closing Consideration is based on the Buyer Stock Price, which is a negotiated, agreed, fixed value, and the trading price of Telix’s Ordinary Shares could be, at the time of the Merger, higher or lower than the Buyer Stock Price.
Pursuant to the Merger Agreement, effective as of the date QSAM files this Information Statement, each QSAM Option will vest in full and become exercisable up to and through the close of regular trading on the Last Exercise Date in accordance with the terms and conditions of such QSAM Option, and such QSAM Option will terminate for no consideration and be of no further force or effect as of immediately prior to closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date. According to the terms of the grants made by the Company, all QSAM Options were fully vested as of March 31, 2024 and will remain exercisable until the Last Exercise Date. As of the Reverse Split and the First Effective Time, the outstanding shares of QSAM Common Stock will constitute the only outstanding equity interests of QSAM, and is currently estimated to be 4,445,469 shares of common stock on a fully diluted basis, excluding 148,611 QSAM Options that have exercise prices that are higher than the estimated consideration payable with respect to each share of QSAM common stock as of the closing.
Subject to the foregoing and the other risks and uncertainties set forth below under Forward-Looking Statements, QSAM management currently estimates the value payable at the Closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that will be outstanding prior to the Reverse Split will be approximately $6.63, which would equate to an Exchange Ratio of approximately 0.876 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. This estimate is based on QSAM’s estimate of its expenses through the closing of the Merger in excess of certain expenses, in an amount equal to $500,000 in the aggregate, that Telix has agreed to assume, as well as QSAM’s estimate of the total shares of QSAM Common Stock outstanding immediately prior to the Reverse Split and the closing of the Merger.
| 44 |
As described above, the amount of cash and/or shares of Telix Ordinary Shares payable with respect to each outstanding share of QSAM Common Stock as of the date hereof is subject to adjustment prior to closing based on, among other things, (i) the amount of indebtedness, unpaid expenses, change-of-control, and similar payments, in each case in connection with the Merger, as well as (ii) the fully diluted number of shares of QSAM common stock issued and outstanding as of the date of closing of the Merger, which could increase if certain stock options are exercised or other reasons. As a result, the amount of Closing Consideration payable with respect to each outstanding share of QSAM Common Stock could be lower on the date of Closing than was previously estimated as of the date of the Merger Agreement and as of the date of this Information Statement. For illustration purposes only, if indebtedness, expenses or other payables at closing is $500,000 greater than the current estimate of $3.62 million, then the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.52, which would equate to an Exchange Ratio of 0.861 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. Similarly, if the outstanding shares of QSAM Common Stock are greater by 100,000 shares at the time of Closing due to exercise of stock options, the value payable at the closing of the Merger and/or in the Reverse Split with respect to each share of QSAM Common Stock that is outstanding prior to the Reverse Split would be approximately $6.49, which would equate to an Exchange Ratio of 0.856 Telix Ordinary Shares for each whole share of QSAM Common Stock prior to giving effect to the Reverse Split. These two examples of possible changes to the Closing Consideration payable to QSAM shareholders are further illustrated in the table below:
| Assumptions | Estimated Value per QSAM Share | Estimated Exchange Ratio | ||||||
| Current estimates of total QSAM shares outstanding and total closing indebtedness | $ | 6.63 | 0.876 | |||||
| Closing indebtedness $500,000 greater than current estimate | $ | 6.52 | 0.861 | |||||
| Total QSAM shares outstanding 100,000 greater than current estimate | $ | 6.49 | 0.856 | |||||
In connection with and as a condition to the Merger, QSAM will effect a reverse stock split of all the issued and outstanding shares of QSAM Common Stock, in a ratio between 1:1000 and 1:2000 (the “Reverse Split”) (as determined by the QSAM Board prior to closing), in which any outstanding fractional shares of QSAM Common Stock (determined after determining the whole number of shares of QSAM Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (i) the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration and (ii) one (1) CVR for each share of QSAM Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares) pursuant to the Reverse Split.
In connection with the Reverse Split, all per share values and exchange ratios set forth above would be proportionately increased. For instance, in the case of a 1:1000 Reverse Split, where every 1,000 shares of a holder would be consolidated into one share, the currently estimated value per share of QSAM Common Stock would be $6.63 X 1,000 = $6,630, and the estimated Exchange Ratio would be 0.876 X 1,000 = 876.
Treatment of Fractional Shares
No fractional ordinary shares of Telix will be issued to the QSAM Stockholders under the Merger Agreement, and any fraction of an ordinary share of Telix shall be rounded to the nearest whole number.
| 45 |
Treatment of Stock Options
Upon the terms and subject to the conditions set forth in the Merger Agreement, outstanding QSAM equity awards will be treated as follows:
Pursuant to the Merger Agreement, effective as of the date QSAM files the definitive Information Statement, each QSAM Option will vest in full and become exercisable up to and through the close of regular trading on the Last Exercise Date in accordance with the terms and conditions of such QSAM Option, and such QSAM Option will terminate for no consideration and be of no further force or effect as of immediately prior to Closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date. According to the terms of the grants made by the Company, all QSAM Options were fully vested as of March 31, 2024 and will remain exercisable until the Last Exercise Date.
All QSAM Options have exercise prices that are higher than the estimated Closing Consideration being paid by Telix per share of QSAM Common Stock and the current trading price of QSAM on OTCQB.
Closing and Effective Time of the Merger
Subject to the terms and conditions of the Merger Agreement, the closing of the First Merger (the “Closing”) shall take place by the electronic exchange of executed counterpart documents as soon as practicable on or after the execution and delivery of the Merger Agreement, but in any event no later than the date which is two (2) Business Days after the date on which all conditions set forth in Merger Agreement shall have been satisfied or waived (other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of such conditions) or such other time and place as Telix and QSAM may mutually agree in writing. The First Merger shall become effective at the time when the First Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by Telix and QSAM in writing and specified in the First Certificate of Merger (the “First Effective Time”). Promptly following the First Effective Time, but in no event later than two (2) Business Days thereafter, Telix, the First Step Surviving Corporation and Merger Sub II shall cause the Second Certificate of Merger to be filed with the Secretary of State of Delaware (the “Second Effective Time”).
Organizational Documents; Directors and Officers
Certificate of Incorporation and Bylaws. At the First Effective Time, the QSAM charter shall be amended to read in its entirety as the certificate of incorporation of Merger Sub I reads as in effect immediately prior to the First Effective Time, provided, that such certificate of incorporation shall reflect, as of the First Effective Time, “Telix QSAM, Inc.” as the name of the First Step Surviving Corporation, and, as so amended, shall become the certificate of incorporation of the First Step Surviving Corporation until thereafter amended in accordance with the applicable provisions of the DGCL and such certificate of incorporation. At the Second Effective Time, the certificate of incorporation of the First Step Surviving Corporation shall be amended as of the Second Effective Time to read in its entirety as the certificate of incorporation of Merger Sub II reads as in effect immediately prior to the Second Effective Time, provided, that such certificate of incorporation shall reflect, as of the Second Effective Time, “Telix QSAM, Inc.” as the name of the Final Surviving Corporation, and, as so amended, shall become the certificate of incorporation of the Final Surviving Corporation until thereafter amended in accordance with the applicable provisions of the DGCL and such certificate of incorporation.
Directors and Officers. The directors of Merger Sub I immediately prior to the First Effective Time shall be the directors of the First Step Surviving Corporation immediately after the First Effective Time. The officers of Merger Sub I immediately prior to the First Effective Time shall be the officers of the Final Surviving Corporation immediately after the First Effective Time. The directors of Merger Sub II immediately prior to the Second Effective Time shall be the directors of the Final Surviving Corporation immediately after the Second Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation until their respective successors are duly elected or appointed and qualified or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation. The officers of Merger Sub II immediately prior to the Second Effective Time shall be the officers of the Final Surviving Corporation immediately after the Second Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation until their respective successors are duly appointed or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation.
| 46 |
Exchange of Shares in the Merger
By virtue of the First Merger, each share of QSAM Capital Stock held by Telix, Merger Subs or QSAM in treasury or otherwise, shall be cancelled and retired and shall cease to exist, and no consideration shall be delivered or receivable in exchange therefor (such shares, “Cancelled Shares”). Each share of QSAM Capital Stock (a “Share”) that is issued and outstanding immediately prior to the First Effective Time, other than (A) Cancelled Shares and (B) shares of Company Capital Stock held by persons who validly exercise their statutory appraisal rights, shall thereupon be cancelled and converted into and become the right to receive a number of ordinary shares of Telix and CVRs equal to such Share’s pro rata share of the transaction consideration, in each case subject to the terms of the Merger Agreement.
At the First Effective Time, each share of common stock, par value $0.0001 per share, of Merger Sub I issued and outstanding immediately prior to the First Effective Time shall be cancelled and, in exchange for the cancellation of such shares of Merger Sub I common stock and the payment of the Merger Consideration by Telix, the First Step Surviving Corporation shall issue an equivalent number of shares of common stock, par value $0.0001 per share, all of which shares shall be held by Telix, and which shall constitute the only outstanding shares of common stock of the First Step Surviving Corporation immediately following the First Effective Time.
At the Second Effective Time, each share of common stock, par value $0.0001 per share, of the First Step Surviving Corporation issued and outstanding immediately prior to the Second Effective Time shall be cancelled and, in exchange for the cancellation of such shares of First Step Surviving Corporation common stock, the Final Surviving Corporation shall issue an equivalent number of shares of common stock, par value $0.0001 per share, all of which shares shall be held by Telix, and which shall constitute the only outstanding shares of common stock of the Final Surviving Corporation immediately following the Second Effective Time.
Representations and Warranties
The Merger Agreement contains customary representations and warranties made by QSAM, Telix and Merger Subs. The representations and warranties described below and included in the Merger Agreement were made only for purposes of the Merger Agreement and as of specific dates, may be subject to a contractual standard of materiality different from what might be viewed as material to stockholders, and may be subject to limitations agreed upon by the parties, including being qualified by disclosures filed with or furnished to the SEC and the confidential disclosure letters exchanged by the parties in connection with the execution of the Merger Agreement. The representations and warranties contained in the Merger Agreement were solely for the benefit of the parties to the Merger Agreement and should not be relied upon as characterizations of the actual state of facts or condition of any party or any of their respective subsidiaries, affiliates or businesses. The following is a description of certain of the representations and warranties of the parties contained in the Merger Agreement:
| ● | Organization, standing and power; | |
| ● | Corporate power and authority; | |
| ● | Subsidiaries; | |
| ● | No conflicts, consents and approvals; | |
| ● | Government consents; | |
| ● | Capitalization, preliminary allocation schedules; |
| 47 |
| ● | SEC reports and financial statements; | |
| ● | Undisclosed liabilities; | |
| ● | Litigation and proceedings; | |
| ● | Compliance with laws; | |
| ● | FDA matters; | |
| ● | Contracts, no defaults; | |
| ● | Company benefit plans; | |
| ● | Employment and labor relations; | |
| ● | Taxes; | |
| ● | Broker fees; | |
| ● | Insurance; | |
| ● | Licenses, permits and authorizations; | |
| ● | Real property; | |
| ● | Intellectual property; | |
| ● | Environmental matters; | |
| ● | Data privacy; | |
| ● | Absence of changes; | |
| ● | Affiliate matters; | |
| ● | Accredited investors; and | |
| ● | No additional representations and warranties. |
Definition of “Material Adverse Effect”
Certain representations and warranties of QSAM are qualified by a “material adverse effect” standard. “Material Adverse Effect” means (i) with respect to QSAM, any event, occurrence, fact, condition or change that, individually or in the aggregate, (x) has had or would reasonably be expected to have a material adverse effect on the business, assets, liabilities, results of operations or condition (financial or otherwise) of QSAM and its subsidiaries, taken as a whole; provided, however, that, in no event will any of the following (or the effect of any of the following), alone or in combination, be deemed to constitute, or be taken into account in determining whether there has been or will be, a “Material Adverse Effect” on or in respect of QSAM, in each case to the extent first arising after the date hereof: (A) any change in Law, regulatory policies, accounting standards or principles (including GAAP) or any guidance relating thereto or interpretation thereof, (B) any change in interest rates or economic, political, business or financial market conditions generally (including any changes in credit, financial, commodities, securities or banking markets), (C) any change generally affecting any of the industries in which QSAM operates or the economy as a whole, (D) any natural disaster, (E) any acts of terrorism, sabotage, war, the outbreak or escalation of hostilities, weather conditions, change in geopolitical conditions or other force majeure events, or (F) any failure of QSAM to meet any projections or forecasts, provided that this clause (F) shall not prevent a determination that any change or effect underlying such failure to meet projections or forecasts has resulted in a Material Adverse Effect (to the extent such change or effect is not otherwise excluded from this definition of Material Adverse Effect); except, in the case of clauses (A), (B), (C), (D) and (E) above, to the extent that any such change, condition, event or effect has a materially disproportionate and adverse effect on the business of QSAM relative to other businesses in the industries in which the Company operates or (y) would or would reasonably be expected to prevent or materially delay or impair QSAM from consummating the transactions contemplated by this Agreement or from performing its material obligations under this Agreement; and (ii) with respect to Telix or Merger Subs, any event, occurrence, fact, condition or change that would or would reasonably be expected to prevent or materially delay or impair Telix from consummating the transactions contemplated by the Merger Agreement or from performing its material obligations under the Merger Agreement.
| 48 |
Conduct of QSAM’s Business Pending the Merger
QSAM has agreed to certain covenants in the Merger Agreement restricting the conduct of its business between the date of the Merger Agreement and the First Effective Time. In general, except as contemplated by the Merger Agreement, as would constitute a violation in applicable law or with the prior written consent of Telix, QSAM has agreed to operate its business in the ordinary course of business and maintain in all material respects satisfactory relationships with its material business relationships.
In addition to the foregoing, between the date of the Merger Agreement and the Effective Time, except as would constitute a violation in applicable law, or as otherwise consented to by Telix in writing or otherwise contemplated by the Merger Agreement, QSAM will not, and will cause each of its subsidiaries not to:
| ● | except as required to effect the Reverse Split, (A) change or amend its charter, bylaws or other organizational documents of QSAM or any of its subsidiaries, except as otherwise required by law; or (B) authorize for issuance, issue, grant, sell, deliver, dispose of, pledge or otherwise encumber any equity securities of QSAM, or any of its Subsidiaries except for shares of QSAM common stock upon the exercise of outstanding QSAM Options or upon the conversion of QSAM preferred stock into QSAM common stock; | |
| ● | except for the Reverse Split, split, combine or reclassify any shares of its capital stock; declare, set aside or pay any dividend or other distribution (whether in cash, stock or property or any combination thereof) in respect of its capital stock; or enter into any agreement with respect to voting of any shares of QSAM capital stock; | |
| ● | make or declare any dividend or distribution to the stockholders of QSAM; | |
| ● | adopt a plan of complete or partial liquidation or dissolution, recapitalization or other reorganization; | |
| ● | hire any new officers or, except in the ordinary course of business, any new employees or consultants or terminate, other than for cause, any officer or employee; |
| ● | create, incur or assume any indebtedness; assume, guarantee, endorse or otherwise become liable or responsible (whether directly, contingently or otherwise) for the obligations of any other person; or make any loans, advances or capital contributions to, or investments in, any other person; | |
| ● | subject any of its material property or assets to any lien, other than any permitted lien; | |
| ● | incur any capital expenditure or commitment for capital expenditures; | |
| ● | (A) modify or terminate (excluding any expiration in accordance with its terms) any contract of a type required to be listed on QSAM’s disclosure schedules or providing for aggregate payments of more than $50,000, or any material insurance policy required to be listed on QSAM’s disclosure schedules, outside of the ordinary course of business; (B) enter into any contract outside of the ordinary course of business or (C) take or omit to take any action that would constitute a material violation of or material default under, or waive any material rights under, any contract of a type required to be listed on QSAM’s disclosure schedules; | |
| ● | change the nature or scope of its business being carried on as of the date of the Merger Agreement or commence any new business not being ancillary or incidental to such business or take any action to alter its organizational or management structure; |
| 49 |
| ● | materially change its accounting methods, principles or practices, except insofar as may be required by a generally applicable change in GAAP; | |
| ● | institute or settle any legal action; | |
| ● | sell, assign, transfer, convey, lease, license, sublicense or otherwise dispose of (A) any QSAM intellectual property, or (B) outside of the ordinary course of business, any assets or properties of QSAM with a value, individually or in the aggregate, of $50,000; | |
| ● | (A) adopt, enter into, terminate or amend any employment, severance, retention or change in control plan or contract, any QSAM benefit plan or any collective bargaining agreement, (B) increase the compensation or fringe benefits of, or pay any bonus to, any director, officer, employee or consultant, (C) pay any benefit to any employee or consultant of QSAM or any of its Subsidiaries except as required as of the date of the Merger Agreement under any QSAM benefit plan, (D) grant any awards to any employee or consultant of QSAM or any of its Subsidiaries under any bonus, incentive, performance or other compensation plan or arrangement or benefit plan, including the grant of equity or equity-based compensation, or the removal of existing restrictions in any benefit plans or agreements or awards made thereunder, or (E) take any action to fund or in any other way secure the payment of compensation or benefits to any employee or consultant of QSAM or any of its Subsidiaries under any employee plan, agreement, contract or arrangement or QSAM benefit plan, other than payment of premiums due or contributions owed in the ordinary course of business; | |
| ● | acquire by merger or consolidation, or merge or consolidate, with any corporation, partnership, association, joint venture or other business organization or division thereof, or acquire any business, assets or property, or make any investment in, any person; | |
| ● | make any material loans or material advances of money to any person (other than the Company), except for advances to employees or officers of QSAM for expenses incurred in the ordinary course of business; | |
| ● | (A) make or change any material tax election, change an annual accounting period, file any material amended tax return, enter into any closing agreement, settle or compromise any claim, notice, audit report or assessment in respect of taxes or consent to any extension or waiver of the statute of limitations period applicable to any tax claim or assessment, or take any other similar action relating to the filing of any tax return or the payment of any tax or (B) except as required or permitted by GAAP, make any material change to any accounting principles, methods or practices; | |
| ● | other than in the ordinary course of business, abandon, or fail to prosecute or maintain, any QSAM owned intellectual property, or any QSAM licensed intellectual property that QSAM has the right to prosecute or maintain; or | |
| ● | enter into any agreement, or otherwise become obligated, to do any action prohibited under the Merger Agreement. |
Covenants of the Parties
The Merger Agreement contains certain covenants and agreements made jointly by the parties, including:
| ● | Using commercially reasonable efforts to: (a) assemble, prepare and file any information (and, as needed, to supplement such information) as may be reasonably necessary to obtain as promptly as practicable all governmental and regulatory consents required to be obtained in connection with the transactions contemplated hereby, (b) obtain all material consents and approvals of third parties that are required to be obtained in order to consummate the First Merger, and (c) take such other action as may reasonably be necessary or as another party may reasonably request to satisfy the conditions or otherwise to comply with the Merger Agreement and to consummate the transactions; |
| 50 |
| ● | Executing and delivering, or causing its Affiliates to execute and deliver, such further instruments, and take (or cause its Affiliates to take) such other action, as may be reasonably necessary to carry out the purposes and intents of the Merger Agreement; | |
| ● | Not knowingly taking any action that could reasonably be expected to cause the Merger to fail to qualify as a “reorganization” within the meaning of Section 368 of the Code or to be subject to Section 367(a)(1) of the Code; | |
| ● | Taking all reasonably necessary action on such that the issuance of Telix Ordinary Shares pursuant to the Merger Agreement constitutes a transaction exempt from registration under the Securities Act under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder; and | |
| ● | Taking all reasonably necessary action on its part to ensure that a duly qualified Rights Agent executes and delivers the CVR Agreement at or prior to the Closing. |
The Merger Agreement also contains certain covenants and agreements made by QSAM, including:
| ● | Promptly obtaining and delivering to Telix a true, correct and complete copy of an irrevocable written consent of holders of at least a majority of the issued and outstanding shares of QSAM capital stock to adopt the Merger Agreement and the amendment of QSAM’s Charter to effect the Reverse Split; | |
| ● | Covenants not to solicit a competing acquisition proposal involving QSAM; and | |
| ● | Covenants regarding the filing of this Information Statement. |
Directors’ and Officers’ Indemnification and Insurance
The Merger Agreement provides for certain indemnification and insurance rights in favor of QSAM’s former and current officers, directors and employees. Specifically, Telix has agreed to maintain provisions in QSAM’s certificate of incorporation, bylaws or other organizational documents concerning the indemnification and exoneration (including provisions relating to expense advancement) of such persons that are no less favorable to those persons than the provisions of the certificate of incorporation, bylaws or other organizational documents of QSAM, in each case, as of the date of the Merger Agreement, and not to amend, repeal or otherwise modify such provisions in any respect that would adversely affect the rights of those persons, in each case, except as required by law. In addition, QSAM has agreed to obtain a prepaid, non-cancelable six-year “tail” policy containing terms not less favorable than the terms of QSAM’s current directors’ and officers’ liability insurance coverage with respect to matters existing or occurring at or prior to the First Effective Time.
Conditions to Completion of the Merger
The obligations of each of the parties to consummate the Merger are subject to the satisfaction (or waiver by each of QSAM and Telix if permissible under applicable law), prior to the First Effective Time, of the following condition:
| ● | No governmental authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, executive order, decree, injunction or other order (whether temporary, preliminary or permanent) which is in effect and which prohibits, restrains, enjoins or makes illegal the consummation of the Merger, and there shall not be any threatened, instituted or pending action by a governmental authority seeking to prohibit, restrain or enjoin the consummation of the Merger or other transactions under the Merger Agreement. |
| 51 |
In addition, the obligation of each of Telix and the Merger Subs to consummate the Merger is subject to the satisfaction (or waiver by Telix) prior to the Effective Time, of each of the following additional conditions:
| ● | The representations and warranties of QSAM (other than certain “fundamental representations” of QSAM) shall be true and correct (without giving regard to any qualifications or limitations as to “materiality” or “Material Adverse Effect”, and words of similar import set forth therein) as of the date of the Merger Agreement and at and as of the Closing with the same effect as though made at and as of such time, except where the failure to be true and correct would not reasonably be expected to have a Material Adverse Effect; | |
| ● | The fundamental representations of QSAM will be true and correct in all respects (other than fundamental representations of QSAM with respect to QSAM’s capitalization or affiliate matters, which will be true and correct in all but de minimis respects) as of the date of the Merger Agreement and at and as of the Closing with the same effect as though made at and as of such time; provided, however, that representations and warranties that are made as of a particular date or period will be true and correct (in the manner set forth above) only as of such date or period; | |
| ● | Each of the covenants of QSAM to be performed at or prior to the Closing shall have been performed in all material respects. | |
| ● | QSAM shall have delivered to Telix a certificate signed by an officer of the Company, dated as of the Closing Date, certifying that the certain conditions specified in the Merger Agreement have been fulfilled (the “Closing Certificate”); | |
| ● | No Material Adverse Effect on QSAM shall not have occurred; | |
| ● | The written consent of QSAM’s stockholders to the transactions contemplated by the Merger Agreement shall have been validly obtained; | |
| ● | The Reverse Split shall have been effected; | |
| ● | QSAM shall have used reasonable best efforts to obtain completed and signed investor questionnaires from each QSAM stockholder and shall have delivered all such investor questionnaires to Telix; Telix shall have no reason to believe that the statements set forth in the investor questionnaires are not true; and Telix shall be reasonably satisfied that the issuance of the Telix Ordinary Shares pursuant to the Merger Agreement is exempt from the registration requirements of the Securities Act; | |
| ● | There shall be no more than 27 QSAM stockholders that are not accredited investors (as defined in Rule 501(a) of Regulation D promulgated under the Securities Act); | |
| ● | The aggregate number of dissenting shares, together with the shares of QSAM capital stock eligible to become dissenting shares, shall not exceed two percent (2%) of the number of outstanding shares of QSAM capital stock as of the First Effective Time; | |
| ● | The aggregate amount of QSAM’s indebtedness, together with all transaction expenses in excess of amounts that the parties agreed could be incurred by QSAM, to be paid by Buyer shall not exceed $500,000; | |
| ● | Telix shall have received copies of written consents evidencing the receipt of the QSAM stockholders’ consent to the Merger and the other transactions contemplated by the Merger Agreement; | |
| ● | QSAM shall have delivered to Telix a signed certification that the shares of QSAM capital stock are not United States real property interests as defined in Section 897(c) of the Code, together with a notice to the IRS (which shall be filed by Telix with the IRS following the Closing), in accordance with the Treasury Regulations under Sections 897 and 1445 of the Code; | |
| ● | Telix shall have received duly executed counterparts to the CVR Agreement from the other parties thereto; |
| 52 |
| ● | Telix shall have received a duly executed copy of an option acknowledgement agreement executed by each holder of outstanding QSAM Options, which agreements describe the treatment of the QSAM Options under the terms of the Merger Agreement; | |
| ● | Telix shall have received other items specified in the Merger Agreement to be delivered by QSAM in connection with the Closing; and | |
| ● | Telix shall have received such other certificates and instruments (including certificates of good standing of QSAM and its Subsidiaries in their respective jurisdictions of organization) as it shall reasonably request in connection with the Closing. |
In addition, the obligation of QSAM to consummate the Merger is subject to the satisfaction (or waiver by QSAM) prior to the Effective Time, of each of the following additional conditions:
| ● | The representations and warranties of Telix and Merger Subs (other than certain “fundamental representations” of Telix) shall be true and correct (without giving regard to any qualifications or limitations as to “materiality” or “Material Adverse Effect”, and words of similar import set forth therein) as of the date of the Merger Agreement and at and as of the Closing with the same effect as though made at and as of such time, except where the failure to be true and correct would not reasonably be expected to have a Material Adverse Effect on Telix; | |
| ● | The fundamental representations of Telix will be true and correct in all respects as of the date of the Merger Agreement and as of the Closing with the same effect as though made at and as of such time; provided, however, that representations and warranties that are made as of a particular date or period will be true and correct only as of such date or period; | |
| ● | Each of the covenants of Telix and the Merger Subs to be performed at or prior to the Closing shall have been performed in all material respects; | |
| ● | Telix shall have delivered to QSAM a certificate signed by an officer of Telix, dated as of the Closing Date, certifying that the conditions specified in Section 9.2(a) and Section 9.2(b) have been fulfilled; and | |
| ● | Telix shall have delivered a duly executed counterpart to the CVR Agreement to the other parties thereto. |
Termination of the Merger Agreement
The Merger Agreement may be terminated at any time prior to the Effective Time under the following circumstances:
| ● | by duly authorized mutual written consent of Telix and QSAM. | |
| ● | by written notice to QSAM from Telix if: |
| ○ | there is any breach of any representation, warranty, covenant or agreement on the part of QSAM set forth in this Agreement, such that the conditions specified in Section 9.1(a) or Section 9.1(b) of the Merger Agreement would not be satisfied at the Closing, except that under certain conditions specified in the Merger Agreement, QSAM will have a limited right to cure such breach and such termination will not be effective unless and until such right to cure has expired; or | |
| ○ | the Closing has not occurred on or before August 7, 2024, unless Telix’s or Merger Subs’ breach is the primary reason for the Closing not occurring on or before such date; or | |
| ○ | the consummation of any of the transactions contemplated hereby is permanently enjoined, prohibited or otherwise restrained by the terms of a final, non-appealable order or judgment of a court of competent jurisdiction; or |
| 53 |
| ○ | if the Merger Consent shall not have been obtained prior to 5:00 p.m., New York time, on the first (1st) business day immediately following the date of the Merger Agreement. |
| ● | by written notice to Telix from QSAM if: |
| ○ | there is any breach of any representation, warranty, covenant or agreement on the part of Telix or Merger Subs set forth in this Agreement, such that the conditions specified in Section 9.2(a) or Section 9.2(b) would not be satisfied at the Closing, except that under certain conditions specified in the Merger Agreement, Telix will have a limited right to cure such breach and such termination will not be effective unless and until such right to cure has expired; or | |
| ○ | the Closing has not occurred on or before August 7, 2024, unless QSAM’s breach is the primary reason for the Closing not occurring on or before such date; or | |
| ○ | the consummation of any of the transactions contemplated hereby is permanently enjoined, prohibited or otherwise restrained by the terms of a final, non-appealable order or judgment of a court of competent jurisdiction. |
Effect of Termination
In the event of the termination of the Merger Agreement, the Merger Agreement shall forthwith become void and have no effect, without any liability on the part of any party hereto or its respective Affiliates, officers, directors, employees or stockholders, other than liability of QSAM, Telix or Merger Subs, as the case may be, for any intentional and willful breach of the Merger Agreement occurring prior to such termination; provided, however, that any such termination shall not relieve any party from liability for damages for any willful breach on the part of Telix or QSAM, as the case may be, including such party’s obligation to close if it was otherwise obligated to do so under the terms of the Merger Agreement.
Indemnification
From and after the Closing, the QSAM stockholders will severally (and not jointly) indemnify Telix and its affiliates for any and all losses incurred by them relating to or constituting:
| ● | any breach of any representation or warranty QSAM made in the Merger Agreement or in the Closing Certificate; | |
| ● | any breach by QSAM of any of its covenants or agreements in this Merger Agreement that, by its terms, provides for performance by QSAM prior to the Closing; | |
| ● | any indebtedness or transaction expenses of QSAM, in each case to the extent not taken into account in the purchase price adjustment process; | |
| ● | any inaccuracy in QSAM’s calculation of the allocation of the purchase price among the QSAM stockholders; | |
| ● | any taxes of QSAM relating to the period prior to the Closing; | |
| ● | any claim by an actual or purported current or former stockholder or option holder of QSAM seeking to assert rights to ownership of QSAM equity, rights as a QSAM equityholder, any claim to consideration in excess of amounts payment under the Merger Agreement or any breach of fiduciary duty by any officer or director of QSAM at or prior to the Closing; | |
| ● | any fraud on the part of QSAM in connection with the transactions contemplated by the Merger Agreement; and | |
| ● | any claim for indemnification, exculpation and/or the advancement or reimbursement of expenses by any person who was an officer or director of the Company at any time prior to the Closing to the extent the Losses arising therefrom exceed amounts actually recovered (net of the costs and expenses of collection) under the director and officer tail insurance policy that will be obtained by QSAM. |
| 54 |
Any amounts owed or claimed in good faith to be owed by any QSAM stockholder to Telix or any of its affiliates pursuant to Telix’s indemnification rights under the Merger Agreement will be automatically offset or set off against any amount that is or may become payable to the QSAM stockholders pursuant to the CVR Agreement.
Telix’s rights to indemnification under the Merger Agreement are subject to customary limitations.
Fees and Expenses; No Termination Fee
Except as provided below, all fees and expenses incurred in connection with the Merger Agreement, the CVR Agreement and the transactions contemplated by the Merger Agreement will be paid by the party incurring such expenses, whether or not the Merger is consummated. Additionally, Telix has agreed to pay $500,000 in excess transaction expenses to the extent left unpaid by QSAM at the time of Closing. Neither party will pay the other a termination fee; however, in the instance of a termination, the $2 million option and pre-closing collaboration fee paid to QSAM by Telix upon the signing of the term sheet shall be automatically deemed an equity investment in QSAM made by Telix Pharmaceuticals (US) Inc., and QSAM will promptly issue to Telix Pharmaceuticals (US) Inc. 298,507 duly authorized, validly issued, fully paid, nonassessable shares of Company Common Stock, free of preemptive rights, in respect thereof.
Governing Law; Jurisdiction; Waiver of Jury Trial
The Merger Agreement is governed by the laws of the State of Delaware (without regard to the laws of any other jurisdiction that might be applied because of the conflicts of laws principles of Delaware). Each of the parties irrevocably submits, for itself and with respect to its property, to the exclusive jurisdiction of the Court of Chancery, or, if such court finds it lacks subject matter jurisdiction, any federal or state court in the State of Delaware. Each party also irrevocably waives any right it may have to a trial by jury in respect of any litigation arising out of or relating to the Merger Agreement or the transactions contemplated by the Merger Agreement.
Amendment; Waiver
The Merger Agreement may be amended or modified in whole or in part, only by a duly authorized agreement in writing executed in the same manner as the Merger Agreement and which makes reference to the Merger Agreement. The approval of the Merger Agreement by the stockholders of QSAM shall not restrict the ability of the Board of Directors of QSAM to terminate the Merger Agreement or to cause QSAM to enter into an amendment to the Merger Agreement to the extent permitted under Section 251(d) of the DGCL.
Any party to the Merger Agreement may, at any time prior to the Closing, by action taken by its Board of Directors, or officers thereunto duly authorized, waive any of the terms or conditions of the Merger Agreement or agree to an amendment or modification to the Merger Agreement by an agreement in writing executed in the same manner (but not necessarily by the same persons) as the Merger Agreement. No waiver by any of the parties to the Merger Agreement of any default, misrepresentation or breach of representation, warranty, covenant or other agreement hereunder, whether intentional or not, shall be deemed to extend to any prior or subsequent default, misrepresentation or breach or affect in any way any rights arising by virtue of any prior or subsequent such occurrence. No waiver by any of the parties to the Merger Agreement of any of the provisions hereof shall be effective unless explicitly set forth in writing and executed by the party sought to be charged with such waiver.
| 55 |
AGREEMENTS RELATED TO THE MERGER
The Contingent Value Rights Agreement
The following is a summary of the material terms of the CVR Agreement, which will be entered into at or prior to the time the Merger becomes effective by and between Telix, QSAM and a rights agent mutually designated by Telix and QSAM, substantially in the form attached as Appendix B to this Information Statement, subject to any revisions that are reasonably requested by such rights agent or are required by applicable law. This summary and the descriptions of the CVR Agreement included elsewhere in this Information Statement are qualified in their entirety by reference to the complete text of the form of the CVR Agreement which is attached as Appendix B to this Information Statement and is incorporated by reference herein. The rights and obligations of the parties and of holders of CVRs are governed by the express terms and conditions of the CVR Agreement and not by the following summary or any other information contained in this Information Statement. The CVR Agreement should not be read alone, but should instead be read in conjunction with the Merger Agreement attached as Appendix A and the other information provided elsewhere in this Information Statement, including the appendices and the documents incorporated by reference into this Information Statement. The CVR Agreement is described in this Information Statement only to provide you with information regarding its terms and conditions and this summary is not intended to provide any factual information about Telix, QSAM or their respective businesses.
Overview
At or prior to the Effective Time, Telix, QSAM and a rights agent mutually agreeable to Telix and QSAM (the “Rights Agent”) will enter into the CVR Agreement substantially in the form attached as Appendix B to this Information Statement, subject to any revisions to the CVR Agreement that are reasonably requested by such rights agent or are required by applicable law. Pursuant to the CVR Agreement and as provided in the Merger Agreement, each share of QSAM Common Stock that is issued and outstanding immediately prior to the Reverse Split (excluding shares owned by Telix, Merger Sub, Company, or any wholly-owned subsidiary of the Company, which shares will automatically be cancelled and extinguished without consideration being delivered in exchange therefor, and any shares where the holder properly demands their statutory appraisal rights) will be automatically converted into the right to receive, in addition to the Closing Consideration, one CVR at the time of Closing.
Each CVR represents the non-transferable contractual right to receive one or more contingent payments, if any, upon the achievement of certain milestones, subject to and in accordance with the terms of the CVR Agreement. If any milestone is achieved during the ten year period from the date of the CVR Agreement (the “Milestone Period”), then, in each case, on a date that is no later than thirty (30) days following the achievement of such milestone, Telix will deliver to the Rights Agent (i) a notice (a “Milestone Notice”) indicating (A) the achievement of such milestone, and (B) a calculation of the amount of cash and/or number of Telix Ordinary Shares, as applicable, payable as the applicable milestone payment (the “Milestone Payment”), including, if applicable, the amount of any permitted deductions from such Milestone Payment (including the IGL License Fee and any transaction bonuses payable to the QSAM Key Employees and Directors), and (ii) for payment to the shareholders, cash and/or shares equal to the applicable Milestone Payment (in each case less any applicable withholding tax, if any).
The Rights Agent shall promptly, and in no event later than ten (10) business days after receipt of a Milestone Notice, send each CVR holder at its address set forth in the CVR Register (defined below) a copy of such Milestone Notice. At the time the Rights Agent sends a copy of such Milestone Notice to CVR holders, the Rights Agent shall also pay to each CVR holder, subject to any applicable withholding tax, the applicable Milestone Payment. The portion of each such Milestone Payment payable to each CVR holder will be calculated as (i) (A) the applicable Milestone Payment divided by (B) the aggregate number of CVRs registered in the CVR Register at such time, multiplied by (ii) the number of CVRs held by such CVR holder as reflected on the CVR Register.
| 56 |
Characteristics of the CVRs; Restrictions on Transfer
The CVRs may not be sold, assigned, transferred, pledged, encumbered or in any other manner transferred or disposed of, in whole or in part, other than pursuant to any of the following: (i) upon death of a holder, by will or intestacy; (ii) by instrument to an inter vivos or testamentary trust in which the CVRs are to be passed to beneficiaries upon the death of the trustee; (iii) pursuant to a court order (such as in connection with divorce, bankruptcy or liquidation); (iv) by operation of law (including a consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other entity; (v) in the case of CVRs payable to a nominee, from a nominee to a beneficial owner (and, if applicable, through an intermediary) or from such nominee to another nominee for the same beneficial owner, in each case as permitted by The Depository Trust Company; or (vi) upon abandonment of a CVR by the holder thereof in accordance with the CVR Agreement.
The CVRs will not be evidenced by a certificate or any other instrument. The CVRs will not have any voting or dividend rights, and interest will not accrue on any amounts payable in respect of the CVRs. The CVRs will not represent any equity or ownership interest in Telix, any constituent company to the Merger, or any of their affiliates. The sole right of each holder of a CVR is the right to receive the Milestone Payment amounts, if any, in accordance with the CVR Agreement. The Rights Agent will maintain an up-to-date register (the “CVR Register”) for the purposes of registering the CVRs and permitted transfers thereof for each holder.
Milestones and Payment
Pursuant to the CVR Agreement, a CVR holder is entitled to receive payments in cash or Telix Ordinary Shares (each a “Milestone Payment”) from Telix upon the achievement of up to four milestone events. If applicable, each Milestone Payment would be paid only once, upon first achievement of the corresponding milestone, regardless of the number of times such event is achieved. Telix shall use commercially reasonable efforts to achieve the milestones.
There are up to four potential milestone events that trigger Milestone Payments. Such milestones, if achieved, and the associated the Milestone Payments are as follows:
| ● | Milestone 1 – Upon the first achievement of successful completion with respect to any acquired product, including CycloSam® for one or more indication(s), a USD $10.0 million Milestone Payment is triggered. Successful completion of an acquired product means generation of significant results from a pivotal clinical trial which results meet or exceed the primary endpoint(s) and secondary endpoint(s) set forth in the protocol prior to the filing of a regulatory approval from any federal or state authority of a pharmaceutical or biologic product in any country or territory. | |
| ● | Milestone 2 – Upon the first commercial sale of an approved acquired product in any major market country for any indication, a USD $20.0 million Milestone Payment is triggered. First commercial sale means, with respect to an acquired product, the first sale for monetary value for use or consumption by the end user in a major market country. Major market countries for purposes of the CVR Agreement consist of the United States, France, Germany, Italy, Spain, Japan, United Kingdom, Australia, Canada, Brazil or China. | |
| ● | Milestone 3 – Upon the first commercial sale of an acquired product in any major market country after receipt of regulatory approval for an indication other than the indication which resulted in the achievement of Milestone 2, a USD $10.0 million Milestone Payment is triggered. | |
| ● | Net Sales Milestone – Upon cumulative worldwide net sales of any product candidate of QSAM of USD $500.0 million, a USD $50.0 million Milestone Payment is triggered. |
| 57 |
Composition of Milestone Payments
All Milestone Payments after the fifth anniversary of closing will be payable exclusively in cash. For payments during the first five years of the CVR Agreement term, Milestone Payments will generally be payable in Telix Ordinary Shares (with the number of shares determined using the volume weighted average price at which Telix Ordinary Shares traded on the ASX over the twenty trading-day period ending on the business day immediately prior to the date on which Telix delivers a Milestone Notice, as converted from AUD to USD in accordance with CVR Agreement), except for in certain specified circumstances where the holders of certain CVRs will be paid exclusively in cash, including CVRs issued in lieu of fractional shares of QSAM common stock resulting from the Reverse Split.
Withholding
Telix and the rights agent will be entitled to deduct and withhold, or cause to be deducted and withheld, from any Milestone Payment otherwise payable pursuant to the CVR Agreement, such amounts as each is required to deduct and withhold with respect to the making of such payment under any provision of law. To the extent that amounts are so deducted and withheld, such deducted and withheld amounts will be treated for all purposes of the CVR Agreement as having been paid to the holder of CVRs in respect of which such deduction and withholding was made.
Audit Rights
Until the earlier of achievement of the Net Sales Milestone or the expiration of the Milestone Period, upon reasonable advance written notice, at the cost and election of the QSAM Stockholder Representative, Telix shall permit an independent certified public accounting firm of nationally recognized standing mutually agreed by the QSAM Stockholder Representative and Telix (the “Independent Accountant”) to access the books and records of Telix and its affiliates to evaluate and verify Telix’s calculation of the Net Sales Milestone.
The Independent Accountant will keep all books and records of Telix and its affiliates strictly confidential, and will provide only a report of the results of its findings to QSAM Stockholder Representative. The Independent Accountant shall provide Telix with a copy of all disclosures made to the QSAM Stockholder Representative. The decision of such accounting firm shall be final, conclusive and binding on Telix, the QSAM Stockholder Representative and the QSAM stockholders, shall be non-appealable and shall not be subject to further review, absent manifest error. This audit right may not be exercised by the QSAM Stockholder Representative more than once; provided however, if a discrepancy greater than 10% in Telix’s favor is uncovered in the audit, the QSAM Stockholder Representative may exercise these audit rights a second time no sooner than 12 months after the completion of the first audit.
Commercially Reasonable Efforts
The CVR Agreement provides that Telix will use “Commercially Reasonable Efforts” (as defined below) to achieve each of the milestones.
The CVR Agreement defines Commercially Reasonable Efforts as, with respect to Telix’s obligations to develop or commercialize the acquired products, the level of efforts consistent with the efforts normally used by similarly situated biotechnology or biopharmaceutical company relating to the development and commercialization of a product with similar market potential as the acquired product at a similar stage of development or commercialization and taking into all relevant factors, including: (a) efficacy and safety clinical data; (b) patent and regulatory exclusivity; (c) target product profile; (d) market competition (including generic or biosimilar competition); (e) anticipated or approved labelling; (f) present and future market potential; (g) the likelihood of and scope obtained for regulatory approval; (h) the likelihood of and scope obtained for pricing and reimbursement; (i) the profitability and commercial potential of the product; and (j) all necessary medical, sales, marketing and other costs required for successful commercialization. For the avoidance of doubt, Commercially Reasonable Efforts shall be determined on a market-by-market and product-by-product basis, and it is anticipated that the level of effort will be different for different markets, and will change over time, reflecting changes in the status of the product and the market(s) involved.
| 58 |
Amendment and Termination of the CVR Agreement
Telix may, at any time and from time to time, unilaterally enter into one or more amendments to the CVR Agreement for any of the following purposes without the consent of any of the holders of CVRs or the Rights Agent:
| ● | to evidence the appointment of another person as a successor Rights Agent and the assumption by any successor Rights Agent of the covenants and obligations of the Rights Agent in accordance with the provisions thereof; | |
| ● | to add to the covenants of Telix such further covenants, restrictions, conditions or provisions as Telix shall consider to be for the protection of the CVR holders; | |
| ● | to cure any ambiguity, to correct or supplement any provision herein that may be defective or inconsistent with any other provision therein or in the Merger Agreement, or to make any other provisions with respect to matters or questions arising under the CVR Agreement; | |
| ● | as may be necessary or appropriate to ensure that CVRs are not subject to registration under the Securities Act, the Exchange Act or any applicable state or foreign securities laws; and | |
| ● | any other amendment hereto which would provide any additional rights or benefits to the CVR holders or QSAM Stockholder Representative or that does not adversely affect the legal rights or intended economic benefits under the CVR Agreement of the CVR holders or QSAM Stockholder Representative. |
Promptly after the execution by Telix and the Rights Agent of any amendment pursuant to the provisions of the CVR Agreement, Telix shall mail or otherwise transmit (or cause the Rights Agent to mail or otherwise transmit) a notice thereof through the facilities of DTC in accordance with DTC’s procedures and/or by first class mail to QSAM stockholders at their addresses as set forth on the CVR Register, setting forth in general terms the substance of such amendment.
With the consent of QSAM Stockholder Representative, Telix and the Rights Agent may enter into one or more amendments for the purpose of adding, eliminating or changing any provisions of the CVR Agreement, even if such addition, elimination or change is adverse to the interests of the CVR holders.
The CVR Agreement shall terminate upon the earlier to occur of (a) the payment of all Milestone Payments and (b) the expiration of the Milestone Period.
The Lock-Up Agreements
Concurrently with the execution of the Merger Agreement, each of QSAM’s directors, executive officers, a key employee, and certain stockholders have entered into a lock-up agreement, pursuant to which each of them agreed that, subject to limited exceptions, each of them will not (i) lend, offer, pledge, hypothecate, encumber, donate, assign, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any Telix Ordinary Shares such person will be issued in connection with the Merger (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of such ownership, or (iii) publicly disclose the intention to do any of the foregoing for a period of 12 months following the Closing under the Merger Agreement.
| 59 |
Reasons for the Reverse Stock Split
On February 6, 2024, the Board approved and recommended that its stockholders approve, and on February 6, 2024, the Majority Stockholders took action by written consent to approve an amendment to the Company Charter to effect a reverse stock split of outstanding shares of the QSAM Common Stock at a ratio in the range of 1:1000 to 1:2000 (the “Reverse Split”), such ratio to be determined by the Board at a later date at its sole discretion, but prior to the First Effective Date, and only in connection with the Merger. The Board may choose to not undertake the Reverse Split at all if it finds it to be unnecessary to complete the Merger.
Purpose of the Reverse Split and Timing
As indicated above in this Information Statement, QSAM stockholders will receive Telix Ordinary Shares upon closing of the Merger. The Telix Ordinary Shares will be issued pursuant to an exemption from registration of shares under Section 4(a)(2) or Regulation D of the Securities Act to “accredited investors” as that term is defined thereunder. In accordance with Regulation D, QSAM and Telix do not intend to issue Telix Ordinary Shares to more than 35 non-accredited investors, and in any case, to no more than 27 non-accredited investors pursuant to the terms of the Merger Agreement. Accordingly, the QSAM Board deemed it advisable and in the best interest of the Company and its stockholders to conduct a reverse stock split to reduce the number of non-accredited investors holding QSAM Common Stock. Based on the receipt of accredited investor questionnaires from approximately 90 of QSAM’s stockholders, QSAM Board determined the appropriate range of reverse stock split to be between 1:1000 and 1:2000. Notwithstanding the Reverse Split, Telix may, with the approval of the QSAM Stockholder Representative, elect to pay a non-accredited investor in lieu of Telix Ordinary Shares, cash in an amount equal to the stockholder’s pro rata share of the Closing Consideration.
Pursuant to the Reverse Split, any outstanding fractional shares of QSAM Common Stock (determined after determining the whole number of shares of QSAM Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (i) the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration and (ii) one (1) CVR for each share of QSAM Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares) pursuant to the Reverse Split.
The Reverse Split will be effected immediately prior to Closing of the Merger and at least 20 calendar days after the mailing of this Information Statement.
Our Board authorized, and the Majority Stockholders approved the Reverse Split under the condition that it may be effected only in connection with the Merger immediately preceding the First Effective Date.
Effects of the Reverse Stock Split
The principal result of the Reverse Split will be to decrease proportionately the number of outstanding shares of Common Stock based on the reverse stock split ratio determined by the Board and to cause the holders of all fractional shares resulting from the Reverse Split to receive cash consideration in lieu of Telix Ordinary Shares.
The Reverse Split would affect all holders of the Company’s Common Stock uniformly (i.e. regardless of their status as accredited or non-accredited investor), except that holders of a number of shares of QSAM common stock less than the Reverse Split ratio will not hold any shares following the Reverse Split, and as a result, would receive the non-CVR portion of their transaction consideration exclusively in cash. The Reverse Split will be effectuated after exercise of outstanding stock options, if any. Due to the retirement of fractional shares resulting from the Reverse Split, the voting power of remaining QSAM’s stockholders will increase proportional to the reduction of issued and outstanding shares of QSAM during the short period between the effectiveness of the Reverse Split and the Closing; however, the Company does not anticipate any matters would be subject to a stockholder vote during such period.
| 60 |
The Reverse Split is not intended as, and will not have the effect of, a “going private transaction” covered by Rule 13e-3 promulgated under the Exchange Act. The Reverse Split is not intended to modify the rights of existing stockholders in any material respect.
Fractional Shares
No fractional shares will be issued in the Reverse Split. If the Reverse Split is effected, each fractional share of Common Stock, as applicable, will be eligible to receive the cash consideration and the CVRs described above and elsewhere in this Information Statement. See “The Merger Agreement – Merger Consideration” on page 44.
Accounting Matters
Pursuant to the Reverse Split, the par value of our Common Stock will remain $0.0001 per share. As a result of the Reverse Split, upon the effectiveness of a certificate of amendment to the Company Charter, the stated capital on the Company’s balance sheet attributable to the Common Stock will be reduced to the aggregate par value of the issued shares of such class, and the additional paid-in capital account shall be credited with the amount by which the stated capital is reduced, if any. The Company’s stockholders’ equity (deficit), in the aggregate, will remain unchanged.
Also, if the Reverse Split is effected, the reported per share net loss would be higher because there will be fewer shares of the Company’s Common Stock outstanding. The Reverse Split would be reflected retroactively for all periods presented in the Company’s financial statements prepared following the effectiveness of the Reverse Split. The Company does not anticipate that any other material accounting consequences, including changes to the amount of stock-based compensation expense to be recognized in any period, would arise as a result of the Reverse Split.
Procedures
The Reverse Split, if effected, would become effective upon the filing of a certificate of amendment to the Company Charter with the Secretary of State of the State of Delaware. The following are descriptions of how the Reverse Split would be effected for beneficial holders and registered book entry holders.
Beneficial Holders of Common Stock
Upon the implementation of the Reverse Split, we intend to treat shares held by stockholders through a bank, broker or other agent in the same manner as registered stockholders whose shares are registered in their names. Banks, brokers and other agents would be instructed to effect the Reverse Split for their beneficial holders holding shares of Common Stock in street name. However, these banks, brokers and other agents may have different procedures than registered stockholders for processing the Reverse Split. Stockholders who hold shares of Common Stock with a bank, broker or other agent and who have any questions in this regard are strongly encouraged to contact their banks, brokers or other agents for more information.
Registered “Book-Entry” Holders of Common Stock
Certain registered holders of common stock may hold some or all their shares electronically in book-entry form with Transfer Online, Inc. (“Transfer Online”), the Company’s transfer agent. These stockholders do not have stock certificates evidencing their ownership of the Common Stock. They are, however, provided with a statement reflecting the number of shares registered in their accounts. If the Reverse Split is effected, stockholders who hold shares electronically in book-entry form with Transfer Online will not need to take action to receive whole shares of post-Reverse Split common stock as the exchange will be automatic.
| 61 |
MATERIAL FEDERAL INCOME TAX CONSEQUENCES OF THE REVERSE STOCK SPLIT
The following general summary discusses the material U.S. federal income tax consequences of the Reverse Split to U.S. Holders (as defined below) whose shares of Common Stock are exchanged for (i) shares of Common Stock, and/or (ii) in lieu of a fractional share of Common Stock, the right to receive an amount of cash equal to such fractional share’s pro rata share of the Closing Consideration (the “Company Share Cash Value”) and CVRs, pursuant to the Reverse Split. This discussion is for general information only and is not tax advice. This summary is based upon the Code, applicable U.S. Treasury regulations promulgated thereunder, judicial authority, and administrative rulings effective as of the date hereof. These laws and authorities are subject to change, possibly with retroactive effect, or different interpretations. Any such change could alter the tax consequences to U.S. Holders as described herein. No ruling from the IRS has been or will be requested in connection with the Reverse Split. The discussion below does not address any aspects of U.S. taxation other than U.S. federal income taxation, and as such does not address any state, local or foreign tax consequences or any estate, gift or other non-income tax consequences of the Reverse Split.
This discussion is for general information only and does not purport to address all aspects of U.S. federal income taxation that may be relevant to a particular beneficial owner of Common Stock (“Holder”) in light of its particular facts and circumstances. This discussion applies only to U.S. Holders that hold their shares of Common Stock as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Holder’s particular circumstances, including the impact of the alternative minimum tax or the unearned income Medicare contribution tax. This discussion also does not address consequences relevant to Holders that are subject to special rules under U.S. federal income tax laws including, without limitation, banks or other financial institutions; dealers or traders in securities or currencies; insurance companies; tax-exempt entities; entities or arrangements treated as partnerships for U.S. federal income tax purposes or other flow-through entities (and investors therein); retirement plans, individual retirement accounts or other tax-deferred accounts; real estate investment trusts; regulated investment companies; mutual funds; controlled foreign corporations; passive foreign investment companies; certain former citizens, former long-term residents of the United States, or entities covered by the anti-inversion rules under the Code; Holders having a functional currency other than the U.S. dollar; Holders who hold shares of Common Stock as part of a hedge, straddle, constructive sale, conversion transaction or other integrated transaction; Holders who own (or are deemed to own) 5% or more of the outstanding stock of QSAM; Holders subject to special tax accounting as a result of any item of gross income with respect to Common Stock being taken into account in an “applicable financial statement” (as defined in the Code); Holders that are non-U.S. persons or entities; and Holders who acquired their shares of Common Stock through the exercise of employee stock options or otherwise as compensation or through a tax-qualified retirement plan.
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of Common Stock that is for U.S. federal income tax purposes:
| ● | an individual citizen or resident of the United States; | |
| ● | a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or | |
| ● | a trust if (a) its administration is subject to the primary supervision of a court within the United States and one or more U.S. persons has the authority to control all substantial decisions of the trust or (b) it has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds shares of Common Stock, the tax treatment of a person treated as a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Partnerships, and other entities treated as a partnership for U.S. federal income tax purposes, that hold Common Stock and the partners in such entities should consult their tax advisors regarding the tax consequences of the Reverse Split to them.
| 62 |
THIS DISCUSSION IS PROVIDED FOR GENERAL INFORMATION ONLY AND DOES NOT CONSTITUTE LEGAL OR TAX ADVICE TO ANY HOLDER. THIS DISCUSSION IS NOT A COMPLETE DESCRIPTION OF ALL POTENTIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE REVERSE SPLIT AND DOES NOT ADDRESS TAX CONSEQUENCES THAT MAY VARY WITH, OR ARE CONTINGENT ON, INDIVIDUAL CIRCUMSTANCES. A HOLDER SHOULD CONSULT ITS OWN TAX ADVISORS CONCERNING THE U.S. FEDERAL INCOME TAX CONSEQUENCES AND ANY CONSEQUENCES ARISING UNDER FEDERAL NON-INCOME TAX LAWS, INCLUDING ESTATE AND GIFT TAXES, OR THE LAWS OF ANY TERRITORY, STATE, LOCAL OR NON-U.S. TAXING JURISDICTION IN LIGHT OF ITS PARTICULAR CIRCUMSTANCES RELATING TO THE REVERSE SPLIT.
General Tax Treatment of the Reverse Stock Split
If the Reverse Split is effected, it is intended to qualify as a “reorganization” under Section 368 of the Code and a “recapitalization” under Section 368(a)(1)(E) of the Code for U.S. federal income tax purposes. Assuming the Reverse Split so qualifies, then for U.S. federal income tax purposes, a U.S. Holder will not recognize gain or loss on the Reverse Split, except with respect to the receipt of the Company Share Cash Value and CVRs received in lieu of a fractional share of Common Stock, as described below. A U.S. Holder’s aggregate adjusted tax basis in the shares of Common Stock received pursuant to the Reverse Split will equal the aggregate adjusted tax basis of the shares of the Common Stock surrendered (excluding any portion of such basis that is allocated to any fractional share of Common Stock), and such U.S. Holder’s holding period in the shares of Common Stock received will include the holding period in the shares of Common Stock surrendered. U.S. Treasury Regulations provide detailed rules for allocating the tax basis and holding period of the shares of Common Stock surrendered to the shares of Common Stock received in the Reverse Split. U.S. Holders of Common Stock acquired on different dates and at different prices should consult their tax advisors regarding the allocation of the tax basis and holding period of such shares.
Company Share Cash Value and CVR
Subject to the discussion below under the heading “Tax Treatment of the CVRs,” and assuming Closed Transaction Treatment as described below applies to the CVR, a U.S. Holder that receives the Company Share Cash Value and CVRs in lieu of a fractional share of Common Stock pursuant to the Reverse Split will generally recognize capital gain or loss in an amount equal to the difference, if any, between (i) the sum of the Company Share Cash Value and the fair market value of the CVRs as determined for U.S. federal income tax purposes received in lieu the fractional share and (ii) such U.S. Holder’s adjusted tax basis in the Common Stock surrendered that is allocated to such fractional share of Common Stock. As discussed below, if Open Transaction Treatment applies to the CVRs, no gain will be recognized with respect to the CVRs until payments are made with respect to the CVRs. Subject to the discussion below related to imputed interest under the heading “Tax Treatment of the CVRs – Open Transaction Treatment,” any such gain or loss will be long-term capital gain or loss if, as of the effective time of the Reverse Split, the U.S. Holder’s holding period for such fractional share exceeds one year. Long-term capital gains of certain non-corporate taxpayers, including individuals, are generally taxed at preferential rates. The deductibility of capital losses is subject to limitations.
Tax Treatment of the CVRs
The amount of gain or loss a U.S. Holder recognizes in the Reverse Split, and the timing and potentially the character of a portion of such gain or loss, depends in part on the U.S. federal income tax treatment of the CVRs, with respect to which there is uncertainty.
| 63 |
Receipt of the CVRs and Payments Thereunder
The receipt of the CVRs pursuant to the Reverse Split may be treated as a “closed transaction” or an “open transaction” for U.S. federal income tax purposes. There is no legal authority directly on point addressing whether contingent value rights with characteristics similar to the CVRs should be taxed as an “open transaction” or “closed transaction,” and such question is inherently factual in nature. Pursuant to U.S. Treasury Regulations dealing with contingent payment obligations analogous to the CVRs, if the fair market value of the CVRs is “reasonably ascertainable,” a U.S. Holder should treat the transaction as a “closed transaction.” On the other hand, if the fair market value of the CVRs cannot be reasonably ascertained, a U.S. Holder should treat the transaction as an “open transaction.” These Treasury Regulations state that only in “rare and extraordinary” cases would the value of contingent payment obligations not be reasonably ascertainable. The following sections discuss the possible consequences if the receipt of CVRs pursuant to the Reverse Split is treated as an “open transaction” or a “closed transaction” for U.S. federal income tax purposes. U.S. Holders are urged to consult their tax advisors with respect to the proper characterization of the receipt of the CVRs.
It is possible that either Telix or QSAM may be required to take a position for income tax, withholding, and/or information reporting purposes that the Reverse Split, including the receipt of the CVRs, is either a “closed transaction” or an “open transaction.” Each U.S. Holder is urged to consult its tax advisor regarding the impact, if any, of the position that may be taken by Telix or QSAM on such U.S. Holder’s characterization of the Reverse Split.
Closed Transaction Treatment
If the value of the CVRs can be “reasonably ascertained,” the receipt of the CVRs as part of the Reverse Split should be treated as a “closed transaction” for U.S. federal income tax purposes and a U.S. Holder would recognize gain or loss upon the receipt of the Company Share Cash Value and the CVRs as part of the Reverse Split taking into account the fair market value of the CVRs, determined on the date of the receipt of the CVRs. The proper method to determine the fair market value of a CVR for U.S. federal income tax purposes is not clear, but it is possible that the trading value of the Common Stock would be considered along with other factors in making that determination. Telix and its affiliates and QSAM do not intend to obtain or report any valuation of the CVRs that may be used by U.S. Holders for this purpose. If the receipt of the CVRs as part of the Reverse Split is a “closed transaction” for U.S. federal income tax purposes, a U.S. Holder’s initial tax basis in the CVRs will equal the fair market value of the CVRs on the date of the receipt of the CVRs. The holding period of the CVRs will begin on the day following the date of the receipt of the CVRs.
There is no legal authority directly addressing the U.S. federal income tax treatment of payments that may be received pursuant to the CVRs if the receipt of the CVRs pursuant to the Reverse Split is a “closed transaction” for U.S. federal income tax purposes. Accordingly, the amount, timing, and character of any gains, income or loss with respect to payments received with respect to the CVRs are uncertain. For example, payments received with respect to a CVR may be treated, in whole or in part, as a non-taxable return of a CVR holder’s adjusted tax basis in the CVR.
To the extent that payments received with respect to a CVR by a U.S. Holder are not treated as a return of basis or if such payments exceed such basis, they could be treated as (1) capital gain (which gain would be long-term capital gain if, as of the date of such payment, the U.S. Holder’s holding period for the CVR exceeds one year), (2) income taxable at ordinary rates, or (3) dividends.
In the event a payment received with respect to a CVR gives rise to capital gain, a portion of any payment due more than six months following receipt of the CVR may constitute imputed interest taxable as ordinary income under Section 483 of the Code, as described below under the heading “Open Transaction Treatment.” There is no legal authority directly addressing the U.S. federal income tax treatment of the expiration of any rights to receive a payment with respect to the CVRs. Accordingly, a CVR holder may not be able to recognize a loss with respect to the expiration of a right to receive a payment under the CVRs until the holder’s right to receive all CVR payments terminates.
| 64 |
It is possible, although neither Telix nor QSAM expect it to be the case, that the CVRs could be treated as one or more “debt instruments” for U.S. federal income tax purposes. If that is the case, then payments received with respect to the CVRs generally will be treated as payments in retirement of a “debt instrument,” except to the extent interest is imputed under the rules of Sections 1274 and 1275 of the Code. If those rules were to apply, interest generally would be imputed under complex rules at a rate that corresponds to Telix’s borrowing rate for similar instruments. In such case, a U.S. Holder would generally be required to include the interest in income on an annual basis, whether or not currently paid.
Open Transaction Treatment
The receipt of the CVRs would generally be treated as an “open transaction” if the value of the CVRs cannot be “reasonably ascertained.” If the receipt of CVRs were treated as an “open transaction” for U.S. federal income tax purposes, a U.S. Holder would not immediately take the CVRs into account in determining its capital gain on the receipt of the Company Share Cash Value and CVRs pursuant to the Reverse Split, and a U.S. Holder would take no tax basis in the CVRs. Rather, subject to the imputed interest rules under Section 483 of the Code as discussed below, the U.S. Holder would recognize gain as payments with respect to the CVRs are received or deemed received in accordance with the U.S. Holder’s regular method of accounting, but only to the extent the sum of such payments (and all previous payments under the CVRs), together with the Company Share Cash Value, exceeds such U.S. Holder’s adjusted tax basis in the Common Stock surrendered pursuant to the Reverse Split that is allocated to any fractional share of Common Stock. Subject to the imputed interest rules discussed below, a U.S. Holder who does not receive consideration in respect of a fractional share of Common Stock pursuant to the Reverse Split (including for this purpose the Company Share Cash Value and any Telix Ordinary Shares and cash received as payments pursuant to the CVRs) with a fair market value at least equal to such U.S. Holder’s basis in its Common Stock that is allocated to such fractional share should recognize a capital loss in the year that the U.S. Holder’s right to receive further payments under the CVR terminates.
If the transaction is treated as an “open transaction” for U.S. federal income tax purposes, a portion of any payment with respect to a CVR due more than six months following the consummation of the Reverse Split may constitute imputed interest taxable as ordinary income under Section 483 of the Code. The portion of any such CVR payment treated as imputed interest under Section 483 of the Code generally would equal the excess of the amount of the CVR payment over the present value of such amount as of the closing date calculated using the applicable federal rate as the discount rate. A CVR holder must include in its taxable income interest imputed pursuant to Section 483 of the Code using such holder’s regular method of accounting.
Information Reporting and Backup Withholding
Payments of cash made in lieu of a fractional share of Common Stock may, under certain circumstances, be subject to information reporting and backup withholding. To avoid backup withholding, each U.S. Holder that does not otherwise establish an exemption should furnish its taxpayer identification number and comply with the applicable certification procedures.
Backup withholding is not an additional tax. Any amounts withheld will be allowed as a credit against the U.S. Holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided the required information is timely furnished to the IRS. U.S. Holders should consult their tax advisors regarding their qualification for an exemption from backup withholding and the procedures for obtaining such an exemption.
holders of Common Stock are urged to consult their tax advisers regarding the tax treatment of the REVERSE SPLIT, INCLUDING THE issuance of the CVRs and any future payments under the CVRS.
THE FOREGOING IS INTENDED ONLY AS A SUMMARY OF CERTAIN FEDERAL INCOME TAX CONSEQUENCES OF THE REVERSE STOCK SPLIT AND DOES NOT CONSTITUTE A TAX OPINION. QSAM has not sought and will not seek any opinion of counsel or any ruling from the IRS with respect to the matters discussed herein. QSAM urges holders of QSAM Common Stock to consult with their tax advisers with respect to the specific tax consequences to them in connection with the REVERSE STOCK SPLIT in light of their own particular circumstances, including the tax consequences under state, local, non-U.S. and other tax laws.
| 65 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the beneficial ownership of the Company’s Common Stock as of March 31, 2024, unless otherwise noted below for the following:
| ● | each person, or group of affiliated persons, who we know to beneficially own more than 5% of our Common Stock; | |
| ● | each of our named executive officers; | |
| ● | each of our directors; and | |
| ● | all our executive officers, and directors as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to such securities. Except as otherwise indicated, all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws. Common stock subject to options exercisable on or within 60 days after March 31, 2024, are deemed outstanding for the purpose of computing the percentage ownership of the person holding those options but are not deemed outstanding for computing the percentage ownership of any other person. According to the terms of the grants made by the Company, all QSAM Options were fully vested as of March 31, 2024 and will remain exercisable until the Last Exercise Date. All QSAM Options have exercise prices that are higher than the estimated Closing Consideration being paid by Telix per share of QSAM Common Stock and the current trading price of QSAM on OTCQB.
The beneficial ownership of voting securities of the Company is based on 4,445,469 shares of QSAM’s Common Stock issued and outstanding as of March 31, 2024.
| Name of beneficial owner | Amount beneficially owned(1) | Percent of Class Beneficially Owned(5) | ||||||
| 5% or Greater Stockholders | ||||||||
| David H. Clarke | 733,972 | (2) | 16.5 | % | ||||
| Checkmate Capital Group LLC, Checkmate Strategic Capital 2, LLC, Checkmate Strategic Capital Holdings LLC, and Charles Thomas Paschall | 313,291 | (3) | 7.0 | % | ||||
| Strategic Planning Assets Limited | 255,370 | (4) | 5.7 | % | ||||
| Named Executive Officers and Directors | ||||||||
| C. Richard Piazza | 393,487 | 8.8 | % | |||||
| Douglas Baum | 392,674 | 8.8 | % | |||||
| Christopher Nelson | 197,690 | 4.4 | % | |||||
| Adam King | 33,450 | 0.8 | % | |||||
| Charles J. Link, Jr. | 108,929 | 2.4 | % | |||||
| Adriann Sax | 35,455 | 0.8 | % | |||||
| All Officers and Directors (as a group, 6 persons) | 1,163,685 | 26.2 | % | |||||
| (1) | The number of shares beneficially owned includes any shares over which the person has sole or shared voting power or investment power and also any shares that the person can acquire within 60 days of March 31, 2024 through the exercise of any stock options or other right. Unless otherwise indicated, each person has sole investment and voting power (or shares such power with his or her spouse) over the shares set forth in the table. For each person, the number of shares that is included in the table because the person has options to acquire the shares is set forth below. |
| 66 |
| Name | QSAM Options | |||
| C. Richard Piazza | 15,625 | |||
| Douglas Baum | 15,825 | |||
| Christopher Nelson | 15,690 | |||
| Adam King | 10,500 | |||
| Charles J. Link Jr. | 26,375 | |||
| Adriann Sax | 25,000 | |||
| (2) | The information is based on a Schedule 13G/A, filed with the SEC on February 15, 2024. Mr. Clarke is the Vice-President and Director of GSB Holdings, Inc. (“GSB”) and makes investment decisions on its behalf. Further, Mr. Clarke makes investment decisions on behalf of Bounty Hunter, LLC (“Bounty Hunter”), in his capacity as its managing director and certain shares held of record by his grandson, Mr. August Gaines (“Gaines”). As such, Mr. Clarke may be deemed to be the beneficial owner of the shares of the Company held by each of GSB (621,744), Bounty Hunter (58,672) and Gaines (13,334). Except for the 40,222 shares that Mr. Clarke owns, all the remaining shares are owned by GSB, Bounty Hunter and Gaines. Mr. Clarke disclaims beneficial ownership with respect to shares of common stock of the Company not held by him. The address for each holder is 14179 Laurel Trail, Wellington, FL 33414. | |
| (3) | The information is based on a Schedule 13D/A, filed with the SEC on February 20, 2024. Their address is 595 E. Colorado Blvd., Suite 530, Pasadena, CA 91101. | |
| (4) | The information is based on a Schedule 13G/A, filed with the SEC on February 20, 2024. Their address is Rm 1201, 12/F Wing On Centre 111 Connaught Road Central, Hong Kong. | |
| (5) | The percentages shown are based on the 4,445,469 shares of our common stock outstanding as of March 31, 2024, plus the number of shares that the named person or group has the right to acquire within 60 days of March 31, 2024. For purposes of computing the percentages of outstanding shares of common stock held by each person, any shares that the person has the right to acquire within 60 days after March 31, 2024 are deemed to be outstanding with respect to such person but are not deemed to be outstanding for the purpose of computing the percentage of ownership of any other person. We also deemed outstanding any shares issued to officers and directors that contain a forfeiture clause which is not tied to a specific vesting date. |
| 67 |
Overview
We are developing next-generation nuclear medicines for the treatment of cancer. Our technology is Samarium-153 DOTMP, a/k/a CycloSam® (“CycloSam®” or the “QSAM Technology”), a clinical-stage bone targeting radiopharmaceutical. CycloSam® features a patented, low specific activity form of Samarium-153, a beta-emitting radioisotope with a short 46-hour half-life, and the chelating agent DOTMP, which selectively targets sites of high bone mineral turnover and reduces off-site migration of the tumor-killing radiation. We believe improvements in formulation and manufacturing from a prior FDA-approved drug utilizing the same radioisotope (Quadramet®) has resulted in our drug candidate demonstrating significantly less impurities, lower costs and more frequent availability. In early clinical testing, the QSAM Technology is demonstrating meaningful pain palliation effects in patients with metastatic prostate and breast cancer; and in future studies, may demonstrate disease modifying results in primary bone cancer patients, including children and young adults suffering from osteosarcoma.
In August 2021, the Food & Drug Administration (FDA) cleared our Investigational New Drug (IND) application to commence Phase 1 clinical trials for CycloSam® as a treatment for cancer that has metastasized to the bone from the lung, breast, prostate and other areas. We initiated this trial at our first site (Houston, TX) in November 2021 and to date we have dosed five patients.
Also in August 2021, the FDA granted Orphan Drug Designation for the use of CycloSam® to treat a primary bone cancer called osteosarcoma, a devastating disease that mostly affects children and young adults; and in February 2022, the FDA granted Rare Pediatric Disease Designation for the same indication. In May 2020, CycloSam® was also utilized in a Single Patient Investigational New Drug for Emergency Use at the Cleveland Clinic. We believe the study we conducted at the Cleveland Clinic showed promising safety results in connection with a bone marrow ablation procedure, including patient tolerability at high dosages. To date, CycloSam® has completed animal studies in both small and large animals, including treating bone cancer in patient dogs at a university veterinary clinic.
As disclosed above, on February 7, 2024, the Company entered into the Merger Agreement with Telix Pharmaceuticals Limited, a public limited company registered under the laws of the Commonwealth of Australia, and certain subsidiaries of Telix established for the purpose of completing a reverse triangular merger with a second forward merger. As of the date of this Information Statement, Telix has a multi-billion-dollar capitalization, and is a publicly traded pharmaceutical company with significant operations in the U.S., Europe and other areas globally. Further, Telix has a deep pipeline of radiopharmaceutical assets, one of which is currently commercialized and several others in late stages of clinical trials. As such, Telix has significant experience in the development, commercialization and sales of both diagnostic and therapeutic radiopharmaceutical technologies. Pursuant to the Merger, all of the operations and assets of the Company will be owned and controlled by Telix upon closing, including the clinical trials for and overall strategic direction of CycloSam®. As a result, the clinical pathway developed by the Company’s management and described in part in this business overview of QSAM, may not apply or be relevant to the future direction of the QSAM Technology.
Background of the QSAM Technology
What is CycloSam®. CycloSam® is a targeted, bone seeking radiopharmaceutical that combines the beta-emitting radioisotope Samarium-153 (153Sm) with a chelating agent, DOTMP (1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetramethylenephosphonic acid). Samarium-153 is acquired from a nuclear reactor from a third party and the chelating agent is supplied in the form of kits. Chelating agents are organic compounds capable of linking together metal ions to form complex ring-like structures. This combination forms a stable complex which delivers a radioactive dose to sites of rapid bone mineral turnover such as bone cancers and tumors. CycloSam® has a physical half-life of 46 hours (radiation decreases by half in 46 hours) and emits both medium-energy beta particles that produce the therapeutic effect, and gamma photons that make it possible to take images of the skeleton and locate and characterize the size and nature of tumors. The use of radioisotopes to both diagnose and treat disease is called “theranostics” and is a rapidly growing area of medical discovery.
| 68 |
How CycloSam® Works – Mechanism of Action & Administration. CycloSam® utilizes a chelating agent called DOTMP that seeks out bone locations of high mineral turnover, typical in cancer cells and tumor growth. DOTMP is taken up by calcium turnover locations in bones and carries the radioactive “payload” along with it. The radioisotope Samraium-153 emits radiation as it decomposes in the form of beta particles. Approximately 50% of the radioactivity concentrates in bone mineral with a very high lesion-to-normal bone ratio. We believe this provides a radiation dose to the adjacent tumor cells. The absorbed radiation dose produces the presumed therapeutic effect to the tumor, killing the cancer cells or slowing their growth by damaging their DNA. Metastatic bone cancer patients who have been dosed with CycloSam® have also reported material reductions in pain lasting for several months after treatment. Our pre-clinical studies and single patient IND performed at the Cleveland Clinic has demonstrated that the remaining half of the administered activity is rapidly excreted through the kidneys.
Generally, radiation therapy does not immediately kill cancer cells and more than one treatment is expected to eradicate a tumor, dramatically reduce its size, or slow its growth. CycloSam® has a short half-life of 46 hours and is rapidly eliminated from the body. This avoids an undesirable radioactive buildup in healthy tissues and organs when used in multiple treatments, which we believe, is an important feature of CycloSam® over predecessor drugs. CycloSam® has also not demonstrated saturation of the bone sites in animal studies, which supports a multi-dosage treatment regimen.
The final drug product of CycloSam® is prepared from DOTMP kits and 153SmCl in 0.1 N HCl at a nuclear pharmacy local to the patient administration site. The final drug product is then delivered to the physician for use as an intravenous (IV) injection within 72 hours.
How is CycloSam® Made – Method of Manufacturing. CycloSam® uses a patented, low-specific-activity Samarium-153 which is produced in the lower flux region (beryllium reflector) of the nuclear reactor and can be accessed with a pneumatic tube on a daily basis. Once prescribed by radiation oncologists and nuclear medicine physicians, we order the radioisotopes from Missouri University Research Reactor (MURR) or the University of Texas at Austin (NETL) to be sent overnight to an onsite or nearby (to the patient) nuclear pharmacy to be compounded with a DOTMP “cold kit” and delivered to the treating physician for administration.
The DOTMP “cold kit” is patented in the U.S. and other jurisdictions, and was developed by IsoTherapeutics LLC, the inventors of CycloSam®. Although we believe the IsoTherapeutics’ cGMP manufacturing facility has the capacity to manufacture sufficient supply for our initial rollout, and such manufacturing is currently covered under our master services agreement, we plan to secure secondary manufacturing partners for the kits in the future. MURR has been our source of Samarium-153 used in our animal studies, our Single Patient IND for Emergency Use at the Cleveland Clinic, and for part of our current clinical trials. We also now use NETL for the supply of irradiated Samarium-153, especially as needed to dose patients at our Houston, Texas clinical trial site. We plan to qualify additional suppliers of both the raw non-irradiated Samarium, and the irradiation of these materials, as part of our supply chain and general business risk diversification strategy.
| 69 |
What are CycloSam®’s anticipated competitive advantages. We believe CycloSam® has competitive advantages over current radiopharmaceutical offerings in the marketplace. Such potential competitive advantages include:
| ● | CycloSam®’s radioisotope, Samarium-153, emits beta particles that travel farther than alpha particles with what we believe is sufficient energy to slow the growth or decrease the size of target cancer cells. We believe beta particles penetrate bone matter deeper than the alpha emitting radiopharmaceuticals currently in the marketplace and may be more effective in treating tumors that form in or metastasize to bones, as well as the often debilitating pain that results from bone metastasis. | |
| ● | CycloSam®’s delivery agent, DOTMP, compared to other chelating agents such as EDTMP used in Quadramet®, has shown in animal and other pre-clinical testing to have a high bone binding affinity allowing for the maximum delivery of the radioactive “payload” adjacent to the tumor without saturation of the bone, as observed from our pre-clinical trials. | |
| ● | Our method of manufacturing Samarium-153 compared to Quadramet®, has shown in our pharmacopeial limits studies to produce a 30-fold reduction in levels of the long-lived radioactive impurity Europium-154. We believe this may mitigate toxicity issues with the patient. | |
| ● | Our initial studies show CycloSam® has fewer toxicities and a short 46 hour half-life that may allow for more frequent and repeated dosing of our radiopharmaceutical. |
The competitive advantages we believe to be important to CycloSam® are based on pre-clinical animal and other studies including our single patient IND at the Cleveland Clinic and the initial patients dosed in our Phase 1 safety trial. We cannot be sure that our technology will perform similarly in future clinical trials. Failure to achieve these competitive advantages could negatively affect our ability to achieve FDA approval as a new drug, or our ability to market CycloSam®.
License Agreement and Intellectual Property
License Agreement. The Company, through its wholly-owned subsidiary QSAM Therapeutics, entered into an exclusive worldwide Patent and Technology License Agreement and Trademark Assignment (the “License Agreement”) with IGL Pharma, Inc. (“IGL”) on April 20, 2020 with respect to the innovative work of Jim Simon, PhD and R. Keith Frank, PhD, at IsoTherapeutics on Samarium-153 DOTMP. IGL is an affiliated company with IsoTherapeutics. Until January 2024, our Executive Chairman also served as President of IGL. We amended the License Agreement on November 24, 2021, and then again on February 2, 2024 (the “Second Amendment”).
Our License Agreement with IGL, as modified in the first amendment in 2021, is for 20 years or until the expiration of the multiple patents covered under the license and requires multiple milestone-based payments including: up to $410,000 as CycloSam® advances through Phase 3 of clinical trials, and $2 million upon commercialization. IGL has also received 12,500 shares of the Company as additional compensation. Upon commercialization, IGL will receive an on-going royalty equal to 4.5% of Net Sales, as defined in the License Agreement, and 5% of any consideration we receive pursuant to a sublicense, sale of the asset, or sale of QSAM Therapeutics (the “IGL License Fee”). We will also pay for ongoing patent filing and maintenance fees, and we have certain requirements to defend the patents against infringement claims. The parties have agreed to mutual indemnification. Pursuant to the Second Amendment, which was entered into as a condition to the execution of the Merger Agreement, the parties: (i) made modifications to sublicense fees, royalties and other amounts payable to IGL; (ii) made modifications to the definitions of “Commercially Reasonable Efforts,” “Products” and “Patents” described in the License Agreement; and (iii) added an additional payment to IGL of $100,000, payable half upon the execution of the Second Amendment and the balance upon the closing of the Merger. The Second Amendment will only become effective upon the closing of the Merger, and shall become null and void if the closing does not occur.
Either party may terminate the License Agreement 30 days after notice in the event of an uncured breach, or immediately in the case of bankruptcy or insolvency of the other party. QSAM Therapeutics may terminate for any reason upon 30 days’ notice. In the case IGL terminates due to an uncured breach, IGL will repay to us 25% of our direct clinical costs to assume ownership of data and other information gained in that process.
In connection with the License Agreement, QSAM Therapeutics signed a two-year Consulting and Confidentiality Agreement (the “Consulting Agreement”) with IGL, which provided IGL with payments of $8,500 per month that continued through April 2022. We now contract directly with IsoTherapeutics for monthly consulting services at $8,500 per month under our Master Services Agreement. Pursuant to this arrangement, IsoTherapeutics provides us with additional consulting and advisory services from the technology’s founders to assist in the clinical development of CycloSam®. Until January 2024, our Executive Chairman served as President of IGL, and received a $500 per month fee from IGL. On February 27, 2024, Telix announced that it had entered into an agreement with IsoTherapeutics to acquire the latter.
Patents. Pursuant to the License Agreement, our IP estate includes 14 total patents issued and pending across three distinct patent families that we believe provide protection for the use of CycloSam® as a radiopharmaceutical in the U.S. and internationally. Under the License Agreement, the Company holds multiple issued, allowed or granted patents in the US, Japan, Canada, and Europe (which have subsequently been granted in approximately 22 countries in Europe). The balance of these patent applications are pending. Notably, the patents cover the use of low-specific activity Samarium-153, allowing for daily supply of the highly toxicity-reduced formulation of the isotope, which we believe is the key to allowing for multi-dose regimens of CycloSam® that could have a positive therapeutic effect. Also, the CycloSam® kit that will be commercialized is protected by the patent estate that broadly protects DOTMP kit formulations for radioisotopes, potentially allowing for efficient distribution of the product and widespread use. Finally, methods relating to repeat dosing regimens for therapeutic radiopharmaceutical agents, which suggest increased efficacy based on prior research, is also covered under patent applications. Taken together, management believes that the patent family provides for a significant barrier to entry for a competitor as it is expected to prevent a generic product from being developed; however, we cannot guarantee that a competitor will not or cannot challenge our patents or otherwise circumvent our patents, or that we would have the resources to defend any patent infringement.
| 70 |
A list of our patents and status of prosecution is included in the following table:
| Country / Region | Owner | Status | App No | Filing Date | Pub No | Pub Date | Patent No | Issue Date | Expiration Date | |||||||||||
| ITG-16 AT | Austria | IGL PHARMA, INC. | ISSUED | 5-May-16 | 3054996 | 21-Jun-23 | 7-Oct-34 | |||||||||||||
| ITG-16 BE | Belgium | IGL PHARMA, INC. | ISSUED | 5-May-16 | 3054996 | 21-Jun-23 | 7-Oct-34 | |||||||||||||
| ITG-16 BG | Bulgaria | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 CA | Canada | IGL PHARMA, INC. | ISSUED | CA2926652A1 | 7-Oct-14 | CA2926652A1 | 16-Apr-15 | CA2926652 | 20-Jul-20 | 7-Oct-34 | ||||||||||
| ITG-16 CZ | Czech Republic | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 DK | Denmark | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 EP | Europe | IGL PHARMA, INC. | GRANTED | EP14852866A | 7-Oct-14 | EP3054996A1 | 17-Aug-16 | 3054996 | 21-Jun-23 | n/a | ||||||||||
| ITG-16 FI | Finland | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 FR | France | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 DE | Germany | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 GR | Greece | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 HU | Hungary | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 IS | Iceland | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 IE | Ireland | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 IT | Italy | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 JP | Japan | IGL PHARMA, INC. | ISSUED | JP 2016-521278 | 7-Apr-16 | JP2016-532652 | 20-Oct-16 | JP 6787781 B2 | 02-Nov-20 | 7-Oct-34 | ||||||||||
| ITG-16 JP 1 | Japan | IGL PHARMA, INC. | ISSUED | 2019-061398 | 27-Mar-19 | JP2019123731A | 25-Jul-19 | JP 7068222 B2 | 06-May-22 | 7-Oct-34 | ||||||||||
| ITG-16 LU | Luxembourg | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 NL | Netherlands | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 NO | Norway | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 PL | Poland | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 PT | Portugal | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 SK | Slovakia | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 SI | Slovenia | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 ES | Spain | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 SE | Sweden | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 CH | Switzerland | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 TR | Turkiye | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 GB | United Kingdom | IGL PHARMA, INC. | ISSUED | 3054996 | 21-Jun-23 | 7-Oct-34 | ||||||||||||||
| ITG-16 US | United States | IGL PHARMA, INC. | ISSUED | 15/027,280 | 5-Apr-16 | US2016/0250359 A1 | 1-Sep-16 | US 10,172,965 B2 | 08-Jan-19 | 19-Nov-34 | ||||||||||
| ITG-16 US 1 | United States | IGL PHARMA, INC. | ISSUED | 16/194,324 | 17-Nov-18 | US2019/0083661 A1 | 21-Mar-19 | US 10,596,277 B2 | 24-Mar-2020 | 7-Oct-34 | ||||||||||
| ITG-16 US P | United States | IGL PHARMA, INC. | EXPIRED | 61/887,603 | 7-Oct-13 | n/a | n/a | n/a | n/a | 7-Oct-14 | ||||||||||
| ITG-16 WO | Patent Cooperation Treaty | IGL PHARMA, INC. | INACTIVE | PCT/US2014/059385 | 7-Oct-14 | WO2015/054173 | 16-Apr-15 | n/a | n/a | 7-Apr-16 | ||||||||||
| ITG-17 BE | Belgium | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 CA | Canada | IGL PHARMA, INC. | ALLOWED | 2,987,242 | 24-May-16 | CA2987242A1 | 1-Dec-16 | CA2987242 | ||||||||||||
| ITG-17 EP | Europe | IGL PHARMA, INC. | ISSUED | 16800631 | 24-May-16 | EP3302496 | 11-Apr-18 | EP3302496A4 | n/a | n/a | ||||||||||
| ITG-17 FR | France | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 DE | Germany | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 IE | Ireland | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 JP | Japan | IGL PHARMA, INC. | ISSUED | 2017-561326 | 24-May-16 | 2018-515585 | 14-Jun-18 | JP 6930922 B2 | 01-Sep-21 | 24-May-36 | ||||||||||
| ITG-17 JP 1 | Japan | IGL PHARMA, INC. | ISSUED | 2021-069039 | 13-Oct-22 | 2021-113207 | 5-Aug-21 | 7305699 | 30-Jun-23 | 24-May-36 | ||||||||||
| ITG-17 NL | Netherlands | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 CH | Switzerland | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 GB | United Kingdom | IGL PHARMA, INC. | ISSUED | EP3302496A4 | 06-Jan-21 | 24-May-36 | ||||||||||||||
| ITG-17 US | United States | IsoTherapeutics Group, LLC | Consolidated into ITG-17 | 15/821,983 | 24-Nov-17 | Consolidated into ITG-17 | n/a | n/a | n/a | |||||||||||
| ITG-17 US | United States | IGL PHARMA, INC. | Abandoned | 15/821,974 | 24-Nov-17 | US2018/0104366 | 19-Apr-18 | n/a | n/a | n/a | ||||||||||
| ITG-17 US 1 | United States | IGL PHARMA, INC. | ISSUED | 16/866,001 | 4-May-20 | US2020/0261607 A1 | 20-Aug-20 | US 11,369,700 B2 | 28-Jun-22 | 25-Jan-38 | ||||||||||
| ITG-17 US 2 | United States | IGL PHARMA, INC. | Published | 17/750,333 | 21-May-22 | US2022/0273828 | 1-Sep-22 | |||||||||||||
| ITG-17 US P | United States | IGL PHARMA, INC. | EXPIRED | 62/166,051 | 25-May-15 | n/a | n/a | n/a | n/a | 25-May-16 | ||||||||||
| ITG-17 US WO | Patent Cooperation Treaty | IGL PHARMA, INC. | INACTIVE | PCT/US2016/33900 | 24-May-16 | WO2016/0191413 | 1-Dec-16 | n/a | n/a | 25-Nov-17 | ||||||||||
| ITG-18 CA | Canada | IGL PHARMA, INC. | Pending | 3,052,973 | 7-Aug-19 | CA3052973A1 | 16-Aug-18 | |||||||||||||
| ITG-18 EP | Europe | IGL PHARMA, INC. | Pending | 18751017 | 22-Aug-19 | EP3579886 | 18-Dec-19 | |||||||||||||
| ITG-18 JP | Japan | IGL PHARMA, INC. | Pending | JP2019-563340 | 20-Sep-19 | JP 2020-506239 A | 27-Feb-20 | |||||||||||||
| ITG-18 JP 1 | Japan | IGL PHARMA, INC. | Pending | JP2022-164581 | 13-Oct-22 | JP 2022-183238 | 8-Dec-22 | |||||||||||||
| ITG-18 US | United States | IGL PHARMA, INC. | ALLOWED | 16/484,706 | 8-Aug-19 | US 2021-0138095 A1 | 13-May-21 | |||||||||||||
| ITG-18 US P | United States | IGL PHARMA, INC. | EXPIRED | 62/456,191 | 8-Feb-17 | n/a | n/a | n/a | n/a | 8-Feb-18 | ||||||||||
| ITG-18 WO | Patent Cooperation Treaty | IGL PHARMA, INC. | INACTIVE | PCT/US2018/017082 | 6-Feb-18 | WO2018/148209 | 16-Aug-18 | n/a | n/a | 8-Aug-19 |
Trademark. Pursuant to the License Agreement, the Company also has the right to use the registered trademark “CycloSam®” for the marketing and sale of the drug candidate. Pursuant to the Second Amendment, the trademark will be formally assigned to QSAM upon closing of the Merger.
| 71 |
Competition
The biotechnology and pharmaceutical industries are characterized by the rapid evolution of technologies and understanding of disease etiology, a strong emphasis on intellectual property and intense competition. We face substantial potential competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, academic research institutions, governmental agencies and public and private research institutions.
In addition to the current methods of care for cancer patients, the field of radiopharmaceuticals is deeply studied, and many parties are pursuing commercial and academic clinical trials. Early results from these trials have fueled continued interest in radiopharmaceuticals, which are pursued by several biotechnology companies as well as by large pharmaceutical companies.
We consider our competitors to be other companies developing targeted radiopharmaceuticals for the treatment of cancer. There are several companies developing targeted alpha-based radiopharmaceuticals for the treatment of cancer, including Bayer AG, or Bayer, Actinium Pharmaceuticals, Inc., RadioMedix, Inc, Orano Med, Telix, Fusion Pharmaceuticals Inc., and RayzeBio, Inc. These companies are targeting a wide range of solid and hematologic malignancies using various alpha emitting isotopes, including Radium-223, Actinium-225 and Thorium-227. The first approved alpha particle-based therapy is Bayer’s Xofigo, a salt of radium that is not currently attached to a targeting molecule, but naturally localizes to regions where cancer cells are infiltrating bone. Xofigo was approved in the United States by the FDA in 2013 for the treatment of bone metastases associated with prostate cancer.
There are several companies with approved or late clinical stage beta-based radiopharmaceuticals, including Novartis AG, Telix, and POINT Biopharma Global. Novartis received FDA approval for Pluvicto in 2022, a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer, and, in 2023, generated approximately $980 million in worldwide sales according to recent company filings. Pluvicto uses the beta-emitter Lutetium-177. Another competitive company, POINT Biopharma, has two indications using beta-emitting particles in Phase 3 trials, and which were recently licensed by Lantheus. The beta emitting isotopes used by these companies include Iodine-131, Lutetium-177, Strontium-89 and Yttrium-90. There are other beta particle-based radiopharmaceuticals in various stages of clinical development by companies including Ipsen S.A., Y-mAbs Therapeutics, Inc. and Clovis Oncology, Inc.
Many of our current or potential competitors, either alone or with their collaboration partners, have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies and materials complementary to, or necessary for, our programs.
We could see a reduction or elimination in our commercial opportunity if our competitors develop and commercialize drugs that are safer, more effective, have fewer or less severe side effects, are more convenient to administer, are less expensive or with a more favorable label than our product candidates. Our competitors also may obtain FDA or other regulatory approval for their drugs more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. The key competitive factors affecting the success of our product candidate, if approved, are likely to be their efficacy, safety, convenience, price, and the availability of reimbursement from government and other third-party payors.
| 72 |
Radiopharmaceutical Market
The global radiopharmaceuticals market size is projected to reach $13.67 billion by 2032, registering a CAGR of 10.2% during the forecast period 2023 to 2032, according to a November 2023 report from Precedence Research, a global provider of market insights. North America dominates the global radiopharmaceuticals market, which is attributed to the rising prevalence of chronic illnesses, extensive research and development initiatives, a supportive regulatory landscape, and the presence of leading pharmaceutical and radiopharmaceutical enterprises. Based on type, the radiopharmaceuticals market is bifurcated into diagnostic nuclear medicine and therapeutic nuclear medicine. The diagnostic nuclear medicine segment is holding a larger share of the market and is anticipated to register a higher CAGR during 2021–2028. By application, the market is segmented into oncology, cardiology, neurology, and others, with the oncology segment holding the largest market share in 2022.
Overall, we believe that the recent technological developments in radiopharmaceuticals, including promising new alpha-emitting candidates, will continue to provide supportive tailwinds to this sector. As a result, management believes that it is well positioned to capitalize on these future opportunities.
History of CycloSam® development and past studies and trials
The Company has an exclusive worldwide patent and technology License Agreement for CycloSam®. The QSAM Technology was developed at IsoTherapeutics Group, LLC (“IsoTherapeutics”) by its founders Jim Simone, PhD and R. Keith Frank, PhD (the “Inventors”). IsoTherapeutics subsequently transferred their technology to IGL Pharma, Inc., an affiliated entity and the Company’s current licensor. The Inventors also developed one of the first commercial radiopharmaceuticals on the market, Quadramet®, approved by the FDA in 1997 for pain palliation. Drs. Simone and Frank each have over 30 years of experience in radiopharmaceuticals, publishing more than 100 papers and authoring over 60 patents in the field. The Inventors spent much of their careers at Dow Chemical Company prior to divestiture of its radiopharmaceutical business. According to the Inventors, CycloSam® was developed to address the shortcomings of other radiopharmaceuticals, including Quadramet, such as toxicity, saturation effects, long-lived impurities, and supply-chain complexities.
Prior generation Quadramet vs Improvements in CycloSam®. CycloSam® is a second-generation bone-seeking radiopharmaceutical based on Quadramet. Although Quadramet was clinically proven to be effective for pain palliation associated with metastatic bone cancer, the toxicity of the drug made repeat doses undesirable and manufacturing complexities made daily availability highly challenging. Therefore, Quadramet’s use was always limited to pain control, not tumor reduction, elimination or disease modification. The loose binding affinity of Quadramet’s chelating agent, EDTMP, means the ratio of chelant to Samarium-153 is extremely high (~300:1). The resulting problem of bone saturation prohibits usage of Quadramet in high doses required for treatment of bone cancer or bone marrow ablation. Additionally, high levels of impurities from the Samarium-153 production, namely Europium-154, made repeated dosing of Quadramet undesirable. Lastly, Quadramet faced supply-chain and distribution limitations because the Samarium-153 it uses could only be accessed from the reactor once per week. We believe that because of these challenges, Quadramet demonstrated limited market success, and to our knowledge, was recently discontinued by its manufacturer and distributor. We believe CycloSam® overcomes these inherent limitations of Quadramet in terms of toxicity, usage, and availability.
The Vienna Protocol – Precedent of Efficacy. In August 2011, Dr. Helmut Sinzinger published a study in the Quarterly Journal of Nuclear Medicine and Molecular Imaging, which demonstrated that despite the described limitations of Samarium-153 EDTMP (Quadramet®), it could still be used to effectively treat bone metastasis.
The Vienna Protocol, as it was labeled, was based on a 550 patient study developed by Dr. Sinzinger to deliver therapeutic doses of Quadramet® on a periodic low dose basis balancing hematological toxicity and europium buildup with clinical results. The specific regimen used very low doses of the predecessor drug on an outpatient basis. The treatment was administered at three month intervals during the first year, followed by another five treatments at six month intervals, then five therapies at nine month intervals, and then annually indefinitely. The dosing schedule was driven by hematological concerns and constant monitoring was required.
| 73 |
During Dr. Sinzinger’s trials, a wide range of positive clinical responses were seen including arrested tumor growth and even regression of the cancer in the bone. Some patients were treated for over five years exhibiting significant clinical response. While effective, this regimen required significant time and safety precautions on the part of both the physician and patient both of which were considered overly burdensome. Although this study was well published and the efficacy results were promising, wide clinical adoption did not occur due to the overall effort that was required to deliver a true therapeutic dose while avoiding the toxicity issues. Quadramet was never approved by the FDA for the treatment of bone cancer, but rather, just for pain management associated with the disease.
Improvements of CycloSam® over Predecessor Drug
CycloSam® is a new, advanced generation Samarium-153 drug with a dramatically different clinical profile than Quadramet®. By producing the Samarium-153 in a different part of the nuclear reactor, the decay by-product Europium has shown in studies to be nearly non-existent, thus eliminating long-term buildup concerns [Source: IsoTherapeutics Group. (2021). Preparation and Stability of CycloSam® Sm-153-DOTMP. (Report No. QSM-1)]. Secondly, the superior binding affinity of the new chelating agent, DOTMP, means more energy can be delivered to the target, thus minimizing off-target concerns. Further, the method of harvesting the patented low specific activity Samarium-153 means it can be accessed on a daily basis, compared to weekly for Quadramet®, at a reduced cost. We believe that all of these clinical and manufacturing improvements were achieved without any reduction in either the tumor killing power of Samarium-153 or its ability to travel deep into the bone tumor.
Potential
Market Indications for CycloSam®
CycloSam’s therapeutic profile and presumed advantages over other radiopharmaceuticals, including Quadramet, translate to several potential key market indications, including treating pain associated with cancer that has metastasized to the bone and certain forms of primary bone cancer, such as osteosarcoma, which mostly affects children and young adults. The following table highlights the prevalences of these diseases in the United States on an annual basis:
| Market | Estimated New Cases Diagnosed Annually (US) | |||
| Bone Metastases (Breast, Prostate, Lung) | 400,000 | |||
| Other Primary Bone Cancers | 2,770 | |||
| Primary Bone Cancer – Osteosarcoma | 1,000 | |||
| Primary Bone Cancer – Ewing’s Sarcoma | 200 | |||
Source: American Cancer Society estimates of new cases reported each year in the United States. Data as of January 2023.
Bone metastases arise in about 5% of all types of cancer, 29% of patients with multiple myeloma (15,000), 16% of lung (37,000), 6% of prostate (48,000) and 7% of breast cancers (70,000). Roughly 70% of patients with bone metastases will experience bone pain, and many are at risk for skeletal-related events including fracture and spinal cord compression. The total annual cost for treatment of metastatic bone disease is approximately $12.7 billion or 17% of the total of $74 billion that was spent on direct medical costs of these cancers [Source: Schulman KL, Kohles J. Economic burden of metastatic bone disease in the U.S. Cancer. 2007 Jun 1;109(11):2334-42. doi: 10.1002/cncr.22678. PMID: 17450591]. In addition to metastatic bone cancers, according to the National Institute of Health SEER, there are approximately 14,000 people living with osteosarcoma in the US at any one time [Source: Damron TA, Ward WG, Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin Orthop Relat Res. 2007 Jun;459:40-7. doi: 10.1097/BLO.0b013e318059b8c9. PMID: 17414166, and National Cancer Institute: Surveilance, E., and End Results Program Cancer Stat Facts: Bone and Joint Cancer, <https://seer.cancer.gov/statfacts/html/bones.html> (2020)] and their cost of care is estimated to exceed $100,000 per patient [Source: American Cancer Society. Key Statistics About Bone Cancer].
| 74 |
Metastatic bone cancer is currently incurable, and therefore palliation and arrest or deceleration of the progress of disease are important near-term goals. Quadramet® (Samarium-153-EDTP) and MetastronTM (89Sr chloride) were approved by the FDA for pain palliation resulting from osteoblastic bone metastases, but their widespread acceptance and use is hampered by concern about the perceived risk of myelosuppression when administered concurrently with chemotherapy. Xofigo, an alpha particle emitter, was approved in May 2013 and initially was expected to capture significant market share rapidly; however, certain safety concerns have limited the product’s applicability. Novartis’ Pluvicto, a beta-emitter, was approved by the FDA in late 2022 for the treatment of prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer. Novartis reported worldwide sales of Pluvicto in 2023 of approximately $980 million according to company filings, and reported in a recent conference that they expect sales to reach $2 billion in the coming years.
Osteosarcoma is the most common childhood and adolescent/young adult (ages 15-39) primary high-grade bone malignancy [Source: Taran SJ, Taran R, Malipatil NB. Pediatric Osteosarcoma: An Updated Review. Indian J Med Paediatr Oncol. 2017;38(1):33-43. doi:10.4103/0971-5851.203513]. Patients can often have metastatic cancer at diagnosis, and metastasis to the lungs is often fatal for these patients. For patients who develop or present with metastatic cancer in this diagnosis, the 5-year survival rate is 66% [Source: Osteosarcoma - Childhood and Adolescence - Statistics.” Cancer.Net, 30 Sept. 2021, https://www.cancer.net/cancer-types/osteosarcoma-childhood-and-adolescence/statistics]. Osteosarcoma standard-of-care usually involves chemotherapy which has substantial negative side effects, or drastic surgeries such as limb salvage or amputation. Osteosarcoma is relatively resistant to External Beam Radiation Therapy (EBRT), and currently approved radiopharmaceutical therapeutics fall short due to myelotoxicity and long-lived radioactive impurities. There is a tremendous unmet need for a better treatment that is more efficacious against pediatric osteosarcoma and better tolerated by patients.
Preclinical and Clinical Studies
Preclinical Studies. Preclinical toxicology studies of CycloSam® in rats and dogs have shown that a single intravenous dose of non-radioactive Samarium-153 DOTMP elicited no significant systemic toxicity. Skeletal uptake has also been studied in rats over a wide range of doses to determine whether CycloSam® displays a similar saturation effect which has been observed in studies of Samarium-153 EDTMP (aka Quadramet). In the rat saturation study, no statistically significant difference was found in uptake as a function of increased dosage of CycloSam®.
In addition to rat and dog toxicological studies, a proof-of-concept study was conducted in ten dogs with spontaneously occurring bone cancer treated with 1-2 mCi/kg of CycloSam®. Treatment was well tolerated with seven dogs treated at a dose of 1 mCi/kg and one dog treated with 2 mCi/kg who did not experience a dose limiting toxicity. One dog treated with 2 mCi/kg and one dog treated with 2.3 mCi/kg experienced grade 4 asymptomatic thrombocytopenia and neutropenia; which refers to a manageable depressed level of platelets and neutrophils in the blood. Results from these preclinical studies suggested CycloSam® has potential as a therapeutic agent in the treatment of primary bone cancer and metastatic bone disease.
Safety/Tox Studies. Non-radioactive CycloSam® has been through a full-scale 14-day, acute toxicological study in both rats and dogs. This study was designed to determine the toxicokinetics of the product at four different dose levels that are higher than expected to be used in the current clinical trials. The studies showed no systemic toxicity in either of the species with a single intravenous administration of CycloSam® (non-radioactive). Some mild to moderate allergic-like responses were seen in dogs at the highest dose, which is much higher than would be expected for clinical use.
Non-clinical testing. Rat and rabbit pharmacology and targeted bone pharmacology studies have been undertaken and published in both patents and in the literature for Samarium-153-DOTMP and their preclinical results demonstrated significant skeletal uptake fractions. We believe these studies suggest CycloSam® has promise as a bone seeking radiopharmaceutical.
| 75 |
Clinical Pharmacology. The majority of non-clinical pharmacology studies with CycloSam® have been done in rats. When administered through the tail vein, much of the Samarium-153-DOTMP binds to the bone; the half-life is 46.3 hours, but the portion of the drug not bound to bone or calcified tissue is completely eliminated through the kidneys within 6 hours of administration. Two additional studies in dogs with osteosarcoma have also elicited promising results, and confirm bone uptake, preliminary safety, and preliminary clinical benefit against bone tumor. Preclinical results demonstrated significant skeletal uptake fractions.
Clinical Studies. We are currently enrolling patients in a Phase 1 multiple center, open label, dose escalation clinical trial intended to determine the maximum tolerated dose of CycloSam® in participants, and also assess early efficacy signals. Participants with bone cancer that has metastasized from the breast, lungs, prostate or other organs, as well as participants with cancer that has originated in the bone such as osteosarcoma and Ewing’s Sarcoma – diseases that mostly affect children and young adults – are eligible subject to the trial’s inclusion and exclusion criteria.
To date, we have completed two of four patient groupings (“cohorts”), with a total of up to 17 participants expected to be enrolled. The completed cohorts are comprised of five participants who received the lowest two dosages of CycloSam® in the study. The total dosage of the active radioisotope Samarium-153 to be received by the third cohort will be twice as high as the second cohort.
Safety data from the first five trial participants in our Phase 1 clinical trial showed no Serious Adverse Events (SAE’s), and no irreversible clinically significant adverse events. Importantly, there was no clinically significant white, red, or platelet blood cell suppression to date, no Grade 3 adverse events, and no irreversible adverse events. Some trial participants also had initial pain relief and reduction from baseline in pain intensity scores using the Visual Analog Scale (VAS) of pain intensity. Additionally, some trial participants had an initial reduction from baseline after treatment in bone tumor size as measured using Response Evaluation Criteria in Solid Tumors (RECIST) criteria in the followed bone tumor. Some trial participants also had an initial reduction in tumor Standard Uptake Values (SUV’s), an imaging measure of bone tumor activity. These results are preliminary and may not be indicative of future results in the trial.
In February 2022, the FDA cleared our amended protocol increasing the age criteria to participants 75 years old from the prior age limitation of 65. This amendment to the enrollment criteria expands the population of potential participants in QSAM’s Phase 1 study evaluating CycloSam® in the treatment of bone cancer.
In 2020, CycloSam® was studied for the first time in humans under a Single Patient Investigational New Drug (IND) for Emergency Use at the Cleveland Clinic. The patient, a 25 year-old male who suffered from myelodysplastic syndrome (MDS) and high-risk osteosarcoma, received a single low dose of 1 mCi/kg of CycloSam® on March 24, 2020 for dosimetry. This was followed seven days later on March 31, 2020 by a single high dose of 32 mCi/kg (1919 mCi) of CycloSam®. No injection site effects were noted at the time of injection. At 48 hours post-injection of the second dose there was no renal toxicity observed. The estimated dose delivered to the skeleton was 40 Gy with bone lesion uptake of 60 Gy. The abbreviation Gy stands for “gray”, which is a measurement of radiation reaching the target. In this instance, a 45 Gy is considered required to deliver the radiation to the target, and therefore, 60 Gy was considered very good. The patient received an allogeneic stem cell transfusion two weeks following high dose injection of Samarium-153 DOTMP; however, the stem cell transplant failed to fully engraft. The patient, who was terminally ill prior to the treatment, passed away on August 18, 2020, a month after bone marrow ablation and after additional procedures not using a radiopharmaceutical were performed, from complications of an infection unrelated to the infusion of CycloSam® according to the investigator.
The investigator concluded that high-dose CycloSam® can be given safely with no apparent renal toxicity and no unexpected adverse events attributable to Samarium-153 DOTMP. Skeletal targeting with sparing of other tissues was observed after the high dose. This was only a single patient human clinical trial, and the patient did not survive long enough for full observation, so additional safety and efficacy clinical trial data will have to be developed.
Contracted Research Organization. In January 2020, our licensor, IGL Pharma, entered into a Master Services Agreement (MSA) with a full-service Contract Research Organization (CRO) with over a 30 year history of service to pharmaceutical and biotechnology clients. The MSA was amended in February 2021 to add QSAM as a party and includes a fixed monthly retainer for regulatory and clinical trial consulting services as well as specific work orders for clinical trial execution services. The CRO has a full-time staff of project managers, statisticians, physicians, nurses and other regulatory and operational personnel to support our FDA interactions, filings and preclinical and clinical trial activities. Specifically, the CRO provides clinical trial management services, clinical study monitoring services, medical coding services, electronic data capture services, data management services, medical monitoring services, safety reporting and medical writing services.
| 76 |
Government Regulation and Product Approval
Clinical trials, the drug approval process, and the marketing of drugs are intensively regulated in the United States and in all major foreign countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act (“FDCA”), and related regulations. Drugs are also subject to other federal, state, and local statutes and regulations. Failure to comply with the applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA Institutional Review Board (“IRB”) of a clinical hold on trials, the FDA’s refusal to approve pending applications or supplements, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and comparable regulatory agencies in state and local jurisdictions and in foreign countries impose substantial requirements upon the clinical development, manufacture and marketing of biopharmaceutical products. These agencies and other federal, state, and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising, and promotion of product we develop in the future.
The FDCA and/or FDA’s policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of any candidate drug product or approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Marketing Approval: The process required by the FDA before new drugs may be marketed in the United States generally involves the following:
| ● | nonclinical laboratory and animal tests; | |
| ● | chemistry, manufacturing, and control testing (CMC), validation and documentation of all synthesis, preparation and production processes for all kit ingredients and finished products; | |
| ● | submission of an Investigational New Drug (IND) application, which must become effective before clinical trials may begin; | |
| ● | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; | |
| ● | pre-approval inspection of manufacturing facilities and clinical trial sites; and | |
| ● | FDA approval of a New Drug Application (NDA) which must occur before a drug can be marketed or sold. |
The testing and approval process requires substantial time and financial resources, and we cannot be certain that any approvals will be granted on a timely basis if at all.
We will need to successfully complete additional clinical trials in order to be in a position to submit an NDA to the FDA. Future trials may not begin or be completed on schedule, if at all. Trials can be delayed for a variety of reasons, including delays in:
| ● | obtaining regulatory approval to commence a study; | |
| ● | reaching agreement with third-party clinical trial sites and vendors and their subsequent performance in conducting accurate and reliable studies on a timely basis; | |
| ● | obtaining institutional review board approval to conduct a study at a prospective site; |
| 77 |
| ● | recruiting subjects to participate in a study; and | |
| ● | supply of the drug. |
We must reach an agreement with the FDA on the proposed protocols for our future clinical trials, post-market safety monitoring, and on a Pediatric Development Plan in the United States. All new drugs now require the presentation to the FDA after Phase II clinical trials have ended of a Pediatric Development Plan outlining the strategy and steps to be taken by us to study CycloSam® in children as appropriate. A separate submission to the FDA must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. Informed consent must also be obtained from each study subject. Regulatory authorities, an IRB, a data safety monitoring board, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the participants are being exposed to an unacceptable health risk. Such risks may include unexpected or serious adverse events, or increased severity or occurrence rate of known potential adverse events.
FDA Post-Approval Requirements: Any products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping and reporting of adverse experiences with the drug and/or additional post-market clinical trials. Drug manufacturers are required to register their facilities with the FDA and certain state agencies and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain quality processes, manufacturing controls, and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality and purity characteristics that it purports to have. Under the federal Prescription Drug Marketing Act, the sampling and distribution and tracking of drugs is regulated. It is designed to discourage the sale of counterfeit, adulterated, misbranded, subpotent, and expired prescription drugs. Certain states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, fail to approve any NDA or other application, require us to recall a drug from distribution, shut down manufacturing operations or withdraw approval of the NDA for that drug. Noncompliance with cGMP or other requirements can result in issuance of warning letters, civil and criminal penalties, seizures, and injunctive action.
Labeling, Marketing and Promotion: The FDA closely regulates the labeling, marketing, and promotion of drugs. While doctors are free to prescribe any drug approved by the FDA for any use, a company can only make claims relating to the safety and efficacy of a drug that are consistent with FDA approval and may only actively market a drug only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions, and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of drugs.
Pediatric Research Equity Act: The Pediatric Research Equity Act (“PREA”) amended the FDCA to authorize the FDA to require certain research into drugs used in pediatric patients. The intent of the PREA is to compel sponsors whose drugs have pediatric applicability to study those drugs in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The Secretary of Health and Human Services may defer or waive these requirements under specified circumstances. The FDA may decide that an NDA will be approved only following completion of additional pediatric studies.
| 78 |
Anti-Kickback and False Claims Laws: In the United States, the research, manufacturing, distribution, sale and promotion of drug products and medical devices are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the U.S. Department of Health and Human Services (e.g., the Office of Inspector General), the U.S. Department of Justice, state Attorneys General, and other state and local government agencies. For example, sales, marketing, and scientific/educational grant programs must comply with the Anti-Kickback Statute, the False Claims Act, as amended, the privacy regulations promulgated under HIPAA, and similar state laws. Pricing and rebate programs must comply with the Medicaid Drug Rebate Program requirements of the Omnibus Budget Reconciliation Act of 1990, as amended, and the Veterans Health Care Act of 1992, as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
In the United States, we are subject to complex laws and regulations pertaining to healthcare “fraud and abuse,” including, but not limited to, the Anti-Kickback Statute, the federal False Claims Act, and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to or approval by the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. Violations of the False Claims Act can result in very significant monetary penalties and treble damages. The federal government is using the False Claims Act, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multi-billion-dollar settlements under the False Claims Act in addition to individual criminal convictions under applicable criminal statutes. In addition, the federal civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws. The federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), also created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the Anti-Kickback Statute a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them in order to have committed a violation.
There are also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, a similar federal requirement Section 6002 of the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (the “Affordable Care Act”) commonly referred to as the “Physician Payments Sunshine Act” requires manufacturers to track and report to the federal government certain payments and “transfers of value” made to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, made in the previous calendar year. There are a number of states that have various types of reporting requirements as well. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
| 79 |
Patient Protection and Affordable Health Care Act: Historically in the United States, policy makers have attempted several healthcare reforms regarding the healthcare system that could expand access to healthcare, improve quality of healthcare, contain healthcare costs, prevent or delay approval of product candidates, restrict or regulate post-approval activities, and affect the profitable sale of drugs.
In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the ACA, was passed, which substantially changed the way healthcare is financed by both governmental and private insurers, and significantly affected the pharmaceutical industry. Since its enactment, there have been judicial and political challenges to certain aspects of the ACA. Most recently on June 17, 2021, the U.S. Supreme Court dismissed a judicial challenge to the ACA brought by several states without specifically ruling on the constitutionality of the ACA. Prior to the Supreme Court’s decision, President Biden issued an executive order to initiate a special enrollment period from February 15, 2021 through August 15, 2021 for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is unclear how the healthcare reform measures of the Biden administration or other efforts, if any, to challenge, repeal or replace the ACA will impact the ACA.
Congress and the Biden administration have generally indicated that they will continue to seek new legislative and/or administrative measures to control drug costs and improve access. Individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
Other Regulations: We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control, and disposal of hazardous or potentially hazardous substances. We may incur significant costs to comply with such laws and regulations now or in the future.
The CycloSam® radioactive product is regulated by the federal Nuclear Regulatory Commission (NRC), and also by similar state regulatory agencies. The handling, packaging, shipping, transportation, and disposal of radioactive materials is highly regulated, and those regulations can change, and the Company would have to comply with all requirements, which could be costly. Additionally, if overnight delivery failures occur, the radioactive compounds cannot be used, and that can result in substantial increased costs and liabilities. The disposal of radioactive materials is also regulated by the Environmental Protection Agency (EPA), and also by similar state regulatory agencies and the Company would have to comply with all of those requirements, which could also be costly.
Radioactive waste from the medical sector is an environmental concern from a global perspective. However, most studies have concluded that radioactive material from the medical sector does not present a significant long term waste management problem when compared to wastes generated from nuclear fuel cycle operations. This is primarily due to the characteristics of biomedical waste, such as its short half-life and low radiotoxicity. For instance, Samarium-153 has a half-life of approximately 46 hours. Biomedical waste also typically contains low energy emitters, such as beta and gamma isotopes, and is generally of low total and specific activity. Further considerations are the volumes of this waste and any other hazardous properties associated with the waste such as biological and chemical risks.
| 80 |
Regardless of the relatively low risks in the preparation, use and disposal of medical isotopes, entities that handle such materials should implement an effective program for biomedical radioactive waste management based on the principles of waste prevention and minimization, while providing for the protection of personnel and the environment, consistent with the requirements of applicable regulatory authorities. This assessment should include an analysis of the total radionuclide inventory and pattern of use, waste types and amounts generated and the potential routes for disposal.
We seek to assure that the nuclear reactor facilities that produce our Samarium-153, as well as the nuclear pharmacies that prepare doses for treatment, have proper and effective waste management procedures in place applicable to the risks presented by the actual material. Further, doctors and trial sites who handle radioactive materials must be educated on the dangers of handling hazardous substances and the proper methods of disposing radioactive or formally radioactive waste, similar to the handling of other medical and bio wastes.
Smaller Reporting Company
We are subject to the reporting requirements of Section 13 of the Exchange Act, and subject to the disclosure requirements of Regulation S-K of the SEC, as a “smaller reporting company.” That designation will relieve us of some of the informational requirements of Regulation S-K.
Sarbanes/Oxley Act
Except for the limitations excluded by the JOBS Act discussed under the preceding heading “Emerging Growth Company,” we are also subject to the Sarbanes-Oxley Act of 2002. The Sarbanes/Oxley Act created a strong and independent accounting oversight board to oversee the conduct of auditors of public companies and strengthens auditor independence. It also requires steps to enhance the direct responsibility of senior members of management for financial reporting and for the quality of financial disclosures made by public companies; establishes clear statutory rules to limit, and to expose to public view, possible conflicts of interest affecting securities analysts; creates guidelines for audit committee members’ appointment, compensation and oversight of the work of public companies’ auditors; management assessment of our internal controls; prohibits certain insiders from trading during pension fund blackout periods; requires companies and auditors to evaluate internal controls and procedures; and establishes a federal crime of securities fraud, among other provisions. Compliance with the requirements of the Sarbanes/Oxley Act will substantially increase our legal and accounting costs.
Exchange Act Reporting Requirements
Section 14(a) of the Exchange Act requires all companies with securities registered pursuant to Section 12(g) of the Exchange Act, like we are, to comply with the rules and regulations of the SEC regarding proxy solicitations, as outlined in Regulation 14A. Matters submitted to shareholders at a special or annual meeting thereof or pursuant to a written consent will require us to provide our shareholders with the information outlined in Schedules 14A (where proxies are solicited) or 14C (where consents in writing to the action have already been received or anticipated to be received) of Regulation 14, as applicable; and preliminary copies of this information must be submitted to the SEC at least 10 days prior to the date that definitive copies of this information are forwarded to our shareholders.
We are also required to file annual reports on Form 10-K and quarterly reports on Form 10-Q with the SEC on a regular basis, and will be required to timely disclose certain material events (e.g., changes in corporate control; acquisitions or dispositions of a significant amount of assets other than in the ordinary course of business; and bankruptcy) in a Current Report on Form 8-K.
Number of Total Employees and Number of Full Time Employees
As of the date of this Information Statement, we have four full-time employees. Our CFO is currently part-time.
In 2024, and subject to adequate funding and assuming the Merger is not consummated, we seek to provide our employees with health care coverage and other benefits to help attract and maintain our workforce. We are not currently obligated to provide health insurance, however, we believe this is an important addition to our benefits package. All employees receive at least three weeks of paid time off per year. We have historically provided incentive stock options and other equity incentives to officers, directors and key employees to provide ownership and alignment of interests with our shareholders. We also use in certain instances performance-based vesting for stock options and restricted stock, whereby we set milestones to reflect important value creating initiatives of the Company. As a company, we seek diversity and inclusion in our workplace.
Available Information
We maintain an internet website at www.qsambio.com. We make available on or through our website, free of charge, our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 as soon as reasonably practical after we electronically file such material with, or furnish it to, the Securities and Exchange Commission. We do not intend the address of our website to be an active link or to otherwise incorporate the contents of our website into this Annual Report. You may also find all of the reports that we have filed electronically with the SEC at their Internet site www.sec.gov.
| 81 |
Unless otherwise indicated or the context otherwise requires, references in this section to “Telix,” and other similar terms refer to Telix Pharmaceuticals Limited and its subsidiaries.
Overview
Telix is a commercial-stage biopharmaceutical company focused on the development and commercialization of diagnostic and therapeutic radiopharmaceuticals. Telix was incorporated on January 3, 2017 under the Corporations Act 2001 (Cth) in Australia, listed on the Australian Securities Exchange (ASX) on November 15, 2017, and currently trades on the ASX under the symbol “TLX.” Telix’s headquarters is located in Melbourne, Australia and it currently maintains commercial operations in the United States, Belgium, Switzerland and Japan. Telix’s registered offices are located at 55 Flemington Road, North Melbourne, Victoria, 3051, Australia and its reception telephone number is +61 3 9093 3855. Telix’s agent for service of process in the United States is Telix Pharmaceuticals (US) Inc., located at 11700 Exit 5 Pkwy, Suite 200, Fishers, Indiana 46037. Find more information about Telix on its website located at www.telixpharma.com.
History and Development of Telix
In 2015, co-founders Dr. Christian Behrenbruch and Dr. Andreas Kluge founded Telix to respond to a rapidly growing level of interest in the field of molecularly targeted radiation (MTR) from the pharmaceutical industry. In January 2017, Telix Pharmaceuticals Limited was incorporated to finance and further develop Telix’s opportunities and hold the portfolio assets and raised A$8.5 million from investors, including strategic partners, to further Telix’s development.
In October 2017, Telix acquired Therapeia GmbH & Co. KG, a privately held German pharmaceutical company, to obtain the core patents and know-how for its theranostic program for brain cancer (glioblastoma). The consideration of the payment was a nominal cash purchase price and the assumption of certain debt.
On September 11, 2018, Telix completed the acquisition of Atlab Pharma SAS. The consideration for the acquisition comprised of A$12.6 million in Company shares and warrants. Further, on December 24, 2018, Telix completed the acquisition of Advanced Nuclear Medicine Ingredients SA. The upfront consideration value was A$3.9 million in Company shares, in addition to cash consideration of A$2.7 million and the fair value of contingent consideration of A$10,591,885. These acquisitions were consummated to advance the technology, intellectual property and talent and materially boosted Telix’s product portfolio, revenues and barrier to entry for competition.
In December 2020, Telix acquired TheraPharm GmbH, a Swiss-German biotechnology, for A$32.7 million comprising of upfront consideration, and future earn-out and royalty payments. Through acquiring TheraPharm, Telix expanded its innovative diagnostic and therapeutic solutions pipeline, providing Telix access to a portfolio of patents, technologies, production systems, clinical data and know-how in relation to the use of MTR in hematology and immunology.
Telix’s first commercial product for prostate cancer imaging, Illuccix®, was approved by the Australian Therapeutic Goods Administration (TGA) in November 2021, the U.S. Food and Drug Administration (FDA) in December 2021, and Health Canada in October 2022. During 2022, Telix achieved a major commercial milestone with the launch of Illuccix® in the U.S. and the subsequent receipt of first commercial revenues from sales of Illuccix® in April 2022.
On December 31, 2022, Telix completed the acquisition of Optimal Tracers, a radiochemistry development business providing radiochemistry process development services and research tracers for use in clinical trials, from Sacramento-based Northern California PET Imaging Center. The consideration comprised of cash consideration of A$973,000 and contingent consideration based on a percentage of sales to existing customers of Optimal Tracers for a period of 24 months. Optimal Tracers was advantageously located to service leading clinical sites along the West Coast of the U.S., with the capability to deliver certain research products across the entire country.
On April 27, 2023, Telix completed the acquisition of Vienna-based Dedicaid GmbH (Dedicaid). The purchase price comprised of A$1.83 million upfront, paid in equity, with an additional future earn out payment subject to the achievement of regulatory approval in the U.S. The acquisition of Dedicaid, pursuant to which Telix obtained ownership over Dedicaid’s clinical decision support software and artificial intelligence platform, will enhance Telix’s artificial intelligence capabilities and accelerate the development of Telix artificial intelligence applications across the pipeline.
| 82 |
On November 1, 2023, Telix completed its acquisition of Lightpoint Medical and its SENSEI ® radio-guided surgery business. The upfront consideration value was $20.0 million (approximately A$30.6 million), of which $19.6 million was paid to Lightpoint Medical Limited in equity through the issuance of 3,298,073 fully paid ordinary Telix shares at A$9.3659 per share, with the balance paid in cash. A further $15.0 million (approximately A$23.6 million) was payable via an earn-out in the form of rights. The acquisition was made to support and expand Telix’s late-stage urologic pipeline and strengthen Telix’s capabilities in deploying molecular imaging in the surgical setting.
On November 13, 2023, Telix announced a strategic investment in Mauna Kea Technologies (Mauna Kea) of USD $10.1 million to develop new hybrid pharmaceutical-device products through the combination of Telix’s cancer-targeting agents with Mauna Kea’s surgical endomicroscopy platform. Following the investment, Telix owns 19.33% of the share capital and 19.01% of the voting rights of Mauna Kea. The investment in Mauna Kea is to further support the development of advanced imaging techniques for minimally invasive surgery, with a specific focus on urologic oncology.
On February 27, 2024, Telix announced entry into an agreement to acquire IsoTherapeutics Group, LLC (IsoTherapeutics), a specialty radiopharmaceutical development and bioconjugation firm based in Angleton, Texas in the United States. Upon completion, the purchase price is expected to comprise of US$8 million upfront, US$5 million in performance-related milestone payments, and a two-year revenue share based on actual revenue earned from existing customers of IsoTherapeutics, estimated to be approximately US$0.6 million. The IsoTherapeutics acquisition will expand Telix’s U.S. manufacturing footprint, allowing for greater control over the isotope supply chain, support the commercialization of Telix’s investigational therapeutic assets, and establish a center for excellence in Good Manufacturing Practice bioconjugation and isotope processing.
On March 5, 2024, Telix announced entry into an agreement to acquire radioisotope production technology firm ARTMS Inc. (ARTMS), based in Vancouver, British Columbia, Canada. The acquisition includes ARTMS’ advanced cyclotron-based isotope production platform, manufacturing plant and stockpile of ultra-pure rare metals required for consumable target production. Upon completion, the purchase price is expected to comprise of approximately US$57.5 million, up to US$24.5 million in contingent future earn out payments, and cash earn-outs representing low single to low double-digit percentage of net sales of ARTMS products or Telix products prepared using ARTMS products. The ARTMS acquisition is expected to further enhance the vertical integration of Telix’s supply chain and manufacturing by providing a greater level of control and security over each of Telix’s diagnostic isotopes.
About Telix’s Business
Telix is a commercial-stage biopharmaceutical company focused on the development and commercialization of therapeutic and diagnostic radiopharmaceuticals. Telix’s pipeline for urologic oncology (prostate and kidney), neuro-oncology (glioma), musculoskeletal oncology (sarcoma) and bone marrow conditioning is underpinned by a global supply, manufacturing and distribution network. Telix has received regulatory approvals from the Australian TGA, U.S. FDA, and Health Canada for its prostate cancer imaging agent.
Telix launched its first commercial product for prostate cancer imaging, Illuccix®, in 2022. Telix aims to build a foundation for long-term sustainable growth to unlock the value in its late-stage theranostic pipeline. Telix intends to leverage its commercial revenues from sales of Illuccix® as a source of funding for the development of additional near-term therapeutic and diagnostic product candidates in its pipeline. Some of these trials are funded directly by Telix, others are funded in collaboration with leading cancer centers and commercial partners.
Telix’s Product Pipeline
Telix’s portfolio includes both therapeutic and diagnostic radiopharmaceutical product candidates designed for use throughout the continuum of the patient journey, from diagnosis to treatment and ongoing care. Telix’s clinical programs include several late-stage product candidates with multiple expected upcoming data readouts and regulatory filings. The following depicts the current status of its core pipeline:
| 83 |

Prostate Cancer and PSMA
Telix’s prostate cancer programs target PSMA, a protein that is overexpressed on the surface of prostate cancer cells and is low or absent on most normal healthy cells. PSMA has become a major breakthrough in the staging, treatment and management of prostate cancer. Imaging with targeted radiation can identify prostate cancer wherever it is in the body and help guide patient treatment. The PSMA receptor is expressed in over 80% of prostate cancer tumors. This expression of PSMA provides a specific target to design therapeutic and diagnostic agents for the treatment and imaging of prostate cancer.
| ● | Telix’s lead therapeutic product candidate, TLX591 (177Lu rosopatamab tetraxetan) is a lutetium-labelled radio antibody-drug conjugate (rADC) that Telix believes has the potential to deliver a better safety and efficacy profile with a more efficient dosing regimen compared to existing small molecule products, including those commercially available and in clinical development. Telix is currently evaluating the efficacy and safety profile of TLX591 in the ProstACT series of clinical trials in prostate cancer, from first recurrence to advanced metastatic disease. | |
| ● | TLX592 (64Cu/225Ac-RADmAb®) is Telix’s investigational next-generation targeted alpha therapy (TAT) based on Telix’s proprietary RADmAb® engineered antibody technology. TLX592 includes an engineered antibody vector designed for faster elimination from circulation than standard antibodies and slower elimination than small molecules that may result in side effects. The Phase I CUPID trial is evaluating 64CU-labelled TLX592 in patients with advanced prostate cancer, prior to commencing therapeutic studies with 225Ac. |
| 84 |
| ● | Telix’s prostate cancer portfolio also includes Illuccix® (68Ga-PSMA-11), its commercially available gallium 68-labelled PSMA positron emission tomography (PET) imaging agent. The “cold kit” format of Illuccix® enables rapid radiolabeling at room temperature with high radiochemical purity and production consistency, which is suited to the commercial and hospital radiopharmacy setting. Illuccix® has validated accuracy compared to other PSMA imaging agents, including lower rates of false positives and strong efficacy in patients with low disease burden. Illuccix® is approved in the United States, Australia, and Canada. Approved indications include staging of high-risk patients, identification of suspected recurrence, and selection for PSMA-directed lutetium therapy. |
Kidney Cancer and CAIX
Currently, there are unmet needs for improvements in the diagnosis of clear cell renal cell carcinoma (ccRCC), the most prevalent and aggressive form of kidney cancer, from indeterminate renal masses, and the staging of advanced disease through more accurate and specific imaging techniques. Despite the transformative impact of immunotherapies on the prognosis of patients with metastatic kidney cancer, a considerable number fail to respond adequately to these and eventually progress. Telix’s target for kidney cancer is carbonic anhydrase IX (CAIX), a scientifically validated target in ccRCC. CAIX is a cell surface protein that is highly expressed in ccRCC, and in many other solid tumors in the hypoxic tumor microenvironment. Telix believes the correlation between hypoxia and disease progression, along with therapeutic resistance, underscores the potential of this target.
| ● | Telix’s CAIX-targeting therapeutic candidate is TLX250 (177Lu-DOTA-girentuximab), a rADC therapy that Telix is developing for the treatment of advanced metastatic kidney cancer. Early clinical trials of TLX250 in patients with advanced ccRCC have demonstrated evidence of promising a safety profile and efficacy outcomes. Animal models also indicate that the combination of TLX250 with checkpoint inhibitor immunotherapies can improve therapeutic response. | |
| ● | Telix’s imaging candidate TLX250-CDx (89Zr-DFO-girentuximab, Zircaix™4) is a PET diagnostic imaging agent for the characterization of renal masses as ccRCC. Telix achieved positive results in the recently completed Phase III ZIRCON trial for TLX250-CDx. In December 2023, Telix submitted a Biologics License Application (BLA) for TLX250-CDx to the FDA for imaging of ccRCC. The BLA was granted a rolling review process. Subject to this regulatory approval, Telix aims to commercialize TLX250-CDx in the second half of 2024. If approved, TLX250-CDx would be the first targeted radiopharmaceutical imaging agent for kidney cancer to be approved in the United States. |
Glioma
Telix’s brain cancer program targets two membrane transport proteins known as large amino acid transporter 1, and large amino acid transporter 2 (LAT1 and LAT2), validated targets that are highly expressed in several solid tumors, including malignancies of the central nervous system (CNS). Telix believes that the LAT1 and LAT2 receptors, which are expressed on both sides of the blood-brain barrier (BBB), are suitable targets for the delivery of radiation to both primary CNS malignancies and metastases from non-CNS cancers such as lung and breast cancer.
| ● | Telix’s therapeutic product candidate, TLX101 (131I-IPA), is a LAT-1 targeting investigational therapy for patients with brain cancer. TLX101 has received orphan drug designation (ODD) in the United States and Europe for the treatment of glioma. Telix is currently evaluating TLX101 in the front-line (Phase I) and recurrent (Phase II) disease settings, where Telix has observed preliminary clinical evidence of anti-tumor effect and disease stabilization. | |
| ● | Telix’s imaging candidate, TLX101-CDx (Pixclara™1), also known as 18F-floretyrosine or 18F-FET, is a PET diagnostic agent designed to image cancerous lesions in the brain. TLX101-CDx has received ODD in the United States for the imaging of glioma and Telix is currently preparing for a New Drug Application (NDA) for TLX101-CDx. |
| 85 |
Soft Tissue Sarcoma
Telix’s product candidates, TLX300 (-olaratumab) and TLX300-CDx (89Zr-DFOsq-olaratumab, including Telix’s proprietary DFO-squaramide chelator) employ antibody-directed targeted radiation for both therapeutic and diagnostic applications, respectively, against platelet-derived growth factor receptor alpha (PDGFRα), which is a tyrosine kinase receptor involved in fibrogenesis. Telix believes that the targeting of activated fibroblasts in the tumor micro-environment is a promising strategy to drive durable treatment responses in certain solid tumors. TLX300 has completed pre-clinical validation. Telix is developing TLX300 and TLX300-CDx as a theranostic pair targeting soft tissue sarcoma.
Bone Marrow Conditioning
Telix’s efforts in bone marrow conditioning (BMC) are designed to explore the potential utility of targeted radiation to ablate bone marrow as part of a pre-conditioning regiment for bone marrow transplantation, novel stem cell therapies and gene therapies, each of which requires conditioning prior to treatment. The current standard of care requires BMC with multi-drug chemotherapy regimens, which are highly toxic and patients may not tolerate treatment. Telix believes that a safe, durable and short internment treatment could be transformative to many facets of cancer and autoimmune disease treatments that require BMC.
TLX66 (90Y-DOTA-besilesomab) is Telix’s investigational therapy for BMC for haematopoietic stem cell transplantation (HSCT) conditioning – a broad clinical indication with potential applicability to many different diseases. TLX66 has been evaluated as a therapeutic bone marrow conditioning agent in approximately 100 patients with promising results. TLX66 has been granted ODD in the United States and Europe for BMC. TLX66-CDx (99mTc-besilesomab, Scintimun®5) is Telix’s imaging agent for osteomyelitis (bone infection). TLX66-CDx has already been commercialized outside of the United States and is sold under license by Curium Pharma as an approved product for imaging osteomyelitis in approximately 30 countries.
Principal Capital Expenditures
Research and Development Costs
Research and development, or R&D, costs relate primarily to the development of new products to add to Telix’s portfolio and costs related to medical affairs, medical information and quality and regulatory functions. Telix’s direct R&D costs consist of costs of materials, a proportion of overhead, direct labor and external service costs, such as fees paid to CROs, CMOs, research laboratories and outside consultants in connection with Telix’s process development, manufacturing and clinical development activities.
R&D costs were A$128.8 million for the year ended December 31, 2023, compared to A$81.0 million for the year ended December 31, 2022.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and other related costs for personnel in field sales, marketing and customer service functions. Other costs in selling and marketing expenses include bad debt expense, the development and printing of advertising and promotional material, professional services, market research and sales meetings.
Selling and marketing expenses were A$54.9 million for the year ended December 31, 2023, compared to A$38 million for the year ended December 31, 2022.
| 86 |
General and Administration Costs
General and administration costs consist of salaries, employee benefit expenses (including share-based payment expenses) and other related costs for personnel in executive, finance, legal, information technology, human resources and other corporate functions. Other costs included in general and administration costs are professional fees for information technology services, external legal fees, consulting and accounting services, as well as certain facility and insurance costs, including director and officer liability insurance.
General and administration costs were A$79.0 million for the year ended December 31, 2023, compared to A$49.1 million for the year ended December 31, 2022.
Principal Capital Expenditures Currently in Progress
| ● | Research and Development Costs: |
| ○ | R&D costs are expected to comprise costs of a similar nature to that recorded to date. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. | |
| ○ | Telix expects that R&D costs will increase in connection with its planned clinical development, manufacturing and regulatory approval activities in the near term and in the future, including as Telix executes its ProstACT GLOBAL clinical trial of TLX591 for the treatment of prostate cancer. Telix also anticipates that it will incur increased labor expenses allocable to R&D costs as it increases headcount to support these manufacturing and clinical development activities. Telix expects additional investment in R&D to be funded by operating cash flow. |
| ● | Selling and Marketing Expenses: Telix expects selling and marketing expenses to continue to decrease as a percentage of revenue as a result of improvements in operating expenditure control and revenue growth exceeding cost base growth. | |
| ● | General and Administration Costs: Telix anticipates that administration expenses will increase in the future as Telix increases headcount to support commercial operations and research and development activities. |
Telix’s Reportable Segments
Telix’s two reportable segments are Commercial Operations and Product Development, which are categorized based on principal activities.
The Commercial Operations segment focuses on the commercial sales of Illuccix® and other products that may obtain regulatory approvals. This segment includes royalties and sales of goods (which account for the majority of Telix’s revenue from operations), as well as the sales and marketing expenses and costs of sales necessary to support those revenues.
For the fiscal year ended December 31, 2023, revenue from contracts with customers for Telix’s commercial operations segment consisted of A$496.2 million (2022: A$156.0 million) in sales of goods, A$0.4 million (2022: A$0.4 million) in royalty revenue and A$0.4 million (2022: A$Nil) in services revenue. U.S. sales of Illuccix® were the main driver of the 218% increase in revenue from contracts with customers for the commercial operations segment compared to 2022.
The Product Development segment focuses on the development of radiopharmaceutical product candidates for commercialization. This segment includes revenue received from license agreements prior to commercialization and research and development services. For the fiscal year ended December 31, 2023, revenue from contracts with customers for our product development segment consisted of A$0.1 million (2022: A$0.4 million) in intellectual property license revenue and A$5.4 million (2022: A$3.4 million) in R&D services revenue. R&D investment during 2023 focused on preparation for commercial launch of Zircaix and Pixclara, including commercial manufacturing process qualification and validation, preparation of FDA filings, commercial launch plans and early access programs. R&D was also directed towards clinical manufacturing to progress the ProstACT GLOBAL trial. The portion of R&D costs that was attributable to employment expenses increased from A$19.2 million in the fiscal year ended December 31, 2022 to A$32.1 million in the fiscal year ended December 31, 2023, reflecting increased activity in Telix’s late-stage assets.
| 87 |
For more information on Telix’s segment reporting, see Note 3 to Telix’s consolidated financial statements appearing elsewhere in this Information Statement.
Telix has operations in the Americas, Asia Pacific, Europe, the Middle East, and Africa. Below is a table including a breakdown of total revenues by geographic market for Telix’s last two financial years:
| 2023 | 2022 | |||||||
Revenue by location of customer | Revenue by location of customer | |||||||
| A$’000 | A$’000 | |||||||
| Australia | 1,166 | 149 | ||||||
| Belgium | 458 | 564 | ||||||
| China | 5,291 | 3,353 | ||||||
| Other countries | 4,669 | 3,979 | ||||||
| United Kingdom | 1,306 | 2,045 | ||||||
| United States | 489,657 | 150,006 | ||||||
| Total | 502,547 | 160,096 |
Global Manufacturing and Supply Chain
Telix is focused on enhancing its existing global manufacturing and supply chain with a balance of external and in-house capabilities, securing a robust and innovative manufacturing infrastructure and supply chain to serve its patients. Manufacturing and supply chain supporting its portfolio broadly cover the following areas: radioisotopes, radiochemistry, biologics, small molecules, fill/finish, packaging and labeling, and storage and distribution.
During 2022 and 2023, Telix made significant progress with the buildout of its radioisotope manufacturing facility in Brussels South. Telix has been granted an updated radiation license by the Belgian Federal Agency for Nuclear Control, enabling site activation subject to the regulatory inspections and approvals.
As a radiopharmaceutical company, Illuccix® and its product candidates are prepared for patient administration using radioisotopes. Gallium-68, or 68Ga, is a necessary component isotope for radiopharmacies to radiolabel Illuccix® for patient administration and is sourced by a radiopharmacy directly. Other important isotopes applicable to its current pipeline of diagnostic and therapeutic product candidates include zirconium-89 or 89Zr, lutetium-177 or 177Lu, yttrium-90, or 90Y, fluorine-18 or 18F, iodine-131 or 131I, and technetium-99m or 99mTc. Telix procures the supply of these isotopes from suppliers based predominately in Canada or Europe. Global isotope supply chains, including obtaining precursor or raw materials necessary to produce many of the synthetic radioisotopes used in nuclear medicine, are commonly sourced from countries such as Russia, Brazil, South Africa and Turkey that may, from time-to-time, be subject to instability, unrest, protests, intergovernmental conflicts and various international trade or monetary sanctions.
| 88 |
Telix has multiple supply agreements with available isotope suppliers and adequate stockpiles to ensure adequate quantities to meet its current pipeline development needs. However, there is a limited supply of some radioisotopes due to the limited supply of starting radioactive raw materials to create the radioisotope or the complexity required to manufacture isotopes to the required quality and purity standards for effective radiolabeling. Telix has supply relationships with all major current suppliers and there are either no or limited alternatives to its current suppliers, depending on the isotope. Telix’s ability to conduct clinical trials to advance its product candidates is dependent on its ability to either self-generate and/or obtain these radioisotopes and other isotopes it may choose to utilize in the future.
Telix is currently dependent on third-party manufacturers and suppliers for many of its isotopes, and its suppliers will be dependent on third parties to supply the raw radioactive materials. Telix currently has long-term supply agreements with its third-party contract manufacturers to manufacture the clinical and commercial supplies of Illuccix® and for its product candidates. Telix currently relies on a single source supplier for its active pharmaceutical ingredient for Illuccix® and its related product manufacturing requirements, although additional sources and back-up suppliers are being validated and implemented. Any changes or interruptions in the current arrangements Telix has to procure its materials for development may affect the price of acquiring these materials.
Telix’s main business is not affected by seasonality. However, any general performance failure on the part of Telix’s existing or future manufacturers could delay clinical development, regulatory approval or commercialization of its product candidates. If Telix’s suppliers or contract manufacturers are so affected, the supply chain could be disrupted, its product shipments could be delayed, its costs could be increased and its business could be adversely affected. If Telix’s current contract manufacturers cannot perform as agreed, Telix may be required to replace those manufacturers. Although Telix believes that there are several potential alternative manufacturers who could manufacture Illuccix® or its product candidates, Telix could incur added costs and delays in identifying and qualifying any such replacement. Consequently, Telix may not be able to reach agreement with third-party manufacturers on satisfactory terms, which could negatively impact revenues from sales of Illuccix® or delay commercialization of any product candidates that are subsequently approved.
Sales and Marketing Operations
Telix’s commercial operations span the Americas, EMEA, and Asia Pacific regions. Illuccix® is approved in the United States, Canada and Australia, and permitted to be sold in New Zealand, and Telix is commercializing this product in these countries through local sales forces, which currently include over 40 associates, and together with distributor partners. Telix has secured a number of commercial partnerships covering certain geographies to enable distribution and/or commercialization of its products.
In the United States, Telix has established a commercial radiopharmacy network of over 220 commercial radiopharmacies to distribute Illuccix®, including partnerships with Cardinal Health, Inc., PharmaLogic Holdings, Corp., and Jubilant Radiopharma. Telix also has a distribution agreement with Isologic Innovative Radiopharmaceuticals Ltd for the Canadian market.
In Asia Pacific, Telix has secured a strategic collaboration with Grand Pharmaceutical Group Limited, or Grand Pharma, in the Greater China area including Mainland China, Taiwan, Hong Kong and Macau. Grand Pharma has been appointed as Telix’s partner for this territory with exclusive development and commercialization rights to Telix’s portfolio. Telix has also secured exclusive distribution agreements in Australia with Global Medical Solutions Australia Pty Ltd and with DuChembio Co., Ltd. in South Korea.
| 89 |
In Europe, Telix has exclusive distribution agreements for the upcoming launch of Illuccix® in a number of geographies, including with Eckert & Ziegler RadioPharma GmbH in Germany, Xiel Ltd in the United Kingdom and Ireland, IRE Elit S.A. in France, Radius S.r.l. in Italy, Nucliber S.A. in Spain, Biokosmos S.A. in Greece and Cyprus, Sociedade Avanço, Unipessoal, LDA in Portugal, THP Medical Products Vertriebs GmbH in Austrian, Czech Republic and Slovak Republic and WIIK Pharma ApS in Denmark, Finland, Norway and Sweden.
Intellectual Property
Like many other companies in the biopharmaceuticals industry, Telix is the licensee of certain core intellectual property assets and is, as a result, dependent on those licenses (and any underlying licenses on which they rely). All of Telix’s licenses carry a risk that the third parties do not adequately or fully comply with its or their respective contractual rights and obligations and that these contractual relationships may be terminated. This could adversely affect Telix’s rights to intellectual property, IP, which could materially adversely affect its operations, financial position and prospects. Additionally, Telix’s business would suffer if the licensors fail to enforce licensed patents against infringing third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if Telix is unable to enter into necessary licenses on acceptable terms. Telix may need to acquire or license IP from third parties to develop and commercialize its own pipeline of IP and products. There is no guarantee such acquisitions or licenses can be obtained or, if obtained, that they will be on reasonable commercial terms.
Telix’s success depends in large part on its ability to obtain and maintain patent protection in the United States and other countries with respect to its proprietary products and product candidates and other discoveries. Telix seeks to protect its proprietary position by filing patent applications in the United States and abroad related to its novel products and product candidates and other discoveries that are important to its business. If Telix is unable to obtain and maintain patent protection for its products or product candidates and other discoveries, or if the scope of the patent protection obtained is not sufficiently broad, Telix’s competitors could develop and commercialize products and other discoveries similar or identical to it, and Telix’s ability to successfully commercialize its products or product candidates and other discoveries may be adversely affected.
Government Regulations
Telix operates under a broad range of legal, regulatory, tax and political systems. Telix’s long-term success and ability to sustain and grow revenue depends on its ability to continue to successfully develop product candidates and obtain regulatory approval to market products both in and outside of the United States. In order to market and sell products in the European Union and many other jurisdictions, Telix must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The U.S. Food and Drug Administration and comparable foreign regulatory authorities, including the European Medicines Agency (EMA) and the Australian Therapeutic Goods Administration (TGA), whose laws and regulations may differ from country to country, impose substantial requirements on the development of product candidates to become eligible for marketing approval, have substantial discretion in the process, and may refuse to accept any application or may decide that the data are insufficient for approval and require additional preclinical studies, clinical trials or other studies and testing.
The approval of Telix’s product candidates for commercial sale could also be delayed, limited or denied or Telix may be required to conduct additional studies for a number of reasons, including, but not limited to, the following:
| ● | regulatory authorities may determine that the product candidates do not demonstrate safety and effectiveness in accordance with regulatory agency standards based on a number of considerations, including adverse events that are reported during clinical trials; | |
| ● | regulatory authorities could analyze and/or interpret data from clinical trials and preclinical testing in different ways than Telix interprets them and determine that Telix’s data is insufficient for approval; |
| 90 |
| ● | regulatory authorities may require more information, including additional preclinical or clinical data or the conduct of new trials, to support approval; | |
| ● | regulatory authorities could determine that Telix’s manufacturing processes are not properly designed, are not conducted in accordance with federal or other laws or otherwise not properly managed, and Telix may be unable to obtain regulatory approval for a commercially viable manufacturing process for its product candidates in a timely manner, or at all; | |
| ● | the size of the patient population required to establish the efficacy of Telix’s product candidates to the satisfaction of regulatory agencies may be larger than Telix or they anticipated; | |
| ● | Telix’s failure or the failure of clinical sites, and the records kept at the respective locations, including records containing clinical trial data, to be in compliance with the FDA’s GCP, requirements or comparable regulations outside of the United States; | |
| ● | regulatory authorities may change their approval policies or adopt new regulations; | |
| ● | regulatory authorities may not be able to undertake reviews of Telix’s marketing applications, conduct applicable inspections or proceed through their approval processes in a timely manner; | |
| ● | regulatory authorities may not agree with Telix’s regulatory approval strategies or components of Telix’s or their regulatory filings, such as the design or implementation of the relevant clinical trials; or | |
| ● | a product may not be approved for the indications that Telix requests or may be limited or subject to restrictions or post-approval commitments that render the approved drug not commercially viable. |
Accordingly, the profitability of Telix’s operations and continued viability may be adversely impacted by the variations in regional specific regulatory regimes, changes in regulatory or fiscal regimes, difficulties in interpreting or complying with local laws and reversal of current political, judicial or administrative policies, including as a result of geopolitical tensions.
Telix’s Subsidiaries
Telix’s subsidiaries as of December 31, 2023, are set out below. Unless otherwise stated, they have share capital consisting solely of ordinary shares that are held directly by Telix. The country of incorporation or registration is also the principal place of business.
| Name of entity | Place of business/country of incorporation | Ownership
interest held by Telix (%) |
Principal activities | |||
| Telix Pharmaceuticals (EST) Pty Ltd | Australia | 100 | Dormant | |||
| Telix Pharmaceuticals (Innovations) Pty Limited (formerly Telix International Pty Ltd)1 | Australia | 100 | Manufacturing and development | |||
| Telix Pharmaceuticals Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals International Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals Australia Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals (ANZ) Pty Ltd1 | Australia | 100 | Commercial operations | |||
| Telix Pharmaceuticals (Corporate) Pty Limited1 | Australia | 100 | Commercial operations | |||
| Telix Pharmaceuticals (Belgium) SRL | Belgium | 100 | Manufacturing and development | |||
| Telix Innovations SA | Belgium | 100 | Commercial operations |
| 91 |
| Telix Pharmaceuticals (Canada) Inc. | Canada | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (France) SAS | France | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Germany) GmbH (formerly Telix Pharmaceuticals Holdings (Germany) GmbH) | Germany | 100 | Clinical R&D | |||
| Rhine Pharma GmbH (formerly Telix Pharmaceuticals (Germany) GmbH) | Germany | 100 | Clinical R&D | |||
| Therapeia GmbH & Co. KG | Germany | 100 | Clinical R&D | |||
| Dedicaid GmbH | Austria | 100 | Software | |||
| Telix Pharma Japan KK | Japan | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (NZ) Limited | New Zealand | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Singapore) Pte Ltd | Singapore | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Switzerland) GmbH | Switzerland | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (UK) Ltd (formerly Telix Life Sciences (UK) Ltd) | United Kingdom | 100 | Clinical R&D | |||
| Lightpoint Surgical Ltd | United Kingdom | 100 | Medical devices | |||
| Lightpoint Medical Espana SLU | Spain | 100 | Medical devices | |||
| Telix Pharmaceuticals (US) Inc. | USA | 100 | Commercial operations | |||
| Telix Optimal Tracers, LLC | USA | 100 | Manufacturing and development |
| 1. | Denotes an entity that is a party to a deed of cross guarantee. |
TheraPharm Deutschland GmbH was wound up during the financial year.
Description of Telix’s Properties
Telix is headquartered in Melbourne, Australia, with regional operations in Sydney and Brisbane, Australia. Telix has international operations in Belgium, Japan, Switzerland, and the U.S.
Telix purchased a radiopharmaceutical production facility in Belgium on April 27, 2020. The site had cyclotrons installed in concrete shielded vaults which also contained some nuclear contamination associated with past manufacturing activities. As part of this transaction, Telix assumed the obligation to remove the cyclotrons and restore the site. Telix removed the cyclotrons from the site during 2022. Other decommissioning activities not required to upgrade the production facility have been deferred to the end of the operating life of the facility in 2041. The facility received an updated operation authorization and environmental permit for the facility from the local Belgian authorities, valid up to October 7, 2042. Telix opened the site in June 2023, which consists of approximately 30,000 square feet. It is one of the largest radiopharmaceutical production facilities in Europe, with nine good manufacturing practice (GMP) lines, two research and development laboratories, quality control labs and warehousing space with capacity to support Telix’s operations and provisions for the installation of two cyclotrons. Telix anticipates that one of the first GMP lines will be dedicated for the use of industry and research partners and collaborators. Telix expects this facility to deliver significant flexibility and reliable supply for Telix’s growing commercial production requirements. It also serves as a vital hub for research and development, specifically in manufacturing scale-up and production of next generation radiopharmaceuticals, including both alpha-emitters and beta-emitters.
| 92 |
Environmental Considerations
Telix’s use of facilities that use and produce radioactive materials subjects Telix to compliance with decommissioning and decontamination (D&D) requirements when Telix closes those facilities, exposing Telix to potentially significant costs. Telix’s product candidates are manufactured using radioactive components. When a cyclotron reaches the end of its useful life at one of Telix’s facilities or if Telix needs to abandon such facility for any other reason, Telix is obligated under the laws and regulatory rules of the various jurisdictions in which it operates to decommission and decontaminate such facility or cyclotron. Estimating the amount and timing of such future D&D costs includes, among other factors, country-specific requirements and projections as to when a facility will retire or the useful life of a cyclotron. If Telix does not conduct D&D properly at any of the sites, it may suffer significant additional costs to remediate any D&D deficiencies, fines, regulatory or criminal charges or other sanction or legal action, any of which could have a material adverse effect upon Telix’s business, financial condition and results of operations. Although Telix has estimated its future D&D costs and recorded a liability for such costs, there can be no assurances that it will not incur material D&D costs beyond such estimates or Telix’s provisions.
Legal Proceedings
Telix is not currently a party to any material legal proceedings or investigations worldwide. From time to time, Telix may become involved in other litigation or legal proceedings particularly relevant to defending its IP rights or in response to any relating to claims arising from the ordinary course of business.
Exchange Controls and Other Limitations Affecting Security Holders
Australia has largely abolished exchange controls on investment transactions. The Australian dollar is freely convertible into U.S. dollars or other currencies. In addition, there are currently no specific rules or limitations regarding the export from Australia of profits, dividends, capital or similar funds belonging to foreign investors, except that certain payments to non-residents must be reported to the Australian Transaction Reports and Analysis Centre, or AUSTRAC, which monitors such transactions, and amounts on account of potential Australian tax liabilities may be required to be withheld unless a relevant taxation treaty can be shown to apply and under such there are either exemptions or limitations on the level of tax to be withheld.
Taxation
See “Material U.S. Federal Income Tax Consequences” beginning on page 119 for a discussion of the material U.S. federal income tax consequences of (i) the Merger to U.S. Holders whose shares of Common Stock are exchanged for Telix Ordinary Shares and CVRs pursuant to the Merger, and (ii) the post-Merger ownership and disposition of Telix Ordinary Shares acquired pursuant to the Merger to U.S. Holders.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following “Management’s Discussion and Analysis of Financial Condition and Results of Operations” should be read together with Telix’s consolidated financial statements and the accompanying notes and other financial information included elsewhere in this Information Statement. This discussion includes both historical information and forward-looking information based upon current expectations that involve risk, uncertainties and assumptions. Telix’s actual results may differ materially from Telix management’s expectations as a result of various factors, including, but not limited to, those discussed in “Risk Factors” and elsewhere in this Information Statement.
Key Factors Affecting Results of Operations
Telix’s operating and financial performance have been, and will continue to be, affected by a number of important factors, including the following:
Strategic Acquisitions
Telix has expanded its pipeline of product candidates through strategic acquisitions. Supporting Telix’s growth strategy through acquisitions continues to be key to strengthening its global supply chain, enhancing the ability to serve patients in all global markets, developing production expertise through in-house manufacturing and leveraging capabilities to identify and develop novel targets, clinical applications and manufacturing technologies for Telix’s future pipeline. Telix has pursued and plans to continue pursuing strategic acquisitions and partnerships to further advance and expand its pipeline, scale production and leverage the expertise and effort of its team.
| 93 |
Successful Commercialization of Telix’s Product Portfolio
Telix’s financial performance is dependent on its ability to manage and develop its business model and global presence to support the commercialization of existing and future products. Commercial sales of Illuccix® have had a significant impact on revenue in the prior and current periods, and the successful continued commercialization of Illuccix® continues to determine Telix’s ability to generate product revenue. Successful commercialization includes the receipt of regulatory approvals, successful product launches, the ability to supply and sell products to customers and the ability to obtain adequate reimbursement coding coverage and payments for products. Success in each of these areas is essential to Telix’s ability to realize and retain value from its product portfolio. The ongoing commercial success of Illuccix® and any other products for which Telix obtains regulatory approval will also depend in part on the impact of new and existing competitive products in the market and Telix’s ability to continue to drive market growth.
Development and Funding of Product Pipeline
Telix has developed a strong research and innovation team and strategy to continuously identify and progress early development on a broad pipeline of pre-clinical and clinical assets. While increased product development activity in a given period results in increases in operating expenses, Telix’s long-term sustainable viability is also determined by its ability to continue successfully identifying, developing and funding a pipeline of products capable of commercialization. Telix’s growth in revenue from the commercialization of assets will affect the amount of funding available for the development of its core pipeline. Telix’s ability to be successful in this area in the context of a dynamic and changing competitive landscape will also be dependent on the protection of its intellectual property position.
Supply Chain Resilience
Nuclear medicine products and technologies have inherently complex manufacturing, supply and logistics chains. Telix is dependent on third parties for the manufacture and supply of a substantial portion of its commercialized products and its products in development. Telix has dual supply surety where possible and continues to seek viable and sustainable opportunities for supply chain integration, including the acquisition and development of in-house manufacturing capability at Telix’s Brussels South, Belgium facility. The impact of expenses or losses attributable to supply chain disruptions or key product component unavailability will depend on the efficacy of Telix’s integration efforts, supplier diligence, vendor management and vendor audit programs in mitigating these risks.
Components of Telix’s Results of Operations
Revenue from Contracts with Customers
Revenue from Telix’s commercial operations consists of sales of Illuccix® and sales-based royalties in connection with the out-licensing of TLX66-CDx outside the United States. Telix expects revenue from these out-licensing arrangements to be nominal in future periods as intellectual property out-licensing is not a core strategy of its business.
Sales are recognized at point-in-time when control of the products has transferred, being when the products are administered to the patient. Revenue from these sales is recognized based on the price specified in the contract, net of the estimated volume discounts, which are estimated and provided for using the expected value method, and revenue is only recognized to the extent it is highly probable that a significant reversal will not occur.
Revenue from Telix’s product development operations consists of out-licenses of intellectual property and research and development services. The transaction price is allocated to the research and development activities based on a cost-plus margin approach. Revenue from research and development services is recognized over time based on the costs incurred to date as a percentage of total forecast costs.
| 94 |
When licenses of intellectual property are distinct from other goods or services promised in the contract, a portion of the transaction price is allocated to the license. The timing of revenue recognition of the transaction price allocated to the license performance obligation is based on the nature of the license. Where Telix performs activities that significantly affect the intellectual property to which the customer has rights, the rights granted by the license directly expose the customer to any positive or negative effects of Telix’s activities, and those activities do not result in the transfer of a good or service to the customer as those activities occur, the nature of the license is a “right to access” license. The transaction price allocable to a right to access license is recognized as revenue over time as activities are performed. Where the license arrangement does not meet the criteria for a right to access license, the license is a “right to use” license and the transaction price allocated to the license is recognized in full upon transfer of control of the license to the customer.
Estimates for rebates and allowances represent Telix’s estimated obligations under contractual arrangements with third parties. Rebate accruals and allowances are recorded in the same period the related revenue is recognized, resulting in a reduction to revenue and the establishment of a liability which is included in accrued expenses. These rebates and allowances result from performance-based offers that are primarily based on attaining contractually specified sales volumes, Medicaid rebate programs for Telix’s products and certain distributor related commissions. Revenue recognized upon administration of Telix’s products to patients is limited to the price specified under Medicaid, Medicare or other government rebate programs where provided under such program. The calculation of the accrual for these rebates and allowances is based on an estimate of the third party’s expected purchases and the resulting applicable contractual rebate to be earned over a contractual period.
Cost of Sales
Cost of sales primarily comprises manufacturing costs of Illuccix® (including direct materials and direct labor), freight, storage and shipping from contract manufacturers to warehouses and radio-pharmacies, fixed and variable overheads and dispensing and administration fees paid to distributors. Overhead expenditure is allocated based on normal operating capacity. Costs are assigned to individual items of inventory using the weighted average cost method. Costs of purchased inventory are determined after deducting rebates and discounts. Other costs in cost of sales expenses include amortization of intangible assets related to commercial products and sales-based royalties paid to licensors.
Research and Development Costs
Research and development, or R&D, costs relate primarily to the development of new products to add to Telix’s portfolio and costs related to its medical affairs, medical information and quality and regulatory functions. Telix’s direct R&D costs consist of costs of materials, a proportion of overhead, direct labor and external service costs, such as fees paid to CROs, CMOs, research laboratories and outside consultants in connection with process development, manufacturing and clinical development activities. Telix has not provided program costs since inception because historically it has not tracked or recorded R&D costs on a program-by-program basis. R&D costs also include:
| ● | expenses incurred in connection with the clinical development of product candidates, including under agreements with third parties, such as consultants and contract research organizations, or CROs; | |
| ● | the cost of manufacturing and purchasing drug products for use in clinical trials, including under agreements with third parties, such as consultants and contract manufacturing organizations, or CMOs; | |
| ● | other research and development related activities, which include pre-clinical expenses and research expenditure on novel targets and technologies; | |
| ● | costs related to compliance with regulatory requirements and patent expenses; | |
| ● | intellectual property costs, such as milestone payments and fees to licensors; and |
| 95 |
| ● | consulting, pre-launch commercialization activities and travel and conferences related to new products in development. |
Telix expenses R&D costs as incurred and have not capitalized any amounts of R&D costs as of December 31, 2023. For the year ended December 31, 2023, Telix made A$11.3 million in advance payments for goods or services to be received in future periods for use in R&D activities. These payments have been recorded as prepayments within current assets in Telix’s consolidated statement of financial position as of December 31, 2023.
Telix does not allocate employee costs associated with research efforts to specific programs. Telix uses internal resources primarily to conduct research activities as well as for managing process development, manufacturing and clinical development activities. These employees work across multiple development programs and, therefore, Telix does not track these costs by program.
R&D costs in fiscal years after December 31, 2023 are expected to comprise costs of a similar nature to that recorded to date. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. Telix expects that its R&D costs will increase in connection with planned clinical development, manufacturing and regulatory approval activities in the near term and in the future, including as Telix executes its ProstACT GLOBAL clinical trial of TLX591 for the treatment of prostate cancer. Telix also anticipates that it will incur increased labor expenses allocable to R&D costs as it increases headcount to support these manufacturing and clinical development activities.
At this time, Telix cannot accurately estimate or know the nature, timing and costs of the efforts that will be necessary to complete the clinical development of its pipeline of product candidates and any future product candidates. Telix may never succeed in achieving regulatory approval for product candidates in its pipeline. The duration, costs and timing of clinical trials and development of Telix’s product candidates will depend on a variety of factors, including:
| ● | the scope, rate of progress and expense of planned clinical trials as well as other R&D activities; | |
| ● | clinical trial results; | |
| ● | the terms and timing of regulatory approvals; | |
| ● | the expense of filing, maintaining, prosecuting, defending and enforcing patent claims and other intellectual property rights; | |
| ● | the ability to raise necessary additional funds, whether through commercial operations or investment; | |
| ● | the ability to market, commercialize and achieve market acceptance for any products that receive regulatory approval; | |
| ● | a continued acceptable safety profile following approval in any indication; and | |
| ● | establishing and maintaining agreements with third-party suppliers and manufacturers for clinical supply and commercial manufacturing for any product candidate, if approved. |
A change in the outcome of any of these factors could significantly change the duration, costs and timing associated with clinical trials and development of Telix’s core product pipeline and research pipeline.
R&D costs also comprise patent expenses related to the cost of outside patent attorneys to manage and prosecute claims for Telix’s patent portfolio, and intellectual property costs to the license and patent assignment costs in respect of its in-license agreements for certain technologies.
Selling and Marketing Expenses
Selling and marketing expenses consist primarily of salaries and other related costs for personnel in field sales, marketing and customer service functions. Other costs in selling and marketing expenses include bad debt expense, the development and printing of advertising and promotional material, professional services, market research and sales meetings.
| 96 |
General and Administration Costs
General and administration costs consist of salaries, employee benefit expenses (including share-based payment expenses) and other related costs for personnel in executive, finance, legal, information technology, human resource and other corporate functions. Other costs included in general and administration costs are professional fees for information technology services, external legal fees, consulting and accounting services as well as certain facility and insurance costs, including director and officer liability insurance.
Telix anticipates that administration expenses will increase in the future as Telix increases headcount to support commercial operations and research and development activities.
Other Losses (Net)
Other losses primarily consist of the remeasurement of contingent consideration liabilities, reflecting the impact of changes in the underlying assumptions and inputs used in the valuation.
Telix acquired Advanced Nuclear Medicine Ingredients SA, or ANMI, in December 2018. Telix is liable for future variable payments which are calculated based on the percentage of net sales of Illuccix® through April 13, 2027, which is five years following the first commercial sale of the product. The applicable percentage of net sales is equal to a percentage in the low teens for sales achieved in the United States and equal to a percentage in the low twenties for sales in the rest of the world. Telix also holds an option to buy out the remaining deferred payments by paying €10 million within 90 days of April 13, 2025. When presenting financial statement information, Telix estimates the fair value of the contingent consideration liability as of the end of the period presented based on a discounted cash flow model based on the risk-adjusted post-tax discount rate, expected sales volumes, net sales price per unit and the exercise of the buy-out option. If it is determined that a remeasurement is needed to adjust the carrying value of the contingent consideration to its fair value, the amount of the remeasurement is recognized in other losses. The carrying value of this contingent consideration as of December 31, 2023 was A$90.5 million.
Other losses also comprise foreign exchange gains and losses, which represent the impact of the variance in exchange rates between the Australian dollar and the U.S. dollar, Euro, British Pound and Canadian dollar on Telix’s cash and cash equivalents, financial assets, financial liabilities and foreign currency denominated transactions.
Finance Income
Finance income comprises interest on cash and cash equivalents.
Finance Costs
Finance costs comprise the unwind of discounts applied to the measurement of contingent consideration, contract liabilities, government grant liabilities and decommissioning liabilities. The discount rate applied to present value liabilities is specific to the liability, with reference to Telix’s weighted average cost of debt or, where appropriate, the risk-free rate of debt.
Other finance costs include interest expense on lease liabilities and bank fees on cash and cash equivalents held with financial institutions.
Income Tax Benefit/(Expense)
Telix operates across multiple tax jurisdictions with varying degrees of activities. As a result, Telix reports a blended effective tax rate reflecting these multiple tax jurisdictions.
| 97 |
Telix expects that it will continue to reflect a blended tax expense or credit from the relevant tax jurisdictions, considering Telix’s tax risk profile and activities in the differing tax jurisdictions.
Telix is eligible under the Australian government’s R&D Tax Incentive Scheme to obtain a cash amount or an R&D tax incentive credit from the Australian Taxation Office. The tax incentive is available to Telix based on specific criteria with which it must comply. In the event that global revenue exceeds A$20 million in a fiscal year, the cash receipt option is not available and Telix is only eligible to receive a non-refundable tax credit, which can be carried forward. The tax incentives may only be offset against Australian taxable income. As such, they are recognized as a component of income tax expense.
Results of Operations for the Fiscal Years Ended December 31, 2022 and 2023
The following table sets forth a summary of Telix’s consolidated statement of profit or loss and other comprehensive income for the periods presented.
| Year ended December 31, | 2023 vs. 2022 | |||||||||||||||
2023 A$ | 2022 A$ | Change A$ | Change % | |||||||||||||
(in thousands, except per share data) | ||||||||||||||||
| Revenue from contracts with customers | 502,547 | 160,096 | 342,451 | 214 | % | |||||||||||
| Cost of sales | (188,157 | ) | (65,170 | ) | 122,987 | 189 | % | |||||||||
| Gross profit | 314,390 | 94,926 | 219,464 | 231 | % | |||||||||||
| Research and development costs | (128,844 | ) | (81,008 | ) | 47,836 | 59 | % | |||||||||
| Selling and marketing expenses | (54,867 | ) | (37,970 | ) | 16,897 | 45 | % | |||||||||
| General and administration costs | (78,985 | ) | (49,128 | ) | 29,857 | 61 | % | |||||||||
| Other losses (net) | (35,854 | ) | (18,750 | ) | 17,104 | 91 | % | |||||||||
| Operating profit/(loss) | 15,840 | (91,930 | ) | 107,770 | 117 | % | ||||||||||
| Finance income | 1,019 | 1 | 1,018 | * | ||||||||||||
| Finance costs | (13,772 | ) | (6,693 | ) | 7,079 | 106 | % | |||||||||
| Profit/(loss) before income tax | 3,087 | (98,622 | ) | 101,709 | 103 | % | ||||||||||
| Income tax benefit/(expense) | 2,124 | (5,457 | ) | 7,581 | 139 | % | ||||||||||
| Profit/(loss) for the year | 5,211 | (104,079 | ) | 109,290 | 105 | % | ||||||||||
| Other comprehensive income/(loss): | ||||||||||||||||
| Items that will not be reclassified to profit or loss in subsequent periods: | ||||||||||||||||
| Changes in fair value of equity investments at fair value through comprehensive income | (895 | ) | — | (895 | ) | — | ||||||||||
| Items to be reclassified to profit or loss in subsequent periods: | ||||||||||||||||
| Exchange differences on translation of foreign operations | (4,852 | ) | 591 | (5,443 | ) | (921 | %) | |||||||||
| Total comprehensive income/(loss) for the year | (536 | ) | (103,488 | ) | 102,952 | 99 | % | |||||||||
| Total comprehensive loss for the year is attributable to: | ||||||||||||||||
| Owners of Telix | (536 | ) | (103,488 | ) | 102,952 | 99 | % | |||||||||
| Basic earnings/(loss) per share after income tax attributable to the ordinary equity holders of Telix (in cents) | 1.63 | (33.50 | ) | |||||||||||||
| Diluted earnings/(loss) per share after income tax attributable to the ordinary equity holders of Telix (in cents) | 1.61 | (33.50 | ) | |||||||||||||
| * | Percentage not meaningful. |
| 98 |
Revenue from Contracts with Customers
Revenue from contracts with customers was A$502.5 million for the year ended December 31, 2023, an increase of A$342.5 million, or 214%, compared to A$160.1 million for the year ended December 31, 2022. This increase was due to a 223% increase in commercial sales volumes of Illuccix® in the United States compared to 2022, which reflected a full year of commercial sales in 2023 and growth in sales during 2023. Average daily demand for doses increased in 2023 while average prices remained relatively consistent compared to 2022.
Cost of Sales
Cost of sales increased by A$123.0 million, or 189%, to A$188.2 million for the fiscal year ended December 31, 2023 from A$65.2 million for the fiscal year ended December 31, 2022. The increase was primarily driven by higher dose administration fees to distributors and kit manufacturing costs and higher royalties driven by higher sales volumes.
Gross margin improved in 2023 relative to 2022, increasing to 63% for 2023 (up from 59% in 2022). This increase reflected improved manufacturing and distribution costs.
Research and Development Costs
R&D costs were A$128.8 million for the year ended December 31, 2023, an increase of A$47.8 million, or 59%, compared to A$81.0 million for the year ended December 31, 2022. This increase was primarily driven by investment in two new diagnostic assets and developing late-stage diagnostic assets, including the prostate cancer therapy program.
Selling and Marketing Expenses
Selling and marketing expenses were A$54.9 million for the year ended December 31, 2023, an increase of A$16.9 million, or 45%, compared to A$38.0 million for the year ended December 31, 2022. This increase was primarily driven by increased investment in Illuccix® commercialization activities, including costs associated with the expansion of Telix’s sales force operations and promotional marketing program costs (including travel costs).
Selling and marketing expenses continue to decrease as a percentage of revenue, reflecting improvements in operating expenditure control and revenue growth exceeding cost base growth.
General and Administration Costs
General and administration costs were A$79.0 million for the year ended December 31, 2023, an increase of A$29.9 million, or 61%, compared to A$49.1 million for the year ended December 31, 2022. This increase was primarily driven by higher employee-related costs and an increased investment in infrastructure to support the expansion of support services for Telix’s commercial operations in each region.
| 99 |
Other Losses
Other losses were A$35.9 million for the year ended December 31, 2023, an increase of A$17.1 million, or 91%, compared to A$18.8 million for the year ended December 31, 2022. This increase was due to higher losses recognized on the remeasurement of contingent consideration.
Finance Income
Finance income was A$1.0 million for the year ended December 31, 2023, an increase of A$1.0 million compared to A$0.0 million for the year ended December 31, 2022. This increase reflects an increase in cash and cash equivalents placed into short term deposits and higher interest rate yields obtained on deposits in the year ended December 31, 2023 compared to the prior year.
Finance Costs
Finance costs were A$13.8 million for the year ended December 31, 2023, an increase of A$7.1 million, or 106%, compared to A$6.7 million for the year ended December 31, 2022. This increase was due to a higher unwind of discount on contingent consideration liability for 2023, reflecting the more significant remeasurement recognized for the year compared to 2022.
Income Tax Benefit/(Expense)
Income tax benefit was A$2.1 million for the year ended December 31, 2023, a change of A$7.6 million, or 139%, compared to a A$5.5 million expense for the year ended December 31, 2022. This resulted from the recognition of A$16.5 million in deferred tax benefits attributable to temporary differences and unused tax losses. Current tax expense increased from A$9.4 million in 2022 to A$14.4 million in 2023 as a result of the increase in taxable profits generated in the United States.
Segments
Telix’s two reportable segments are Commercial Operations and Product Development, which are categorized based on its principal activities. Telix evaluates the performance of its segments based on Adjusted EBITDA, calculated as earnings before interest, tax, depreciation and amortization, adjusted for the effects of the remeasurement of contingent consideration and government grant liabilities and other income and expense items which may have an impact on the degree to which earnings reflect the results of core operations, such as an impairment where the impairment is the result of an isolated, non-recurring event. Telix’s management uses Adjusted EBITDA to assess the core operating performance of segments and to make decisions about the allocation of resources. Telix also believes this measure provides useful information to users of its financial statements by allowing for the assessment of underlying trends in its current operational performance by excluding the impacts of non-cash sunk costs.
Commercial Operations
The Commercial Operations segment focuses on the commercial sales of Illuccix® and other products that may obtain regulatory approvals. This segment includes royalties and sales of goods (which account for the majority of Telix’s revenue from operations), as well as the sales and marketing expenses and costs of sales necessary to support those revenues.
The following table sets forth the results of operations for Telix’s Commercial Operations segment for the periods presented.
| 100 |
| Year ended December 31, | 2023 vs. 2022 | |||||||||||||||
2023 A$ | 2022 A$ | Change A$ | Change % | |||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue from contracts with customers | 497,051 | 156,369 | 340,682 | 218 | % | |||||||||||
| Cost of sales | (188,157 | ) | (65,170 | ) | 122,987 | 189 | % | |||||||||
| Gross profit | 308,894 | 91,199 | 217,695 | 239 | % | |||||||||||
| Research and development costs | (284 | ) | (704 | ) | (420 | ) | 60 | % | ||||||||
| Selling and marketing expenses | (54,437 | ) | (37,756 | ) | 16,681 | 44 | % | |||||||||
| General and administration costs | (36,092 | ) | (17,730 | ) | 18,362 | 104 | % | |||||||||
| Other losses (net) | (863 | ) | (820 | ) | 43 | 5 | % | |||||||||
| Operating profit/(loss) | 217,218 | 34,189 | 183,029 | 535 | % | |||||||||||
| Other losses (net) | 863 | 820 | (43 | ) | 5 | % | ||||||||||
| Depreciation and amortization | 5,665 | 4,694 | 971 | 21 | % | |||||||||||
| Adjusted EBITDA | 223,746 | 39,703 | 184,043 | 464 | % | |||||||||||
For the fiscal year ended December 31, 2023, revenue from contracts with customers for Telix’s commercial operations segment consisted of A$496.2 million (2022: A$156.0 million) in sales of goods, A$0.4 million (2022: A$0.4 million) in royalty revenue and A$0.4 million (2022: A$Nil) in services revenue. U.S. sales of Illuccix® were the main driver of the 218% increase in revenue from contracts with customers for the commercial operations segment compared to 2022. Adjusted EBITDA increased by A$184.0 million, or 464%, to A$223.7 million for the fiscal year ended December 31, 2023, up from A$39.7 million in 2022.
Product Development
The Product Development segment focuses on the development of radiopharmaceutical product candidates for commercialization. This segment includes revenue received from license agreements prior to commercialization and research and development services.
The following table sets forth the results of operations for our Product Development segment for the periods presented.
| Year ended December 31, | 2023 vs. 2022 | |||||||||||||||
2023 A$ | 2022 A$ | Change A$ | Change % | |||||||||||||
| (in thousands) | ||||||||||||||||
| Revenue from contracts with customers | 5,496 | 3,727 | 1,769 | 47 | % | |||||||||||
| Cost of sales | — | — | — | — | ||||||||||||
| Gross profit | 5,496 | 3,727 | 1,769 | 47 | % | |||||||||||
| Research and development costs | (128,517 | ) | (80,304 | ) | 48,213 | 60 | % | |||||||||
| Selling and marketing expenses | — | — | — | — | ||||||||||||
| General and administration costs | — | — | — | — | ||||||||||||
| Other losses (net) | — | 10 | 10 | 100 | % | |||||||||||
| Operating profit/(loss) | (123,021 | ) | (76,567 | ) | (46,454 | ) | 61 | % | ||||||||
| Other losses (net) | — | (10 | ) | (10 | ) | 100 | % | |||||||||
| Depreciation and amortization | 538 | 493 | 45 | 9 | % | |||||||||||
| Adjusted EBITDA | (122,483 | ) | (76,084 | ) | (46,399 | ) | 61 | % | ||||||||
| 101 |
For the fiscal year ended December 31, 2023, revenue from contracts with customers for Telix’s product development segment consisted of A$0.1 million (2022: A$0.4 million) in intellectual property license revenue and A$5.4 million (2022: A$3.4 million) in R&D services revenue. R&D investment during 2023 focused on preparation for commercial launch of Zircaix and Pixclara, including commercial manufacturing process qualification and validation, preparation of FDA filings, commercial launch plans and early access programs. R&D was also directed towards clinical manufacturing to progress the ProstACT GLOBAL trial. The portion of R&D costs that was attributable to employment expenses increased from A$19.2 million in the fiscal year ended December 31, 2022 to A$32.1 million in the fiscal year ended December 31, 2023, reflecting increased activity in our late-stage assets. Adjusted EBITDA for the product development segment was negative A$122.5 million in 2023, compared to negative A$76.1 million in 2022. The year-over-year change in this measure reflects a trend in higher investment in Telix’s R&D expenditure toward new product candidates, paired with relatively low revenue generation attributable to intellectual property licensing and R&D services contracts.
For more information on Telix’s segment reporting, see Note 3 to Telix’s consolidated financial statements appearing elsewhere in this Information Statement.
Liquidity and Capital Resources
Prior to the fiscal year ended December 31, 2023, Telix has incurred operating losses in each year since its founding. Telix anticipates that as it continues to expand through strategic acquisitions, increase sales and marketing efforts and expand investment in R&D, it will need additional capital to fund operations, which Telix may raise through a combination of equity offerings, debt financings, strategic collaborations and other third-party funding arrangements. Telix’s future liquidity and capital resources will depend on product revenue from the successful continued commercialization of Illuccix®, revenue from any future products for which Telix obtains regulatory approval and the R&D costs and other expenditure necessary to support these initiatives and future products. Telix’s total comprehensive loss was A$103.5 million and A$0.5 million for the years ended December 31, 2022 and 2023, respectively. As of December 31, 2023, Telix had cash and cash equivalents of A$123.2 million and accumulated losses of A$263.7 million.
Sources and Uses of Liquidity
Telix’s operations have been financed primarily through cash generated by its commercial operations and the issuance and sale of new ordinary shares. Telix has raised aggregate proceeds of A$271.9 million (including share issuance costs) in the five fiscal years ended December 31, 2023 from the issuance and sale of new ordinary shares. In January 2022, Telix completed an institutional placement of 22,727,273 ordinary shares at a price per share of A$7.70 per share for aggregate gross proceeds of A$175 million. Telix primarily intends to use cash generated from commercial operations to fund its committed development activities and to progress new products towards regulatory approval and commercialization. In the years ended December 31, 2022 and 2023, Telix received A$124.1 million and A$463.7 million, respectively, in collections from sales of Illuccix®. Telix has also received an aggregate of A$51.2 million in the five fiscal years ended December 31, 2023 under the Australian government’s R&D Tax Incentive Scheme for the funding of the development and clinical trials of new products. Telix did not recognize any amounts in relation to the R&D Tax Incentive Scheme in 2022 or 2023, due to global revenue exceeding the threshold of A$20 million.
| 102 |
In the first quarter of 2022, Telix entered two loan agreements whereby BNP Paribas agreed to lend A$9.9 million and IMBC Group agreed to lend A$6.5 million. Each loan is denominated in Euros, in the amounts of €6.1 million and €4 million, respectively, and have been translated to Australian dollars based on the applicable exchange rate as of December 31, 2023. Each loan has a 10-year term and an interest rate of 1.85% per annum, payable monthly, and each is repayable in 96 monthly installments beginning at the end of a two-year grace period. As of December 31, 2023, Telix has drawn down an aggregate of A$9.2 million from these facilities (translated based on the applicable exchange rate as of December 31, 2023). In connection with the loan agreement with BNP Paribas, Telix also entered a roll-over loan agreement whereby BNP Paribas agreed to lend an additional A$3.2 million (€2.0 million, translated based on the applicable exchange rate as of December 31, 2023). The loan has a two-year extendable term and a per annum interest rate calculated by adding the eurozone interbank interest rate as of the determination date to a 1.5% margin, payable based on Telix’s choice of interest period ranging from 1 month to 12 months for each advance (with a default interest period of three months if no alternative is chosen), and it is repayable in full upon its expiration date. As of December 31, 2023, Telix has drawn down A$Nil from this facility. Telix has used the borrowings from these loans in order to fund the renovation and redevelopment of its Brussels South production facility.
Funding Requirements
Telix expects expenses to increase in connection with our ongoing activities, particularly as Telix continues the commercialization of Illuccix® and any other product for which it receives regulatory approval and continues clinical development of its therapeutic product candidates. Until Telix can generate a sufficient amount of revenue from the sale of approved products, if ever, Telix expects to finance its operating activities through cash generated from commercial sales, existing cash and cash equivalents and future financing activities, which may include equity offerings, debt financings, collaborations, strategic alliances and licensing arrangements. To the extent that Telix raises additional capital through the sale of equity or convertible debt securities, stockholder ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect current stockholder rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting Telix’s ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If Telix raises funds through collaborations, strategic alliances or licensing arrangements with third parties, it may have to relinquish valuable rights to its technologies, intellectual property, future revenue streams or product candidates. If Telix is unable to raise additional funds through equity or debt financings when needed, Telix may be required to delay, limit, reduce or terminate product development or future commercialization efforts or grant rights to develop and market product candidates that it would otherwise prefer to develop and market in-house. Telix’s present and future funding requirements will depend on many factors, including, among other things:
| ● | the amount of revenue received from commercial sales of Illuccix® and any of Telix’s product candidates for which it may receive marketing approval; | |
| ● | the initiation, progress, timing, costs and results of clinical trials for product candidates; | |
| ● | the costs associated with in-licensing or acquiring assets to expand Telix’s pipeline, acquiring businesses or assets to vertically integrate Telix’s supply chain and manufacturing and acquiring complementary business; |
| 103 |
| ● | the amount of milestones and royalties that Telix may be required to pay under existing acquisition and licensing agreements; | |
| ● | costs associated with expanding Telix’s organization; | |
| ● | the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims of infringement raised by third parties; and | |
| ● | the time and costs involved in obtaining regulatory approval for Telix’s product candidates and any delays it may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these product candidates. |
Cash Flows
The following table summarizes Telix’s cash flows for the periods presented:
| Year ended December 31, | ||||||||
2023 A$ | 2022 A$ | |||||||
| (in thousands) | ||||||||
| Net cash generated/(used) in operating activities | 23,884 | (63,970 | ) | |||||
| Net cash used in investing activities | (25,489 | ) | (16,997 | ) | ||||
| Net cash provided by financing activities | 10,186 | 174,960 | ||||||
| Net increase in cash and cash equivalents | A$8,581 | A$93,993 | ||||||
Operating Activities
Net cash generated from operating activities was A$23.9 million during the year ended December 31, 2023. The primary sources of cash from operating activities were A$463.7 million received in collections from sales of Illuccix®. The primary uses of cash in operating activities were payments to suppliers and employees, including A$183.1 million spent on dose administration fees, royalties and manufacturing costs, A$118.9 million spent on R&D expenditures, and A$42.5 million spent on selling and marketing efforts. Other operating cash outflows included A$16.3 million in contingent consideration payments to former ANMI shareholders and A$10.3 million in income tax payments.
Net cash used in operating activities was A$64.0 million during the year ended December 31, 2022. The primary sources of cash from operating activities were A$124.1 million received in collections from sales of Illuccix® and A$18.9 million received in R&D tax incentives. The primary uses of cash in operating activities were payments to suppliers and employees, including A$50.6 million spent on manufacturing costs, A$73.2 million spent on R&D expenditures and A$15.2 million spent on selling and marketing efforts.
Investing Activities
Net cash used in investing activities was A$25.5 million during the year ended December 31, 2023. The primary uses of cash in investing activities were A$13.2 million in payments toward Telix’s acquisition of QSAM Biosciences, Inc. and strategic investment in Mauna Kea and A$9.7 million in property, plant and equipment purchases for the buildout of Telix’s manufacturing facility in Belgium.
Net cash used in investing activities totaling A$17.0 million during the year ended December 31, 2022 was primarily comprised of A$6.8 million paid for the in-license to the worldwide rights to develop and commercialize radiolabeled forms of olaratumab for the diagnosis and treatment of human cancers, A$7.0 million paid for the construction of Telix’s manufacturing facilities in Belgium and A$2.2 million paid for the decommissioning and removal of two cyclotrons at Telix’s manufacturing facilities in Belgium.
| 104 |
Financing Activities
For the year ended December 31, 2023, net cash provided by financing activities totaled A$10.2 million. Financing activity cash flows included A$6.7 million received from the issuance of new ordinary shares on the exercise of options previously granted to employees, proceeds of A$5.8 million received from borrowings related to the loan facilities provided for the construction of Telix’s manufacturing facility in Belgium and A$2.2 million paid toward lease liabilities.
For the year ended December 31, 2022, net cash provided by financing activities totaling A$175 million was primarily comprised of A$173.2 million (net of transaction costs) received from the issuance of new ordinary shares in connection with the exercise of options previously granted to employees and a private placement to institutional investors. Other financing activities comprised A$3.0 million received from borrowings related to the loan facilities provided for the construction of Telix’s manufacturing facility in Belgium and A$1.3 million paid toward lease liabilities.
Contractual Obligations
Telix has commitments against existing development activities and capital commitments relating to the purchase of Ytterbium-176 isotopes from a vendor over a three year period. R&D commitments are estimated based on the contractual obligations included within agreements entered into by us, to the extent that a work order has been executed with the vendor.
Certain of Telix’s supply agreements contain minimum purchase commitments in certain situations, the amount and timing of which are not known. Additionally, Telix enters into contracts in the normal course of business with clinical trial sites and clinical supply manufacturers and with vendors for preclinical studies and clinical trials, research supplies and other services and drugs for operating purposes. These contracts generally provide for termination after a notice period, and, therefore, are cancellable contracts.
Telix has entered into collaboration arrangements, including in-licensing arrangements with various companies. Such collaboration agreements may require us to make payments on achievement of stages of development, launch or revenue milestones and may include variable payments that are based on unit sales or profit (e.g. royalty and profit share payments). The amount of variable payments under the arrangements are inherently uncertain and difficult to predict, given the direct link to future sales, profit levels and the range of outcomes These payments are not included in this table of contractual obligations.
The following table summarizes Telix’s contractual obligations as of December 31, 2023 (in thousands), grouped as payments due by period:
| Total | < 1 year | 1-3 years | 3-5 years | > 5 years | ||||||||||||||||
| Capital commitments | A$56,572 | 16,572 | 40,000 | — | — | |||||||||||||||
| R&D commitments | A$48,515 | 28,112 | 20,403 | — | — | |||||||||||||||
| 105 |
Lightpoint Medical Share Sale Agreement
On June 21, 2023, Telix entered into a share sale agreement with Lightpoint Medical Ltd, or Lightpoint Medical, to acquire Lightpoint Medical’s SENSEI radio-guided surgery business. The acquisition is intended to support and expand Telix’s late-stage urologic cancer pipeline. Telix completed the acquisition of Lightpoint Medical’s SENSEI radio-guided surgery business on November 1, 2023. The acquisition was implemented through the purchase of Lightpoint Medical Limited’s wholly owned subsidiary, Lightpoint Surgical Limited, as the then owner of Lightpoint Medical’s business, assets and operation. Telix paid upfront consideration of US$20.0 million, of which it paid US$19.6 million through the issuance of 3.3 million ordinary shares at a price of A$9.3659 per share. Telix is obligated to pay an additional US$15.0 million via an earn-out in the form of performance rights, which may be settled in cash or ordinary shares, at Telix’s option, upon achievement of specified milestones relating to the ongoing development and commercialization of SENSEI.
Agreement and Plan of Merger with QSAM Biosciences, Inc.
On February 7, 2024, Telix entered into an Agreement and Plan of Merger, or the Merger Agreement, with QSAM Biosciences, Inc., a public reporting company in the United States. See a more detailed description of the Merger Agreement under The Merger—The Merger Agreement in this Information Statement.
Agreement and Plan of Merger with IsoTherapeutics Group, LLC
On February 27, 2024, Telix entered into an agreement and plan of merger, or the IsoTherapeutics Agreement, to acquire IsoTherapeutics Group, LLC, or IsoTherapeutics. The purchase price for the acquisition consists of (i) US$8.0 million payable at closing in the form of US$2.0 million in cash and US$6.0 million in Telix ordinary shares, (ii) US$5.0 million in performance-related milestone payments, which are payable in cash and are subject to meeting certain milestone conditions within twelve months of closing, and (iii) a two-year revenue share that is based on actual revenue earned from existing customers of IsoTherapeutics, which Telix estimates will require total cash payments of approximately US$0.6 million. The upfront cash consideration is subject to customary working capital, debt and transaction expense adjustments. The number of shares to be issued at closing will be determined by converting US$6.0 million to Australian dollars using the Reserve Bank of Australia exchange rate at closing and dividing that amount by the volume weighted average price at which Telix ordinary shares trade on the ASX over the 10-trading day period prior to closing.
The closing of the merger with IsoTherapeutics is subject to various conditions set forth in the IsoTherapeutics Agreement. The IsoTherapeutics Agreement also provides the parties with customary rights to terminate the IsoTherapeutics Agreement in certain circumstances, including by mutual written consent of Telix and IsoTherapeutics or by either party if the merger has not been consummated by May 27, 2024, in each case on the terms set forth in the IsoTherapeutics Agreement.
Share Purchase Agreement with ARTMS Inc.
On March 5, 2024, Telix entered into a share purchase agreement, or the ARTMS Agreement, to acquire ARTMS Inc., or ARTMS. The purchase price for the acquisition consists of: (i) US$57.5 million upfront consideration, US$15.0 million of which is payable in cash and the balance of which is payable in the form of 5,674,636 of Telix ordinary shares to be issued at closing, (ii) US$24.5 million in contingent future earn out payments, payable in cash following achievement of certain regulatory and commercial milestones, and (iii) cash earnouts representing low teens percentage royalties based on net sales of ARTMS products and related services and representing low single-digit percentage royalties based on net sales of Telix products prepared using ARTMS products for up to three years depending on the product location where the sale occurs. All earn-out royalties which have not otherwise expired will terminate on the 10-year anniversary following closing of the ARTMS acquisition. The cash upfront consideration is subject to customary working capital, debt and transaction expense adjustments. The shares issued at closing will be subject to escrow restrictions.
The closing of the acquisition of ARTMS is subject to various conditions set forth in the ARTMS Agreement, including regulatory approvals. The ARTMS Agreement also provides the parties with customary rights to terminate the ARTMS Agreement in certain circumstances.
| 106 |
Off-Balance Sheet Arrangements
During the periods presented, Telix did not, and does not currently, engage in off-balance sheet financing arrangements as defined under SEC rules, such as relationships with other entities or financial partnerships, which are often referred to as structured finance or special purpose entities, established for the purpose of facilitating financing transactions that are not required to be reflected on Telix’s consolidated statement of financial position. In addition, Telix does not engage in trading activities involving non-exchange traded contracts.
Critical Accounting Policies and Estimates
Telix believes that the following accounting policies involve a high degree of judgment and complexity. Accordingly, these are the policies Telix believes are the most critical to aid in fully understanding and evaluating Telix’s consolidated financial condition and results of operations. See Note 2 to the Consolidated Financial Statements for a description of other significant accounting policies and Note 2.28 for additional information on Telix’s key judgments and estimates. The preparation of Telix’s consolidated financial statements in conformity with IFRS Accounting Standards requires Telix to make estimates and judgments that affect the amounts reported in those financial statements and accompanying notes. Although Telix believes that the estimates it uses are reasonable, due to the inherent uncertainty involved in making those estimates, actual results reported in future periods could differ from those estimates.
Research and Development Costs
As part of the process of preparing the financial statements, Telix is required to estimate accrued R&D expenses. This process involves reviewing open contracts and purchase orders, communicating with program directors and managers to identify services that have already been performed, estimating the level of services performed with associated costs incurred for the service for which Telix has not yet been invoiced or otherwise notified of the actual cost. The majority of service providers invoice Telix monthly in arrears for services performed or when contractual milestones are met. Telix estimates accrued expenses as of each reporting date based on facts and circumstances known at that time. Telix periodically confirms the accuracy of estimates with the service providers and makes adjustments if necessary. Examples of estimated accrued expenses include fees paid to Contract Research Organizations, or CROs, in connection with clinical studies investigative sites in connection with clinical studies, vendors in connection with preclinical development activities, and vendors related to product manufacturing, process development and distribution of clinical supplies.
Intangible Assets
Goodwill and intangible assets that have an indefinite useful life are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment trigger assessment is performed annually.
Telix has identified the estimate of the recoverable amount of intangible assets as a significant judgment for the year ended December 31, 2023. In determining the recoverable amount of intangible assets, Telix has used discounted cash flow forecasts and key assumptions on risk adjusted post-tax discount rates, regulatory/marketing authorization approval dates, expected sales volumes, sales price per unit, and the probability of approval for marketing authorization. Telix has considered reasonable possible changes in the key assumptions and has not identified any instances that could cause the carrying amounts of the intangible assets as of December 31, 2022 and 2023 to exceed their recoverable amounts.
Contingent Consideration
The contingent consideration liabilities associated with business combinations are measured at fair value which has been calculated with reference to Telix’s judgment of the expected probability and timing of the potential future milestone payments or with reference to percentage of net sales achieved, based upon level 3 inputs under the fair value hierarchy, which is then discounted to a present value using appropriate discount rates with reference to Telix’s weighted average cost of capital.
| 107 |
Contingent consideration in connection with the purchase of individual assets outside of business combinations is recognized as a financial liability only when a non-contingent obligation arises (i.e., when the milestone is met).
The valuation of the contingent consideration has been performed using a discounted cash flow model that uses certain unobservable assumptions. These key assumptions include a risk adjusted post discount rate, marketing authorization dates, expected sales volumes over the forecast period, net sales price per unit and a probability of obtaining approval for marketing authorization. Significant changes in any of the assumptions would result in a significantly lower or higher fair value measurement.
Decommissioning Liabilities
Telix purchased a radiopharmaceutical production facility in Belgium on April 27, 2020. At the time of purchase, the facility had two cyclotrons installed in concrete shielded vaults which also contained some nuclear contamination associated with past manufacturing activities. As part of this purchase, Telix assumed an obligation to remove the cyclotrons and restore the site. Telix removed the cyclotrons from the site during 2022. Other decommissioning activities not required to upgrade the production facility have been deferred to the end of the operating life of the facility in 2041.
Telix has recognized a provision for its obligation to decommission the radiopharmaceutical production facility at the end of its operating life. At the end of the operating life of a facility, Telix incurs costs to remove certain assets involved in the production of radioactive isotopes. For each period presented, the decommissioning costs that Telix expects to incur have been discounted using the Belgium risk-free rate and translated to Australian dollars at the exchange rate as of the date of the consolidated statement of financial position. The provisions recognized in the periods presented represent the best estimate of the expenditures required to settle the present obligation as of December 31, 2023.
While Telix believes it has made its best estimate in establishing the decommissioning liability, because of potential changes in technology as well as safety and environmental requirements, plus the actual timescale to complete decommissioning, the ultimate provision requirements could vary from current estimates. Any subsequent changes in estimate which alter the level of the provision required are also reflected in adjustments to the plant & equipment asset. Each year, the provision is increased to reflect the unwind of discount and to accrue an estimate for the effects of inflation, with the charges being presented in the consolidated statement of comprehensive income or loss. Actual payments for commencement of decommissioning activity are disclosed as provision utilized.
Revenue from Sales of Goods
Sales are recognized at a point-in-time when control of the products has transferred, being when the products are administered to the patient. Revenue from sales is recognized based on the price specified in the contract, net of the estimated volume discounts and government rebates.
Accumulated experience is used to estimate and provide for discounts, using the expected value method, and revenue is recognized to the extent that it is highly probable that a significant reversal will not occur. No element of financing is deemed present as the sales are made with credit terms ranging from 30 to 45 days, which is consistent with market practice.
Where distributors are used to facilitate the supply of a product, a distribution fee is charged. This fee represents a cost of satisfying the performance obligation to the customer and expensed within “Cost of sales” in the Consolidated statement of comprehensive income or loss.
| 108 |
Share-based Payment Transactions
Telix provides benefits to its directors and employees (including key management personnel) in the form of share-based payments, whereby employees render services in exchange for ordinary shares, options or performance rights over ordinary shares (equity-settled transactions). The cost of these equity-settled transactions with employees is measured by reference to the fair value at the date at which they are granted. The Black-Scholes option pricing model is used to determine fair value, with key assumptions being the listed price per ordinary share on the grant date, the option exercise price, the term of the option, the impact of dilution, expected volatility of the underlying ordinary shares based on the historical share price volatility, the expected dividend yield and the risk-free interest rate.
The cost of the equity-settled transactions is recognized, together with a corresponding increase in equity, over the period in which the performance conditions are fulfilled (the vesting period), ending on the date on which the relevant employees become fully entitled to the award (the vesting date). The charge to profit or loss for the period is the cumulative amount less the amounts already charged in previous periods. There is a corresponding credit to equity. Until an award has vested, any amounts recorded are contingent and will be adjusted if more or fewer awards vest than were originally anticipated to do so. If an award is cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognized immediately.
Recently Adopted Accounting Pronouncements
Telix has adopted all relevant new and amended Accounting Standards and Interpretations issued by the IASB that are effective for annual reporting periods beginning on January 1, 2023. The adoption of these Accounting Standards and Interpretations did not have any significant impact on amounts reported in Telix’s consolidated financial statements.
Certain new or amended accounting standards and interpretations have been published that are not yet mandatory for the December 31, 2023 reporting period and have not been early adopted. These standards or interpretations are not expected to have a material impact on Telix’s financial performance or position in the current or future reporting periods or on foreseeable future transactions.
Qualitative and Quantitative Disclosures about Market Risk
Telix is exposed to market risks in the ordinary course of its business. Market risk represents the risk of loss that may impact Telix’s financial position due to adverse changes in financial market prices and rates. Telix’s market risk exposure is primarily attributable to foreign currency exchange rate risk.
Interest Rate Risk
As of December 31, 2023, Telix had cash and cash equivalents of A$123.2 million. Telix has limited exposure to interest rate risk. Telix’s cash and cash equivalents are not locked into long-term deposits at fixed rates so as to mitigate the risk of earning interest below the current floating rate.
Telix’s exposure to market interest rates relates primarily to short-term deposits. The roll-over loan facility totaling A$3.2 million (translated from Euros based on the exchange currency rate as of December 31, 2023) carries an interest rate that is calculated using the eurozone interbank interest rate as of each interest determination date. However, all of Telix’s borrowings that have been drawn down as of December 31, 2023 bear a fixed interest rate.
Telix does not believe that inflation has had a material effect on its business, financial condition, or results of operations. Nonetheless, if Telix’s costs were to become subject to significant inflationary pressures, it may not be able to fully offset such higher costs. Telix’s inability or failure to do so could harm its business, results of operations, or financial condition.
Foreign Currency Exchange Rate Risk
Foreign currency risk is the risk of fluctuation in fair value or future cash flows of a financial instrument as a result of changes in foreign exchange rates. Telix operates internationally and is exposed to foreign exchange risk, primarily related to the U.S. dollar and Euro. Foreign exchange risk arises from commercial activities in the United States and research and development activities in Europe and the United States.
| 109 |
Telix’s treasury risk management policy is to settle all U.S. dollar denominated expenditures with U.S. dollar denominated receipts from sales of Illuccix® in the United States. Telix also manages currency risk by making decisions as to the levels of cash to hold in each currency by assessing future activities which will likely be incurred in those currencies. Any remaining foreign currency exposure has therefore not been hedged.
Telix has both foreign currency receivables and payables, predominantly denominated in U.S. dollar and Euro. Telix had a surplus of foreign currency receivables over payables of A$26.5 million as of December 31, 2023.
Telix’s exposure to the risk of changes in foreign exchange rates also relates to the net investments in foreign subsidiaries, which predominantly include denominations in the Euro and the U.S. dollar. However, given the low level of current investments in foreign subsidiaries, this impact is limited.
As of December 31, 2023, Telix held 6.7% of its cash in Australian dollars, 77.5% in U.S. dollars, 15.4% in Euros, 0.1% in Japanese Yen and 0.3% in Swiss Francs.
Liquidity Risk
Telix is exposed to liquidity and funding risk from operations and from external borrowings, where the risk is that Telix may not be able to refinance debt obligations or meet other cash outflow obligations when required. Vigilant liquidity risk management requires that Telix maintain sufficient liquid assets (mainly cash and cash equivalents). Telix manages liquidity risk by maintaining adequate cash reserves by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities.
Credit Risk
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to Telix. Credit risk arises from cash and cash equivalents and credit exposures to customers, including outstanding receivables.
Credit risk is managed on a group basis. If customers are independently rated, these ratings are used. Otherwise, if there is no independent rating, Telix assesses the credit quality of the customer, taking into account its financial position, past experience and other factors. Individual risk limits are set based on internal or external ratings. The compliance with credit limits by customers is regularly monitored. Telix obtains guarantees where appropriate to mitigate credit risk.
Telix apples the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance for all trade receivables.
To measure the expected credit losses, trade receivables have been grouped based on shared credit risk characteristics and the days past due. The expected loss rates are based on historical payment profiles of sales and the corresponding historical credit losses experienced. The historical loss rates are adjusted to reflect current and forward-looking information on macroeconomic factors affecting the ability of the customers to settle the receivables.
Trade receivables are written off where there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, the failure of a debtor to engage in a repayment plan with us, and the failure to make contractual payments for a period of greater than 120 days past due.
Impairment losses on trade receivables are presented within sales and marketing costs within profit or loss. Subsequent recoveries of amounts previously written off are credited against the same line item. As of December 31, 2023, the expected credit losses were A$0.5 million.
Financial Statements
See Telix’s consolidated financial statements starting on page F-1 of this Information Statement.
| 110 |
COMPARISON OF RIGHTS OF HOLDERS OF TELIX ORDINARY SHARES AND QSAM COMMON STOCK
This section describes the material differences between the rights of the holders of QSAM Common Stock and the rights of holders of Telix Ordinary Shares. QSAM is incorporated under the laws of the State of Delaware. Telix is incorporated under the laws of Commonwealth of Australia. Accordingly, the rights of the stockholders of Telix and QSAM are governed by unique laws, as well as Telix’s Constitution and QSAM’s Certificate of Incorporation and Bylaws, as may be amended from time to time. After the completion of the Merger, the rights of QSAM stockholders who become Telix stockholders will be governed by Telix’s Constitution.
The following description summarizes the material differences between the rights of QSAM stockholders and Telix stockholders, but is not a complete statement of all those differences, or a complete description of the specific provisions referred to in this summary. QSAM stockholders should read carefully the relevant provisions of the Australia’s Corporations Act 2001 (Cth), Telix’s Constitution and the Company’s Certificate of Incorporation and Bylaws as amended. For more information on how to obtain the documents that are not attached to this Information Statement, see “Where You Can Find More Information” beginning on page 129.
| Rights of QSAM Stockholders | Rights of Telix Shareholders | |||
| Authorized Capital Stock | The authorized capital stock of QSAM consists of 305,000,000 shares, of which 300,000,000 shares consist of QSAM Common Stock, par value $0.0001 per share, and 5,000,000 shares of Preferred Stock, par value $0.0001 per share. | Australian law contains no concept of authorized capital stock or par value. The issue price of shares is set by the directors of Telix as at the time of issue. | ||
| Outstanding Capital Stock | 4,445,469 as of March 31, 2024. | 323,989,947 fully paid ordinary shares as of March 31, 2024. | ||
| Rights of Preferred Stock | In connection with the Merger, 100% of outstanding shares of Series A and Series B Preferred Stock was converted into or exchanged for QSAM Common Stock. As such, no preferred stock remains outstanding as of the date hereof. | Telix’s constitution authorizes Telix to issue preference shares, including preference shares which are liable to be redeemed or convertible into ordinary shares (subject to the Corporations Act 2001 (Cth) (the “Corporations Act”)). However, Telix has never issued preference shares. | ||
| Calling Special Meetings of Stockholders | QSAM’s bylaws provide that a special meeting of the stockholders of the Corporation may be called at any time only by the Board of Directors, the Executive Chairman, or the Chief Executive Officer with the concurrence of a majority of the Board of Directors, and may not be called by any other person or persons. | Pursuant to Telix’s constitution, a general meeting of shareholders may only be called by a Telix Board resolution or as otherwise provided in the Corporations Act. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at the general meeting.
The Corporations Act also provides that shareholders with at least 5% of the votes that may be cast at a general meeting may also call and arrange to hold a general meeting. The shareholders calling the general meeting must pay the expenses of calling and holding the general meeting. | ||
| 111 |
| Stockholder Proposals and Nominations of Candidates for Election to the Board of Directors | QSAM’s bylaws allow QSAM stockholders to propose business to be brought before an annual meeting and make nominations for election of directors provided the stockholder is entitled to vote at the meeting and who complies with the notice procedures set forth in the Bylaws. | Pursuant to Telix’s constitution, Telix’s shareholders may by resolution at a general meeting appoint an eligible person to be a director, either as an addition to the existing directors or to fill a casual vacancy. | ||
| Such proposals and nominations, however, may only be brought by a stockholder who has given a timely notice thereof in writing to the Secretary of the Corporation and, in the case of business other than nominations, such business must be a proper subject for stockholder action. | A person is eligible for election as a director at a general meeting only if:
(a) the person is in office as a director immediately before the general meeting; or
(b) the person has been nominated by the Telix Board for election at the general meeting; or
(c) not less than: | |||
| In connection with the annual meeting, to be timely, notice of such proposals and nominations must be delivered by a nationally recognized courier service or mailed by U.S. mail, postage or delivery charges prepaid, and delivered to QSAM’s Secretary at the principal executive offices of the Corporation not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s Annual Stockholders Meeting; provided, however, that in the event that the date of the Annual Stockholders Meeting is more than thirty (30) days before or more than seventy (70) days after such anniversary date, or if no Annual Stockholders Meeting was held in the preceding year, notice by the stockholder to be timely must be so delivered not earlier than the close of business on the 120th day prior to such Annual Stockholders Meeting and not later than the close of business on the later of the 90th day prior to such Annual Stockholders Meeting or the 10th day following the date on which public announcement of the date of such meeting is first made by the Corporation. | (i) Telix shareholders with at least 5% of the votes that may be cast on a resolution; or
(ii) at least 100 Telix shareholders who are entitled to vote,
have, at least 45 business days before the general meeting (or at least 30 business days before the general meeting, if the general meeting has been requested by Telix shareholders), but no more than 90 business days before the general meeting, given Telix:
(iii) a notice signed by the relevant shareholders stating their intention to nominate the person for election as director; and
(iv) a notice signed by the person nominated as director stating his or her consent to the nomination. |
| 112 |
| Stockholder Action by Written Consent | QSAM’s certificate of incorporation provides that the taking of any action by written consent of the stockholders without a meeting of the QSAM Stockholders is allowed. | Australian public companies cannot under the Corporations Act pass resolutions by circulating written resolutions. | ||
| Number of Directors and Size of Board | QSAM’s bylaws provides that the numbers of directors constituting the whole QSAM’s Board is to be determined from time to time by resolution adopted by the affirmative vote of a majority of the QSAM Board then authorized to vote.
There are currently four directors serving on the QSAM Board. |
Telix’s constitution provides that the number of directors (not including alternate directors) shall: (i) not be less than 3; and (ii) not be more than 9, unless the company resolves otherwise at a general meeting.
There are currently six directors serving on the Telix Board. | ||
| 113 |
| Classes and Terms of Directors | QSAM’S board is not divided into classes.
Directors hold office until the term of the director expires and until such directors successor is elected and qualified or until such director’s earlier death, resignation or removal. |
Telix’s constitution provides that no director who is not the chief executive officer may hold office without re-election beyond the third annual general meeting following the meeting at which the director was last elected or re-elected.
Telix’s constitution also provides that if there is more than one chief executive officer, only one of them, nominated by the Board, is entitled not to be subject to the three-year term.
Telix’s constitution further provides that to the extent the listing rules of the Australian Securities Exchange require an election of directors to be held and no director would otherwise be required to submit for election or re-election, the director to retire is any director who wishes to retire (whether or not he or she intends to stand for re-election), otherwise it is the director who has been longest in office since their last election or appointment (excluding the chief executive officer). As between directors who were last elected or appointed on the same day, the director to retire must be decided by lot (unless they can agree among themselves).
Telix’s Board is not divided into classes. | ||
| Election of Directors | Under the DGCL, directors are elected by a plurality of the votes of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. |
Under Telix’s constitution, directors are elected or re-elected by ordinary resolution passed by shareholders at a general meeting. The resolution must be passed by the majority of the votes cast by the shareholders attending. | ||
| Voting Rights | Holders of QSAM Common Stock are entitled to one vote per share. |
Holders of Telix ordinary shares are entitled to one vote per share. | ||
| 114 |
| Removal of Directors | Under QSAM’s bylaws, any director, or the entire Board of Directors, may be removed from office at any time by the affirmative vote of the majority of the voting power of the stock outstanding and entitled to vote thereon. | Under the Corporations Act, Telix shareholders may remove a director by passing an ordinary resolution at a general meeting. The resolution must be passed by the majority of the votes cast by the shareholders attending. | ||
| Vacancies | Under QSAM’s bylaws, any vacancy on the QSAM Board (whether resulting from an increase in the total number of directors or any vacancies in QSAM Board resulting from death, resignation, retirement, disqualification, removal from office or other cause) shall be filled solely by the affirmative vote of a majority of the remaining directors then in office, even though less than a quorum, or the sole remaining director, and any director so chosen shall hold office until the next Annual Meeting of the Company and until his or her successor shall have been duly elected or qualified. | Telix’s constitution provides that the Telix Board may appoint any eligible person to be a director, either as an addition to the existing directors or to fill a casual vacancy, as long as the total number of directors does not exceed the maximum number fixed under Telix’s constitution (being 9 directors). A director who is appointed by the Board to fill a Board vacancy, who is not a chief executive officer, holds office until the conclusion of the next annual general meeting following their appointment. If there is more than one chief executive officer, only one of them, nominated by the Board, is entitled not to be subject to the one-year term.
Telix’s constitution also provides that shareholders may by resolution at a general meeting appoint an eligible person to be a director, either as an addition to the existing directors or to fill a casual vacancy, as long as the total number of directors does not exceed the maximum number fixed under Telix’s constitution (being 9 directors). | ||
| Limitation on Liability of Directors | QSAM’s certificate of incorporation provides that, to the fullest extent permitted by the DGCL, no director of QSAM will be personally liable to QSAM or its stockholders for monetary damages for breach of a fiduciary duty as a director of QSAM, provided, however, that the foregoing shall not eliminate or limit the liability of a director of the Corporation for any breach of the director’s duty of loyalty. | Under the Corporations Act, there is a general prohibition on a company or a related body corporate exempting officers from any liability to the company that is incurred as an officer of the company. Telix’s constitution indemnifies directors against liability as set out below. | ||
| 115 |
| Indemnification of Directors and Officers | QSAM’s certificate of incorporation and bylaws provide that QSAM will, to the fullest extent permitted by law, indemnify any person made party to any action or proceeding by reason of the fact that the person is or was a director or officer of QSAM (or any predecessor), or is or was serving, at the request of QSAM (or any predecessor), as a director, officer, employee or agent of another corporation, partnership, limited liability company, joint venture, trust, certain employee benefit plans or other enterprise.
QSAM’s certificate of incorporation and bylaws permit QSAM to purchase and maintain insurance to protect any person who is or was a director or officer of QSAM, or is or was serving, at the request of QSAM, as a director, officer, employee or agent of another corporation, partnership, joint venture, trust, employee benefit plan or other enterprise, whether or not QSAM would have the power to indemnify such person against such liability under the DGCL. |
Telix’s constitution provides that Telix will, on a full indemnity basis and to the full extent permitted by law, indemnify (i) each person who is or has been a director, alternate director or executive officer of Telix and (ii) such other officers or former officers of Telix or of its related bodies corporate as Telix’s Board in each case determines (each an “Officer”) against all losses, liabilities, costs, charges and expenses (“Liabilities”) incurred by the Officer as an officer of the company or of a related body corporate.
The indemnity is enforceable without the Officer having to first incur any expense or make any payment, is a continuing obligation and is enforceable by the Officer even though the Officer may have ceased to be an officer of the company or its related bodies corporate, and applies to Liabilities incurred both before and after the adoption of the constitution.
The Corporations Act provides that Telix may not indemnify directors and officers for certain classes of liabilities, including liabilities owed to Telix. Further, Telix must not indemnify an officer against legal costs in certain circumstances.
Telix’s constitution permits Telix, to the extent permitted by law, purchase and maintain insurance or pay or agree to pay a premium for insurance for each Officer against any liability incurred by the Officer as an officer of Telix or of a related body corporate, including, but not limited to, a liability for negligence or for reasonable costs and expenses incurred in defending or responding to proceedings, whether civil or criminal and whatever their outcome. |
| 116 |
| Telix has entered into deeds of access, indemnity and insurance with each of its directors, and certain of its officers, by which Telix agrees to: (i) indemnify the relevant person; (ii) provide access to documents to the relevant person for use in connection with any claim or proceeding; and (iii) take out directors’ and officers’ insurance to cover any losses arising out of the acts and omissions of the relevant person. | ||||
| DGCL 203 Election | QSAM has not opted out of the statutory protections of Section 203 of the DGCL, which, in general prohibits QSAM from engaging, under certain circumstances, in a business combination with an interested stockholder for a period of three years following the date the person became an interested stockholder unless the business combination or transaction in which such stockholder became an interested stockholder is approved in a prescribed manner. | The Corporations Act restricts the acquisition by any person of a “relevant interest” in a “voting share” of Telix where, because of the acquisition, that person or someone else’s percentage “voting power” in the company increases above 20% (or, where the person’s voting power was already above 20% and below 90%, increases in any way at all).
There is an exception from these restrictions where the shares are acquired under takeover offers made under the Corporations Act to all shareholders (which must be on the same terms for all shareholders (subject to minor exceptions) and which must comply with the timetable and disclosure requirements of the Corporations Act). There are certain other exceptions provided for in the Corporations Act. | ||
| 117 |
| Amendments to Certificate of Incorporation | QSAM’s certificate of incorporation provides that the affirmative vote of the majority of holders of the voting power of the shares of the capital stock of QSAM entitled to vote generally in the election of directors, shall be required to amend certain provision of the certificate of incorporation. | Telix’s governing document is its constitution. Under the Corporations Act, Telix may modify or repeal its constitution by special resolution. A special resolution is passed by 75% of the votes cast by shareholders entitled to vote on the resolution.
For so long as Telix is admitted to the Australian Securities Exchange (“ASX”), Telix’s constitution is subject to the terms of the ASX Listing Rules. | ||
| Amendments to Bylaws | QSAM’s bylaws and certificate of incorporation provides that, QSAM Board is expressly authorized to adopt, alter, amend or repeal the bylaws, without any action on the part of the stockholders, by the vote of at least the majority of the directors then in office. The stockholders may also adopt, alter, amend or repeal any provision inconsistent with any provision of the bylaws, by the affirmative vote of at least a majority of the voting power of the stock outstanding and entitled to vote thereon, voting together as a single class. | Under the Corporations Act, Telix may modify or repeal its constitution by special resolution. A special resolution is passed by 75% of the votes cast by shareholders entitled to vote on the resolution. | ||
| Special Resolutions | U.S. law contains no concept of special resolutions. | Under the Corporations Act, a special resolution is passed by 75% of the votes cast by shareholders entitled to vote on the resolution.
Approval by special resolution of shareholders is required for actions such as modifying or repealing the company’s constitution, changing the company’s name or type, selectively reducing or buying back capital (in some circumstances), giving financial assistance in connection with the acquisition of shares in the company, and undertaking a voluntary winding up of the company. | ||
| 118 |
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following general summary discusses the material U.S. federal income tax consequences of (i) the Merger to U.S. Holders (as defined below) whose shares of Common Stock are exchanged for Telix Ordinary Shares and CVRs pursuant to the Merger, and (ii) the post-Merger ownership and disposition of Telix Ordinary Shares acquired pursuant to the Merger to U.S. Holders. This discussion is for general information only and is not tax advice. This summary is based upon the Code, applicable U.S. Treasury regulations promulgated thereunder, judicial authority, and administrative rulings effective as of the date hereof. These laws and authorities are subject to change, possibly with retroactive effect, or different interpretations. Any such change could alter the tax consequences to U.S. Holders as described herein. No ruling from the IRS has been or will be requested in connection with the Merger. The discussion below does not address any aspects of U.S. taxation other than U.S. federal income taxation, and as such does not address any state, local or foreign tax consequences or any estate, gift or other non-income tax consequences.
This discussion is for general information only and does not purport to address all aspects of U.S. federal income taxation that may be relevant to a particular beneficial owner of Common Stock or Telix Ordinary Shares, as applicable (“Holder”), in light of its particular facts and circumstances. This discussion applies only to U.S. Holders that hold their shares of Common Stock or Telix Ordinary Shares as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Holder’s particular circumstances, including the impact of the alternative minimum tax or the unearned income Medicare contribution tax. This discussion also does not address consequences relevant to Holders that are subject to special rules under U.S. federal income tax laws including, without limitation, banks or other financial institutions; dealers or traders in securities or currencies; insurance companies; tax-exempt entities; entities or arrangements treated as partnerships for U.S. federal income tax purposes or other flow-through entities (and investors therein); retirement plans, individual retirement accounts or other tax-deferred accounts; real estate investment trusts; regulated investment companies; mutual funds; controlled foreign corporations; passive foreign investment companies; certain former citizens, former long-term residents of the United States, or entities covered by the anti-inversion rules under the Code; Holders having a functional currency other than the U.S. dollar; Holders who hold shares of Common Stock or Telix Ordinary Shares as part of a hedge, straddle, constructive sale, conversion transaction or other integrated transaction; Holders who own (or are deemed to own) 5% or more of the outstanding stock of QSAM; Holders subject to special tax accounting rules as a result of any item of gross income with respect to Common Stock being taken into account in an “applicable financial statement” (as defined in the Code); Holders that are non-U.S. persons or entities; and Holders who acquired their shares of Common Stock or Telix Ordinary Shares through the exercise of employee stock options or otherwise as compensation or through a tax-qualified retirement plan.
For purposes of this discussion, a “U.S. Holder” is a beneficial holder of QSAM Common Stock or Telix Ordinary Shares, as applicable, that is for U.S. federal income tax purposes:
| ● | an individual citizen or resident of the United States; |
| ● | a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| ● | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| ● | a trust if (a) its administration is subject to the primary supervision of a court within the United States and one or more U.S. persons has the authority to control all substantial decisions of the trust or (b) it has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds shares of Common Stock or Telix Ordinary Shares, the tax treatment of a person treated as a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Partnerships, and other entities treated as a partnership for U.S. federal income tax purposes, that hold Common Stock or Telix Ordinary Shares and partners in such entities should consult their tax advisors regarding the tax consequences to them.
| 119 |
THIS DISCUSSION IS PROVIDED FOR GENERAL INFORMATION ONLY AND DOES NOT CONSTITUTE LEGAL OR TAX ADVICE TO ANY HOLDER. This discussion is not a complete description of all potential U.S. federal income tax consequences of the merger OR THE OWNERSHIP AND DISPOSITION OF TELIX ORDINARY SHARES AND does not address tax consequences that may vary with, or are contingent on, individual circumstances. A HOLDER SHOULD CONSULT ITS OWN TAX ADVISORS CONCERNING THE U.S. FEDERAL INCOME TAX CONSEQUENCES AND ANY CONSEQUENCES ARISING UNDER FEDERAL NON-INCOME TAX LAWS, INCLUDING ESTATE AND GIFT TAXES, OR THE LAWS OF ANY TERRITORY, STATE, LOCAL OR NON-U.S. TAXING JURISDICTION IN LIGHT OF ITS PARTICULAR CIRCUMSTANCES RELATING TO THE MERGER, the receipt of any payments under, OR expiration of, a CVR, the receipt of any amounts due to its exercise of statutory appraisal rights, OR THE OWNERSHIP AND DISPOSITION OF TELIX ORDINARY SHARES.
Classification of the Merger
The Merger is intended to qualify as a single, integrated transaction that constitutes a “reorganization” within the meaning of Section 368(a) of the Code and not to be subject to Section 367(a)(1) of the Code. Telix and QSAM have agreed not to report or take any position for U.S. federal, state or local income tax purposes that is inconsistent with the intended tax treatment, except to the extent required by a “determination” as defined in Section 1313(a) of the Code. However, the closing of the Merger is not conditioned on the Merger qualifying for the intended tax treatment, it is not contemplated that an opinion of counsel with respect to the intended tax treatment will be required or delivered as a condition to closing, and neither QSAM nor Telix has requested a ruling from the IRS regarding any of the U.S. federal income tax consequences of the Merger. Accordingly, there can be no assurance that the IRS will not assert, or that a court would not sustain, a position contrary to the intended tax treatment or any of the conclusions set forth herein. U.S. Holders should therefore consult their own tax advisors to determine the U.S. federal income tax consequences of the Merger to them.
Generally, for the Merger to qualify as a “reorganization” within the meaning of Section 368(a) of the Code, numerous requirements, including the requirements in Section 368(a)(2)(D) of the Code, must be satisfied. Under Section 368(a)(2)(D) of the Code, for the Merger to qualify as a “reorganization” within the meaning of Section 368(a) of the Code, Merger Sub II must acquire “substantially all” of the properties held by QSAM immediately prior to the Merger, which is referred to as the “substantially all” requirement in this discussion. For purposes of issuing letter rulings on reorganizations under Section 368 of the Code, the IRS has required that an acquiring corporation acquire at least 90 percent of the fair market value of the net assets and at least 70 percent of the fair market value of the gross assets held by the acquired corporation immediately prior to the acquisition to meet the “substantially all” requirement. The IRS’s ruling requirement does not, however, set the lower limit for what constitutes “substantially all” for purposes of 368(a)(2)(D) of the Code.
In determining whether the “substantially all” requirement has been met, the cash consideration paid and the fair market value of the CVRs treated as paid by QSAM in exchange for fractional shares resulting from the Reverse Split, plus any cash treated as paid by QSAM to dissenters for U.S. federal income tax purposes are considered assets held by the corporation immediately prior to the acquisition.
Additionally, for the Merger to qualify as a “reorganization” within the meaning of Section 368(a) of the Code, the “continuity of interest” requirement as described in U.S. Treasury Regulations must be satisfied. Under the applicable U.S. Treasury Regulations, the “continuity of interest” requirement is satisfied if a proprietary interest in QSAM is preserved, which, under regulatory guidance generally will be the case if the Telix Ordinary Shares constitute at least 40% of the value of the aggregate consideration QSAM Stockholders receive in the Merger.
| 120 |
Furthermore, because Telix is a non-U.S. corporation, Section 367(a)(1) of the Code may apply to cause the Merger not to qualify as a “reorganization” under Section 368(a) of the Code. QSAM and Telix, however, intend that the exception to Section 367(a)(1) of the Code provided by Treasury Regulation Section 1.367(a)-3(c) will apply because, among other things, Telix has conducted a trade or business outside the United States for 36 months prior to the Merger. As a result, the parties believe Section 367(a)(1) of the Code will not apply to the Merger and cause a U.S. Holder to recognize gain in the Merger so long as either (i) the U.S. Holder is not a “five-percent transferee shareholder” (as defined in the Treasury Regulations and computed taking into account direct, indirect and constructive ownership) of Telix (by total voting power or by total value), or (ii) the U.S. Holder is a “five-percent transferee shareholder” (as defined in the Treasury Regulations and computed taking into account direct, indirect and constructive ownership) of Telix and enters into a “gain recognition agreement” with respect to the transferred Common Stock. Any U.S. Holder that will own, actually or constructively, 5% or more of either the total voting power or the total value of the outstanding shares of Telix after the Merger (taking into account, for this purpose, ownership of any Telix Ordinary Shares not acquired in connection with the Merger) should consider entering into a valid “gain recognition agreement” under applicable Treasury Regulations and is strongly urged to consult its own tax advisors to determine the particular consequences to it of the Merger.
There can be no assurance that the IRS will ultimately conclude that the Merger meets the “substantially all” requirement, the “continuity of interest” requirement, or any of the other requirements for qualification as a “reorganization” within the meaning of Section 368(a) of the Code, that the exception to Section 367(a)(1) described above will apply to the Merger, or that any of the other statements made herein would not be challenged by the IRS and, if so challenged, sustained upon review in a court.
If the Merger Qualifies as a “Reorganization” Within the Meaning of Section 368(a) of the Code
Subject to the discussion below under the heading “Tax Treatment of the CVRs,” if the Merger qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, Section 367(a)(1) of the Code does not apply to the Merger, and the transaction is treated as a “closed transaction” rather than an “open transaction” for U.S. federal income tax purposes (as described below), then for U.S. federal income tax purposes:
| ● | generally, capital gain will be recognized by a U.S. Holder in an amount equal to the lesser of (i) the fair market value of the CVRs received as determined for U.S. federal income tax purposes and (ii) the difference, if any, between (x) the sum of the fair market values of the Telix Ordinary Shares received and the CVRs received by a U.S. Holder in the Merger as determined for U.S. federal income tax purposes, and (y) such U.S. Holder’s adjusted tax basis in the Common Stock surrendered; | |
| ● | such gain would be long-term capital gain if such U.S. Holder’s holding period for such shares of Common Stock is more than one year as of the Closing Date of the Merger; | |
| ● | if a U.S. Holder acquired different blocks of Common Stock at different times or at different prices, such U.S. Holder must determine its adjusted tax basis and holding period separately with respect to each block of Common Stock; | |
| ● | generally, no loss will be recognized by a U.S. Holder as a result of exchanging its Common Stock for the merger consideration pursuant to the Merger; | |
| ● | the initial tax basis of the Telix Ordinary Shares that a U.S. Holder receives pursuant to the Merger will generally equal the aggregate adjusted tax basis in the shares of the Common Stock surrendered in exchange therefor pursuant to the Merger plus the amount of gain recognized (as described above), minus the fair market value of the CVRs received as determined for U.S. federal income tax purposes; | |
| ● | the holding period of the Telix Ordinary Shares that a U.S. Holder receives pursuant to the Merger will generally include the holding period of the Common Stock surrendered in exchange therefor pursuant to the Merger; and | |
| ● | a U.S. Holder’s initial tax basis in the CVRs received will equal the fair market value of such CVRs as determined for U.S. federal income tax purposes, and the holding period for such CVRs will begin on the day following the Closing Date. |
| 121 |
If the Merger qualifies as a “reorganization” within the meaning of Section 368(a) of the Code, but Section 367(a)(1) of the Code were to apply to the Merger, a U.S. Holder would recognize gain (but not loss) in an amount equal to the excess, if any, of the fair market value as of the Closing Date of the Telix Ordinary Shares received in the Mergers, plus the fair market value of the CVRs received in the Merger, over such U.S. Holder’s tax basis in the shares of Common Stock surrendered in the Merger. Any such gain would generally be long-term capital gain if such U.S. Holder’s holding period for such shares of Common Stock is more than one year as of the Closing Date of the Merger.
If the Merger Does Not Qualify as a “Reorganization” Within the Meaning of Section 368(a) of the Code
If the Merger does not qualify as a “reorganization” within the meaning of Section 368(a) of the Code, and the transaction is treated as a “closed transaction” rather than an “open transaction” for U.S. federal income tax purposes (as described below), then, for U.S. federal income tax purposes, a U.S. Holder who receives the merger consideration pursuant to the Merger would generally recognize capital gain or loss equal to the difference, if any, between (i) the sum of the fair market values of the Telix Ordinary Shares and the CVRs, as determined for U.S. federal income tax purposes, and (ii) such holder’s adjusted tax basis in the Common Stock surrendered. Such gain or loss generally will be long-term capital gain or loss provided the U.S. Holder’s holding period for the Common Stock surrendered in the Merger exceeds one year as of the Closing Date.
A U.S. Holder’s initial tax basis in the Telix Ordinary Shares received in the Merger will equal the fair market value of such stock upon receipt, and the holding period for such stock will begin on the day following the Closing Date. A U.S. Holder’s initial tax basis in the CVRs received in the Merger will equal the fair market value of such CVRs as determined for U.S. federal income tax purposes, and the holding period for such CVRs will begin on the day following the Closing Date.
Long-term capital gain of certain non-corporate U.S. Holders (including individuals) is currently eligible for U.S. federal income taxation at preferential rates at a maximum rate of 20%. The deductibility of capital losses is subject to limitations under the Code. U.S. Holders that realize a loss should consult their tax advisors regarding the allowance of this loss.
Tax Treatment of the CVRs
The amount of gain or loss a U.S. Holder recognizes in the Merger, and the timing and potentially the character of a portion of such gain or loss, depends in part on the U.S. federal income tax treatment of the CVRs, with respect to which there is uncertainty.
Receipt of the CVRs and Payments Thereunder
The receipt of the CVRs may be treated as a “closed transaction” or an “open transaction” for U.S. federal income tax purposes. There is no legal authority directly on point addressing whether contingent value rights with characteristics similar to the CVRs should be taxed as an “open transaction” or “closed transaction,” and such question is inherently factual in nature. Pursuant to U.S. Treasury Regulations dealing with contingent payment obligations analogous to the CVRs, if the fair market value of the CVRs is “reasonably ascertainable,” a U.S. Holder should treat the transaction as a “closed transaction.” On the other hand, if the fair market value of the CVRs cannot be reasonably ascertained, a U.S. Holder should treat the transaction as an “open transaction.” These Treasury Regulations state that only in “rare and extraordinary” cases would the value of contingent payment obligations not be reasonably ascertainable. The following sections discuss the possible consequences if the receipt of CVRs pursuant to the Merger is treated as an “open transaction” or a “closed transaction” for U.S. federal income tax purposes. U.S. Holders are urged to consult their tax advisors with respect to the proper characterization of the receipt of the CVRs.
| 122 |
It is possible that either Telix or QSAM may be required to take a position for income tax, withholding, and/or information reporting purposes that the Merger, including the receipt of the CVRs, is either a “closed transaction” or an “open transaction.” Each U.S. Holder is urged to consult its tax advisor regarding the impact, if any, of the position that may be taken by Telix or QSAM on such U.S. Holder’s characterization of the Merger.
Closed Transaction Treatment
If the value of the CVRs can be “reasonably ascertained,” the receipt of the CVRs in the Merger should be treated as a “closed transaction” for U.S. federal income tax purposes and a U.S. Holder would recognize gain (but not loss) upon the receipt of the CVRs taking into account the fair market value of the CVRs, determined on the date of the receipt of the CVRs. The proper method to determine the fair market value of a CVR for U.S. federal income tax purposes is not clear, but it is possible that the trading value of the Common Stock would be considered along with other factors in making that determination. Telix and its affiliates and QSAM do not intend to obtain or report any valuation of the CVRs that may be used by U.S. Holders for this purpose. If the receipt of the CVRs is a “closed transaction” for U.S. federal income tax purposes, a U.S. Holder’s initial tax basis in the CVRs will equal the fair market value of the CVRs on the date of the receipt of the CVRs. The holding period of the CVRs will begin on the day following the date of the receipt of the CVRs.
There is no legal authority directly addressing the U.S. federal income tax treatment of payments that may be received pursuant to the CVRs if the receipt of the CVRs is a “closed transaction” for U.S. federal income tax purposes. Accordingly, the amount, timing, and character of any gains, income or loss with respect to the CVRs are uncertain. For example, payments received with respect to a CVR may be treated, in whole or in part, as a non-taxable return of a CVR holder’s adjusted tax basis in the CVR.
To the extent that payments received with respect to a CVR by a U.S. Holder are not treated as a return of basis or if such payments exceed such basis, they could be treated as (1) capital gain (which would be long-term capital gain if, as of the date of such payment, the U.S. Holder’s holding period for the CVR exceeds one year), (2) income taxable at ordinary rates, or (3) dividends.
In the event a payment gives rise to capital gain, a portion of any payment due more than six months following the consummation of the Merger with respect to a CVR may constitute imputed interest taxable as ordinary income under Section 483 of the Code as described below under the heading “Open Transaction Treatment.” There is no legal authority directly addressing the U.S. federal income tax treatment of the expiration of any rights to receive a payment with respect to the CVRs. Accordingly, a CVR holder may not be able to recognize a loss with respect to the expiration of a right to receive a payment under the CVR until the holder’s right to receive all CVR payments terminates.
It is possible, although neither Telix nor QSAM expect it to be the case, that the CVRs could be treated as one or more “debt instruments” for U.S. federal income tax purposes. If that is the case, then payments received with respect to the CVRs generally will be treated as payments in retirement of a “debt instrument,” except to the extent interest is imputed under the rules of Sections 1274 and 1275 of the Code. If those rules were to apply, interest generally would be imputed under complex rules at a rate that corresponds to Telix’s borrowing rate for similar instruments. In such case, a U.S. Holder would generally be required to include the interest in income on an annual basis, whether or not currently paid.
Open Transaction Treatment
The receipt of the CVRs would generally be treated as part of an “open transaction” if the value of the CVRs cannot be “reasonably ascertained.” If the receipt of CVRs were treated as an “open transaction” for U.S. federal income tax purposes, a U.S. Holder would not immediately take the CVRs into account in determining its capital gain on the receipt of CVRs upon consummation of the Merger, and a U.S. Holder would take no tax basis in the CVRs. Rather, subject to the imputed interest rules under Section 483 of the Code as discussed below, the U.S. Holder would recognize gain as payments with respect to the CVRs that are received or deemed received in accordance with the U.S. Holder’s regular method of accounting. Whether any tax basis in the Common Stock surrendered in the Merger can be allocated to the CVR payments will depend on several factors including whether the Merger qualifies as a reorganization, the fair market value of the Telix Ordinary Shares received, and whether such shares have been sold. Each U.S. Holder is urged to consult its tax advisor regarding the tax consequences of the receipt of the CVRs being treated as part of an “open transaction.”
| 123 |
If the transaction is treated as an “open transaction” for U.S. federal income tax purposes, a portion of any payment with respect to a CVR due more than six months following the consummation of the Merger may constitute imputed interest taxable as ordinary income under Section 483 of the Code. The portion of any CVR payment treated as imputed interest under Section 483 of the Code generally would equal the excess of the amount of the CVR payment over the present value of such amount as of the closing date calculated using the applicable federal rate as the discount rate. A CVR holder must include in its taxable income interest imputed pursuant to Section 483 of the Code using such holder’s regular method of accounting.
Reporting Requirements
Each U.S. Holder who receives Telix Ordinary Shares in the Merger is required to retain permanent records pertaining to the Merger and make such records available to any authorized IRS officers and employees. Such records should specifically include information regarding the amount, basis, and fair market value of the Common Stock exchanged and the amount of Telix Ordinary Shares and CVRs received in exchange therefor. U.S. Holders who owned immediately before the Merger at least five percent (by vote or value) of the total outstanding stock of QSAM are required to attach a statement to their tax returns for the year in which the Merger is consummated that contains the information listed in Treasury Regulation Section 1.368-3(b). Such statement must include the U.S. Holder’s tax basis in such holder’s Common Stock surrendered in the Merger, the fair market value of such stock, the date of the Merger and the name and employer identification number of each of QSAM and Telix. U.S. Holders are urged to consult with their tax advisors to comply with these rules.
holders of Common Stock are urged to consult their tax advisers regarding the tax CONSEQUENCES OF THE MERGER, INCLUDING THE treatment of the issuance of the CVRs and any future payments under the CVRS.
Treatment of QSAM Stockholders Who Exercise Statutory Appraisal Rights
The discussion above does not apply to U.S. Holders who properly perfect statutory appraisal rights with respect to their shares of Common Stock.
Generally, a U.S. Holder who perfects appraisal rights and receives cash in exchange for such U.S. Holder’s Common Stock will recognize capital gain or loss measured by the difference between the amount of cash received and such U.S. Holder’s adjusted tax basis in such shares. Such gain or loss will generally be long-term capital gain or loss if the U.S. Holder’s holding period for such shares of Common Stock exceeds one year at the time of payment. The deductibility of capital losses is subject to limitations. If a U.S. Holder acquired different blocks of Common Stock at different times or at different prices, such U.S. Holder must determine gain or loss separately for each such block of stock for which statutory appraisal right have been perfected.
Tax Considerations Related to Ownership and Disposition of Telix Ordinary Shares
Distributions
Telix does not currently anticipate paying any distributions on the Telix Ordinary Shares in the foreseeable future. However, to the extent there are any distributions made with respect to the Telix Ordinary Shares, and subject to the passive foreign investment company, or PFIC, rules discussed below, the gross amount of any such distributions made out of Telix’s current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) will generally be taxable to a U.S. Holder as ordinary dividend income on the date such distribution is actually or constructively received. Distributions in excess of Telix’s current and accumulated earnings and profits, as so determined, will be treated first as a tax-free return of capital to the extent of the U.S. Holder’s adjusted tax basis in the Telix Ordinary Shares and thereafter as capital gain. However, because Telix does not intend to calculate its earnings and profits under U.S. federal income tax principles, it is expected, and U.S. Holders should assume, that any distribution will be reported as a dividend and will constitute ordinary dividend income to a U.S. Holder. Any dividends will generally be treated as foreign-source and will not be eligible for the dividends-received deduction generally allowed to corporate U.S. Holders.
| 124 |
Subject to the discussion under “—Passive Foreign Investment Company Considerations” below, dividends paid to non-corporate U.S. Holders may qualify as “qualified dividend income” eligible for the preferential rates of taxation applicable to long-term capital gains if Telix is a “qualified foreign corporation” and certain other requirements (discussed below) are met. Telix generally will be considered to be a qualified foreign corporation (a) if Telix is eligible for the benefits of the Convention between the Government of the United States of America and the Government of Australia for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, signed on August 6, 1982, as amended and currently in force (the “U.S.-Australia Tax Treaty”), or (b) the Telix Ordinary Shares are readily tradable on an established securities market in the United States. It is not anticipated that the Telix Ordinary Shares will be readily tradable on an established securities market in the United States. However, Telix believes that it qualifies as a resident of Australia for purposes of, and is eligible for the benefits of, the U.S.-Australia Tax Treaty, although there can be no assurance in this regard. Therefore, subject to the discussion under “—Passive Foreign Investment Company Considerations” below, it is anticipated that any dividends on the Telix Ordinary Shares generally will be “qualified dividend income” in the hands of individual U.S. Holders, provided that a holding period requirement (more than 60 days of ownership, without protection from the risk of loss, during the 121-day period beginning 60 days before the ex-dividend date) and certain other requirements are met.
Distributions paid in Australian dollars, including any Australian taxes withheld, will be included in a U.S. Holder’s gross income in a U.S. dollar amount calculated by reference to the spot exchange rate in effect on the date of actual or constructive receipt, regardless of whether the Australian dollars are converted into U.S. dollars at that time. A U.S. Holder will have a tax basis in the Australian dollars equal to their U.S. dollar value on the date of receipt. As a result, if a U.S. Holder converts the Australian dollars into U.S. dollars on the date of receipt, such U.S. Holder generally should not be required to recognize any foreign exchange gain or loss. If Australian dollars so received are not converted into U.S. dollars on the date of receipt, any gain or loss on a subsequent conversion or other disposition of the Australian dollars generally will be treated as ordinary income or loss and generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Subject to certain limitations, a U.S. Holder may be able to claim as a credit against its U.S. federal income tax liability the amount of any Australian tax withheld from any dividends at a rate not exceeding an applicable rate under the U.S.-Australia Tax Treaty. Alternatively, a U.S. Holder may be able to deduct such Australian taxes from its U.S. federal taxable income, provided that the U.S. Holder elects to deduct rather than credit all foreign income taxes paid or accrued for the relevant taxable year. The rules governing U.S. foreign tax credits are complex and latest U.S. Treasury Regulations may further restrict the availability of any such credit based on the nature of the withholding tax imposed by the foreign jurisdiction. Each U.S. Holder should consult its tax advisors regarding the foreign tax credit rules, including regarding the availability of such credit or deductions.
| 125 |
Sale, Exchange or Other Disposition of Ordinary Shares
A U.S. Holder generally will recognize gain or loss for U.S. federal income tax purposes upon the sale or other taxable disposition of the Telix Ordinary Shares in an amount equal to the difference between the U.S. dollar value of the amount realized from such disposition and the U.S. Holder’s adjusted tax basis in the Telix Ordinary Shares, determined in U.S. dollars. Subject to the discussion under “—Passive Foreign Investment Company Considerations” below, any such gain or loss generally will be a capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder’s holding period for the Telix Ordinary Shares is more than one year at the time of such disposition. A U.S. Holder’s adjusted tax basis in the Telix Ordinary Shares generally will be equal to the amount paid for such Telix Ordinary Shares. Any long-term capital gain from the disposition of the Telix Ordinary Shares by a non-corporate U.S. Holder generally is eligible for a preferential rate of taxation. The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations. Any such gain or loss that a U.S. Holder recognizes generally will be treated as U.S.-source gain or loss for foreign tax credit limitation purposes. U.S. Holders should consult their tax advisors regarding the tax consequences if Australian taxes are imposed on or in connection with a sale, exchange or other disposition of the Telix Ordinary Shares and their ability to credit any Australian tax against their U.S. federal income tax liability.
In the case of a U.S. Holder that is a cash basis taxpayer, any units of foreign currency received on a disposition of Telix Ordinary Shares are translated into U.S. dollars at the spot exchange rate on the settlement date of the disposition if the Telix Ordinary Shares disposed of are treated as traded on an established securities market. In such case, no foreign currency exchange gain or loss will result for a cash basis taxpayer from currency fluctuations between the trade date and the settlement date of such a disposition. An accrual basis taxpayer may elect the same treatment required of cash basis taxpayers with respect to dispositions of Telix Ordinary Shares that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. If an accrual basis taxpayer does not make such election, or if the Telix Ordinary Shares are not treated as traded on an established securities market, any units of foreign currency received on a disposition of the Telix Ordinary Shares are translated into U.S. dollars at the spot exchange rate on the trade date of the disposition. In such case, the taxpayer may recognize exchange gain or loss based on currency fluctuations between the trade date and the settlement date. Any foreign currency gain or loss a U.S. Holder recognizes will be U.S.-source ordinary income or loss.
Passive Foreign Investment Company Considerations
If Telix is classified as a PFIC in any taxable year, certain adverse tax consequences could apply to U.S. Holders as a result of that classification. Telix generally will be classified as a PFIC for any taxable year if (i) at least 75% of its gross income for the taxable year consists of certain types of passive income, or (ii) at least 50% of its gross assets during the taxable year, based on a quarterly average and generally determined by value, produce or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions and gains from the disposition of assets that produce or are held for the production of passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. In determining whether Telix is a PFIC, Telix will be treated as owning its proportionate share of the assets and earning its proportionate share of the income of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value).
Based on the expected nature and amount of Telix’s estimated gross income, the anticipated nature and estimated average value of its gross assets, the anticipated cash needs of the group’s operations and the nature and extent of the active businesses conducted by Telix’s “25% or greater” owned subsidiaries, Telix does not expect that it will be classified as a PFIC in the current taxable year or for the foreseeable future. However, Telix’s PFIC status for any taxable year will not be determinable until after the end of the taxable year, and will depend on, among other things, the composition of Telix’s income and assets (which could change significantly during the course of a taxable year) and the market value of Telix’s assets for such taxable year, which may be, in part, based on the market price of the Telix Ordinary Shares (which could be volatile). Accordingly, there can be no assurance that Telix will not be a PFIC for the current or any future taxable year. U.S. Holders should consult their own tax advisors regarding Telix’s PFIC status.
| 126 |
If Telix is a PFIC for any taxable year during which a U.S. Holder holds Telix Ordinary Shares, absent certain elections (including the mark-to-market election or qualified electing fund election described below), such U.S. Holder generally will be subject to adverse rules (regardless of whether Telix continues to be classified as a PFIC) with respect to (i) any “excess distribution” that Telix makes to such U.S. Holder (generally, any distributions on the Telix Ordinary Shares in a taxable year that are greater than 125% of the average annual distributions received by such U.S. Holder in the three preceding taxable years or, if shorter, the U.S. Holder’s holding period) and (ii) any gain recognized from a sale or other disposition (including a pledge) of the Telix Ordinary Shares. Under these special tax rules:
| ● | the excess distribution or gain will be allocated ratably over the U.S. Holder’s holding period for the Telix Ordinary Shares; | |
| ● | the amount allocated to the current taxable year and any taxable year prior to the first taxable year in which Telix was classified as a PFIC will be treated as ordinary income arising in the current taxable year (and would not be subject to the interest charge discussed below); and | |
| ● | the amount allocated to each other taxable year will be subject to income tax at the highest marginal tax rate in effect for individuals or corporations, as applicable, for such year, and the interest charge generally applicable to underpayments of tax will be imposed with respect to the resulting tax attributable to each such year. |
In addition, non-corporate U.S. Holders will not be eligible for reduced rates of taxation applicable to “qualified dividend income” on any dividends that Telix pays if it is a PFIC for either the taxable year in which the dividend is paid or the preceding year.
If Telix is classified as a PFIC in any taxable year with respect to which a U.S. Holder owns Telix Ordinary Shares, Telix generally will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding taxable years, regardless of whether Telix continues to be classified as a PFIC under the tests described above, unless Telix ceases to be classified as a PFIC and such U.S. Holder makes a “deemed sale” election. If Telix ceases to be classified as a PFIC and a U.S. Holder makes the “deemed sale” election, such U.S. Holder will be deemed to have sold Telix Ordinary Shares at their fair market value on the last day of the last taxable year in which Telix was classified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime described above. After the “deemed sale” election, a U.S. Holder’s Telix Ordinary Shares would not be treated as shares of a PFIC unless Telix subsequently becomes a PFIC.
If Telix is a PFIC for any taxable year during which a U.S. Holder holds Telix Ordinary Shares, and one of Telix’s non-U.S. subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions.
If a U.S. Holder owns Telix Ordinary Shares during any taxable year in which Telix is a PFIC, such U.S. Holder generally will be required to file an IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to Telix, generally with its U.S. federal income tax return for that year. U.S. Holders should consult their tax advisors regarding any annual filing requirements.
If Telix is a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on Telix Ordinary Shares if a valid “mark-to-market” election is made by the U.S. Holder for the Telix Ordinary Shares, provided that Telix Ordinary Shares held by such U.S. Holder are “marketable.”
| 127 |
If a U.S. Holder makes a mark-to-market election, it must include in gross income, as ordinary income, for each taxable year that Telix is a PFIC an amount equal to the excess, if any, of the fair market value of the Telix Ordinary Shares that are “marketable stock” at the close of the taxable year over such U.S. Holder’s adjusted tax basis in such Telix Ordinary Shares. If a U.S. Holder makes such election, it may also claim a deduction as an ordinary loss in each such year for the excess, if any, of such U.S. Holder’s adjusted tax basis in such Telix Ordinary Shares over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. The U.S. Holder’s adjusted tax basis in the Telix Ordinary Shares with respect to which the mark-to-market election applies would be adjusted to reflect amounts included in gross income or allowed as a deduction because of such election. If a U.S. Holder makes an effective mark-to-market election, any gain recognized upon the sale or other disposition of the Telix Ordinary Shares in a year that Telix is a PFIC will be treated as ordinary income and any loss will be treated first as ordinary loss (to the extent of any net mark-to-market gains for prior years) and thereafter as capital loss. However, a mark-to-market election will generally not be available with respect to a lower-tier PFIC unless the shares of such lower-tier PFIC are themselves treated as “marketable stock.”
If a U.S. Holder makes a mark-to-market election, it will be effective for the taxable year for which the election is made and all subsequent taxable years unless the Telix Ordinary Shares are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. U.S. Holders are urged to consult their tax advisors about the availability of the mark-to-market election.
Alternatively, in certain cases, a U.S. Holder may be able to avoid the interest charge and the other adverse PFIC tax consequences described above by electing to treat the PFIC as a “qualified electing fund”, or QEF, under Section 1295 of the Code. If a U.S. Holder makes a valid and timely QEF election and Telix provides certain required information to such U.S. Holder, then for each taxable year to which such an election applies, the U.S. Holder will be subject to U.S. federal income tax on its pro rata share of Telix’s net capital gain and ordinary earnings, regardless of whether such amounts are actually distributed to the U.S. Holder in that year or any later year. However, Telix does not anticipate that this election will be available to U.S. Holders because Telix does not expect to provide U.S. Holders with the information that would be necessary to make a valid QEF election.
Backup Withholding Tax and Information Reporting Requirements
U.S. Holders generally will be subject to information reporting requirements with respect to distributions paid on the Telix Ordinary Shares, and on the proceeds from the sale, exchange or other disposition of the Telix Ordinary Shares that are paid within the United States or through U.S.-related financial intermediaries, unless the U.S. Holder is an “exempt recipient.” In addition, U.S. Holders may be subject to backup withholding on such payments, unless the U.S. Holder provides a correct taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Certain U.S. Holders are required to report information relating to an interest in the Telix Ordinary Shares, subject to certain exceptions (including an exception for Telix Ordinary Shares held in accounts maintained by U.S. financial institutions) by filing IRS Form 8938 (Statement of Specified Foreign Financial Assets) with their U.S. federal income tax return. Substantial penalties may be imposed upon a U.S. Holder that fails to comply. U.S. Holders should consult their tax advisors regarding their information reporting obligations, if any, with respect to their ownership and disposition of the Telix Ordinary Shares.
THE FOREGOING IS INTENDED ONLY AS A SUMMARY OF CERTAIN FEDERAL INCOME TAX CONSEQUENCES OF THE MERGER AND THE OWNERSHIP AND DISPOSITION OF THE TELIX ORDINARY SHARES AND DOES NOT CONSTITUTE A TAX OPINION OR TAX ADVICE. NEITHER QSAM NOR TELIX has sought, and NEITHER will seek, any opinion of counsel or any ruling from the IRS with respect to the matters discussed herein. QSAM urges U.S. holders to consult with their tax advisers with respect to the specific tax consequences to them OF the Merger AND THE OWNERSHIP AND DISPOSITION OF THE TELIX ORDINARY SHARES in light of their own particular circumstances, including the tax consequences under state, local, non-U.S. and other tax laws.
| 128 |
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other documents with the SEC. These reports contain additional information about QSAM. Stockholders may read and copy any reports, statements or other information filed by QSAM at the SEC’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. You may also obtain copies of this information by mail from the Public Reference Section of the SEC, 100 F Street, N.E., Washington, D.C. 20549, at prescribed rates. Please call the SEC at (800) SEC-0330 for further information on the operation of the Public Reference Room. QSAM’s SEC filings are made electronically available to the public at the SEC’s website located at https://www.sec.gov/edgar/search-and-access. Stockholders can also obtain free copies of our SEC filings through the “Investors” section of QSAM’s website at https://www.ir.qsambio.com/. Our website address is being provided as an inactive textual reference only. The information provided on, or accessible through, our website, other than the copies of the documents listed or referenced below that have been or will be filed with the SEC, is not part of this information statement, and therefore is not incorporated herein by reference.
Stockholders should not rely on information that purports to be made by or on behalf of QSAM other than that contained in this Information Statement. QSAM has not authorized anyone to provide information on behalf of QSAM that is different from that contained in this Information Statement. This Information Statement is dated [●], 2024. No assumption should be made that the information contained in this Information Statement is accurate as of any date other than that date, and the mailing of this Information Statement will not create any implication to the contrary.
HOUSEHOLDING
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for information statements with respect to two or more stockholders sharing the same address by delivering a single information statement addressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
A number of brokers with account holders who are our stockholders may be “householding” our Information Statement. If householding is in effect, a single Information Statement will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate copy of the Information Statement, please notify your broker or the Company at the following address. Stockholders who currently receive multiple copies of the Information Statement at their address and would like to request “householding” of their communications should contact their broker or the Company at the following address. We will deliver a separate Information Statement promptly upon written or oral request from a stockholder. Please direct any such requests to QSAM Investor Relations, 9442 Capital of Texas Hwy N., Plaza 1, Suite 500, Austin, Texas 78759, or call Investor Relations at (512) 343-4558.
| 129 |
INDEX TO TELIX CONSOLIDATED FINANCIAL STATEMENTS
| F-1 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Telix Pharmaceuticals Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Telix Pharmaceuticals Limited and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of comprehensive income or loss, changes in equity and cash flows for each of the two years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ PricewaterhouseCoopers | |
| Melbourne, Australia | |
| March 14, 2024 |
We have served as the Company’s auditor since 2017.
| F-2 |
Consolidated statement of comprehensive income or loss for the years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||
| Note | A$’000 | A$’000 | ||||||||||
| Continuing operations | ||||||||||||
| Revenue from contracts with customers | 4 | 502,547 | 160,096 | |||||||||
| Cost of sales | (188,157 | ) | (65,170 | ) | ||||||||
| Gross profit | 314,390 | 94,926 | ||||||||||
| Research and development costs | (128,844 | ) | (81,008 | ) | ||||||||
| Selling and marketing expenses | (54,867 | ) | (37,970 | ) | ||||||||
| General and administration costs | (78,985 | ) | (49,128 | ) | ||||||||
| Other losses (net) | 5 | (35,854 | ) | (18,750 | ) | |||||||
| Operating profit/(loss) | 15,840 | (91,930 | ) | |||||||||
| Finance income | 1,019 | 1 | ||||||||||
| Finance costs | 6 | (13,772 | ) | (6,693 | ) | |||||||
| Profit/(loss) before income tax | 3,087 | (98,622 | ) | |||||||||
| Income tax benefit/(expense) | 7 | 2,124 | (5,457 | ) | ||||||||
| Profit/(loss) for the year | 5,211 | (104,079 | ) | |||||||||
| Profit/(loss) for the year attributable to: | ||||||||||||
| Owners of Telix Pharmaceuticals Limited | 5,211 | (104,079 | ) | |||||||||
| Other comprehensive (loss)/income: | ||||||||||||
| Items that will not be reclassified to profit or loss in subsequent periods: | ||||||||||||
| Changes in the fair value of equity investments at fair value through other comprehensive income | 14 | (895 | ) | — | ||||||||
| Items to be reclassified to profit or loss in subsequent periods: | ||||||||||||
| Exchange differences on translation of foreign operations | (4,852 | ) | 591 | |||||||||
| Total comprehensive loss for the year | (536 | ) | (103,488 | ) | ||||||||
| Total comprehensive loss for the year attributable to: | ||||||||||||
| Owners of Telix Pharmaceuticals Limited | (536 | ) | (103,488 | ) | ||||||||
| 2023 | 2022 | |||||||||||
| Note | Cents | Cents | ||||||||||
| Basic earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company | 8.1 | 1.63 | (33.50 | ) | ||||||||
| Diluted earnings/(loss) per share from continuing operations after income tax attributable to the ordinary equity holders of the Company | 8.2 | 1.61 | (33.50 | ) | ||||||||
The above consolidated statement of comprehensive income or loss should be read in conjunction with the accompanying notes.
| F-3 |
Consolidated statement of financial position as at December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||
| Note | A$’000 | A$’000 | ||||||||||
| Current assets | ||||||||||||
| Cash and cash equivalents | 123,237 | 116,329 | ||||||||||
| Trade and other receivables | 11 | 64,777 | 39,354 | |||||||||
| Inventories | 12 | 17,310 | 8,477 | |||||||||
| Other current assets | 13 | 19,524 | 9,073 | |||||||||
| Total current assets | 224,848 | 173,233 | ||||||||||
| Non-current assets | ||||||||||||
| Trade and other receivables | 11 | 586 | 327 | |||||||||
| Financial assets | 14 | 12,260 | — | |||||||||
| Deferred tax assets | 15.1 | 20,452 | 3,971 | |||||||||
| Property, plant and equipment | 16 | 23,170 | 12,032 | |||||||||
| Right-of-use assets | 17 | 7,323 | 6,806 | |||||||||
| Intangible assets | 18 | 109,663 | 58,984 | |||||||||
| Total non-current assets | 173,454 | 82,120 | ||||||||||
| Total assets | 398,302 | 255,353 | ||||||||||
| Current liabilities | ||||||||||||
| Trade and other payables | 20 | 81,704 | 49,519 | |||||||||
| Borrowings | 21 | 964 | — | |||||||||
| Current tax payable | 11,508 | 7,320 | ||||||||||
| Contract liabilities | 22 | 10,995 | 4,940 | |||||||||
| Lease liabilities | 23 | 595 | 641 | |||||||||
| Provisions | 24 | 577 | 402 | |||||||||
| Contingent consideration | 25 | 37,153 | 15,183 | |||||||||
| Employee benefit obligations | 26 | 13,912 | 7,551 | |||||||||
| Total current liabilities | 157,408 | 85,556 | ||||||||||
| Non-current liabilities | ||||||||||||
| Borrowings | 21 | 8,209 | 3,312 | |||||||||
| Contract liabilities | 22 | 12,162 | 22,522 | |||||||||
| Lease liabilities | 23 | 7,677 | 6,493 | |||||||||
| Provisions | 24 | 8,004 | 7,482 | |||||||||
| Contingent consideration | 25 | 55,601 | 49,766 | |||||||||
| Employee benefit obligations | 26 | 330 | 215 | |||||||||
| Total non-current liabilities | 91,983 | 89,790 | ||||||||||
| Total liabilities | 249,391 | 175,346 | ||||||||||
| Net assets | 148,911 | 80,007 | ||||||||||
| Equity | ||||||||||||
| Share capital | 27.1 | 446,268 | 370,972 | |||||||||
| Share capital reserve | 27.2 | (62,829 | ) | (26,909 | ) | |||||||
| Foreign currency translation reserve | (5,414 | ) | (562 | ) | ||||||||
| Share-based payments reserve | 27.3 | 35,446 | 9,321 | |||||||||
| Financial assets at FVOCI reserve | 27.4 | (895 | ) | — | ||||||||
| Accumulated losses | (263,665 | ) | (272,815 | ) | ||||||||
| Total equity | 148,911 | 80,007 | ||||||||||
The above consolidated statement of financial position should be read in conjunction with the accompanying notes.
| F-4 |
Consolidated statement of changes in equity for the years ended December 31, 2023 and 2022
Share capital | Share capital reserve | Foreign currency translation reserve | Share-based payments reserve | Financial assets at FVOCI reserve | Accumulated losses | Total equity | ||||||||||||||||||||||||||
| Note | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||||||||||||||
| Balance as at January 1, 2023 | 370,972 | (26,909 | ) | (562 | ) | 9,321 | — | (272,815 | ) | 80,007 | ||||||||||||||||||||||
| Profit for the year | — | — | — | — | — | 5,211 | 5,211 | |||||||||||||||||||||||||
| Other comprehensive loss | — | — | (4,852 | ) | — | (895 | ) | — | (5,747 | ) | ||||||||||||||||||||||
| Total comprehensive income/(loss) | — | — | (4,852 | ) | — | (895 | ) | 5,211 | (536 | ) | ||||||||||||||||||||||
| Issue of shares on acquisitions | 27.1 | 32,724 | — | — | — | — | — | 32,724 | ||||||||||||||||||||||||
| Issue of shares on exercise of options | 27.1, 27.2 | 42,572 | (35,920 | ) | — | — | — | — | 6,652 | |||||||||||||||||||||||
| Share based payments | 27.3 | — | — | — | 8,786 | — | — | 8,786 | ||||||||||||||||||||||||
| Share based payments associated with acquisitions | 27.3 | — | — | — | 21,278 | — | — | 21,278 | ||||||||||||||||||||||||
| Transfer on exercise of options | 27.3 | — | — | — | (3,939 | ) | — | 3,939 | — | |||||||||||||||||||||||
| 75,296 | (35,920 | ) | — | 26,125 | — | 3,939 | 69,440 | |||||||||||||||||||||||||
| Balance as at December 31, 2023 | 446,268 | (62,829 | ) | (5,414 | ) | 35,446 | (895 | ) | (263,665 | ) | 148,911 | |||||||||||||||||||||
| Balance as at January 1, 2022 | 170,840 | — | (1,153 | ) | 5,942 | — | (173,471 | ) | 2,158 | |||||||||||||||||||||||
| Loss for the year | — | — | — | — | (104,079 | ) | (104,079 | ) | ||||||||||||||||||||||||
| Other comprehensive income | — | — | 591 | — | — | 591 | ||||||||||||||||||||||||||
| Total comprehensive loss | — | — | 591 | — | — | (104,079 | ) | (103,488 | ) | |||||||||||||||||||||||
| Contributions of equity | 27.1 | 175,000 | — | — | — | — | 175,000 | |||||||||||||||||||||||||
| Transaction costs arising on new share issues | (7,816 | ) | — | — | — | — | (7,816 | ) | ||||||||||||||||||||||||
| Issue of shares on exercise of options | 27.1, 27.2 | 32,948 | (26,909 | ) | — | — | — | 6,039 | ||||||||||||||||||||||||
| Share based payments | 27.3 | — | — | — | 8,114 | — | 8,114 | |||||||||||||||||||||||||
| Transfer on exercise of options | 27.3 | — | — | — | (4,735 | ) | 4,735 | — | ||||||||||||||||||||||||
| 200,132 | (26,909 | ) | — | 3,379 | — | 4,735 | 181,337 | |||||||||||||||||||||||||
| Balance as at December 31, 2022 | 370,972 | (26,909 | ) | (562 | ) | 9,321 | — | (272,815 | ) | 80,007 | ||||||||||||||||||||||
The above consolidated statement of changes of equity should be read in conjunction with the accompanying notes.
| F-5 |
Consolidated statement of cash flows for the years ended December 31, 2023 and 2022
| 2023 | 2022 | |||||||||||
| Note | A$’000 | A$’000 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Receipts from customers | 463,654 | 124,095 | ||||||||||
| Receipts in relation to R&D tax incentive | — | 18,909 | ||||||||||
| Payments to suppliers and employees | (414,079 | ) | (204,289 | ) | ||||||||
| Payments for contingent consideration | (16,282 | ) | — | |||||||||
| Income taxes paid | (10,253 | ) | (2,278 | ) | ||||||||
| Interest received | 1,629 | 1 | ||||||||||
| Interest paid | (785 | ) | (408 | ) | ||||||||
| Net cash generated from/(used in) operating activities | 29.1 | 23,884 | (63,970 | ) | ||||||||
| Cash flows from investing activities | ||||||||||||
| Payments for investments in financial assets | (13,155 | ) | — | |||||||||
| Payments for acquisition of subsidiary, net of cash acquired | — | (973 | ) | |||||||||
| Purchases of intangible assets | (1,115 | ) | (6,823 | ) | ||||||||
| Payments for contingent consideration | (1,484 | ) | — | |||||||||
| Purchases of property, plant and equipment | (9,679 | ) | (7,038 | ) | ||||||||
| Payments for decommissioning liability | (56 | ) | (2,163 | ) | ||||||||
| Net cash used in investing activities | (25,489 | ) | (16,997 | ) | ||||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from borrowings | 5,756 | 3,014 | ||||||||||
| Repayment of borrowings | — | (13 | ) | |||||||||
| Principal element of lease payments | (2,222 | ) | (1,264 | ) | ||||||||
| Proceeds from issue of shares and other equity | 6,652 | 181,039 | ||||||||||
| Transaction costs of capital raising | — | (7,816 | ) | |||||||||
| Net cash provided by financing activities | 10,186 | 174,960 | ||||||||||
| Net increase in cash held | 8,581 | 93,993 | ||||||||||
| Net foreign exchange differences | (1,673 | ) | 299 | |||||||||
| Cash and cash equivalents at the beginning of the financial year | 116,329 | 22,037 | ||||||||||
| Cash and cash equivalents at the end of the financial year | 123,237 | 116,329 | ||||||||||
The above consolidated statement of cash flows should be read in conjunction with the accompanying notes.
| F-6 |
Notes to the Consolidated Financial Statements
| 1. | Corporate information |
Telix Pharmaceuticals Limited (Telix or the Company) is a for profit company incorporated and domiciled in Australia. It is limited by shares that are publicly traded on the Australian Securities Exchange (ASX: TLX). These consolidated financial statements comprise the results of Telix and its subsidiaries (together referred to as the Group). The consolidated financial statements were authorized for issue in accordance with a resolution of the Directors on March 14, 2024.
| 2. | Summary of significant accounting policies |
The significant accounting policies that have been used in the preparation of these financial statements are summarized below.
| 2.1. | Going concern |
For the year ended December 31, 2023, the Group generated a profit of $5,211,000 (2022: loss of $104,079,000) and cash generated from operating activities of $23,884,000 (2022: cash used in operating activities of $63,970,000). As at December 31, 2023 the net assets of the Group were $148,911,000 (2022: $80,007,000), with cash on hand of $123,237,000 (2022: $116,329,000).
Cash on hand and future cash inflows from commercial activities is considered sufficient to meet the Group’s forecast cash outflows in relation to research and development activities currently underway and other committed business activities for at least 12 months from the date of these financial statements.
On this basis, the Directors are satisfied that the Group continues to be a going concern as at the date of these financial statements. Further, the Directors are of the opinion that no asset is likely to be realized for an amount less than the amount at which it is recorded in the consolidated statement of financial position as at December 31, 2023.
As such, no adjustment has been made to the financial statements relating to the recoverability and classification of the asset carrying amounts or the classification of liabilities that might be necessary should the Group not continue as a going concern.
| 2.2. | Basis of preparation |
Telix Pharmaceuticals Limited is a for-profit entity for the purpose of preparing the financial statements.
These general purpose financial statements have been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (IFRS).
The financial statements have been prepared on a historical cost basis, except for certain financial instruments, which have been measured at fair value.
| a. | Comparatives |
Where necessary, comparative information has been re-classified to achieve consistency in disclosure with current financial amounts and other disclosures.
| F-7 |
| b. | New and amended standards adopted by the Group |
The Group has adopted all relevant new and amended standards and interpretations issued by the International Accounting Standards Board which are effective for annual reporting periods beginning on January 1, 2023. The new standards and amendments did not have any impact on the amounts recognized in the current and prior periods.
| c. | New standards and interpretations not yet adopted |
Certain new accounting standards and interpretations have been published that are not mandatory for December 31, 2023 reporting periods and have not been early adopted by the Group. These standards are not expected to have a material impact on the Group in the current or future reporting periods or on foreseeable future transactions.
| 2.3. | Significant changes in the current reporting period |
The Group updated the classification of expenses to make the consolidated statement of comprehensive income more relevant to users of the financial statements, particularly as the Group has moved to commercial operations. This has resulted in the reclassification of some expenses for the year ended December 31, 2022, however has not impacted the reported loss for the year or earnings per share.
From 2023, the Group has determined that a functional presentation of its consolidated statement of comprehensive income or loss is most appropriate. In accordance with IAS 1 Presentation of Financial Statements, within a functional consolidated statement of comprehensive income or loss, costs directly associated with generating revenues are included in cost of sales. Cost of sales includes direct material and labor costs, distribution fees incurred to ensure delivery of the product to the end customer and indirect costs that are directly attributed to generating revenue, such as amortization of intangible assets associated with commercialized products.
| 2.4. | Principles of consolidation |
Subsidiaries are all entities (including structured entities) over which the Group has control. The Group controls an entity when the Group is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Group. If the Group loses control of a subsidiary, the Group derecognizes the assets and liabilities of the former subsidiary from the consolidated statement of financial position and recognizes the gain or loss associated with the loss of control attributable to the former controlling interest.
Intercompany transactions, balances and unrealized gains on transactions between Group companies are eliminated on consolidation. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment of the transferred asset. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
| 2.5. | Foreign currency translation |
a. Functional and presentation currency
Items included in the financial statements of each of the Group’s entities are measured using the currency of the primary economic environment in which the entity operates (the functional currency). The consolidated financial statements are presented in Australian dollars.
| b. | Transactions and balances |
Foreign currency transactions are translated into the functional currency using the exchange rates at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of monetary assets and liabilities denominated in foreign currencies at year end exchange rates are generally recognized in profit or loss. Foreign exchange gains and losses that relate to borrowings are presented in the consolidated statement of comprehensive income or loss, within finance costs. All other foreign exchange gains and losses are presented in the consolidated statement of comprehensive income or loss on a net basis within other income or other expenses.
Non-monetary items that are measured at fair value in a foreign currency are translated using the exchange rates at the date when the fair value was determined. Translation differences on assets and liabilities carried at fair value are reported as part of the fair value gain or loss.
| F-8 |
| c. | Group companies |
The results and financial position of foreign operations (none of which has the currency of a hyperinflationary economy) that have a functional currency different from the presentation currency are translated into the presentation currency as follows:
| ● | assets and liabilities for each consolidated statement of financial position presented are translated at the closing rate at the date of that consolidated statement of financial position |
| ● | income and expenses for each consolidated statement of comprehensive income or loss are translated at actual exchange rates at the dates of the transactions, and |
| ● | all resulting exchange differences are recognized in other comprehensive income. |
On consolidation, exchange differences arising from the translation of any net investment in foreign entities, and of borrowings and other financial instruments designated as hedges of such investments, are recognized in other comprehensive income. When a foreign operation is sold or any borrowings forming part of the net investment are repaid, the associated exchange differences are reclassified to profit or loss, as part of the gain or loss on sale. Goodwill and fair value adjustments arising on the acquisition of a foreign operation are treated as assets and liabilities of the foreign operation and translated at the closing rate.
| 2.6. | Business combinations |
The acquisition method of accounting is used to account for all business combinations, regardless of whether equity instruments or other assets are acquired. The consideration transferred for the acquisition of a subsidiary comprises the:
| ● | fair values of the assets transferred |
| ● | liabilities incurred to the former owners of the acquired business |
| ● | equity interests issued by the Group |
| ● | fair value of any asset or liability resulting from a contingent consideration arrangement, and |
| ● | fair value of any pre-existing equity interest in the subsidiary. |
Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are, with limited exceptions, measured initially at their fair values at the acquisition date. Acquisition-related costs are expensed as incurred. The excess of the consideration transferred, amount of any non-controlling interest in the acquired entity, and acquisition-date fair value of any previous equity interest in the acquired entity over the fair value of the net identifiable assets acquired is recorded as goodwill. If those amounts are less than the fair value of the net identifiable assets of the subsidiary acquired, the difference is recognized directly in profit or loss as a bargain purchase.
| F-9 |
Where settlement of any part of cash consideration is deferred, the amounts payable in the future are discounted to their present value as at the date of exchange. The post-tax discount rate used is the entity’s incremental borrowing rate, being the rate at which a similar borrowing could be obtained from an independent financier under comparable terms and conditions. Contingent consideration is classified either as equity or a financial liability. Amounts classified as a financial liability are subsequently remeasured to fair value with changes in fair value recognized in profit or loss.
The acquisition date carrying value of the Group’s previously held equity interest in the acquiree is remeasured to fair value at the acquisition date. Any gains or losses arising from such remeasurement are recognized in profit or loss. If the initial accounting for a business combination is incomplete by the end of the reporting period in which the combination occurs, the Group reports provisional amounts for the items for which the accounting is incomplete. Those provisional amounts are adjusted during the measurement period (see below), or additional assets or liabilities are recognized, to reflect new information obtained about facts and circumstances that existed as of the acquisition date that, if known, would have affected the amounts recognized as of that date. The measurement period is the period from the date of acquisition to the date the Group obtains complete information about facts and circumstances that existed as of the acquisition date and is subject to a maximum of one year.
| 2.7. | Current and non-current classification |
Assets and liabilities are presented in the consolidated statement of financial position based on current and non- current classification.
An asset is current when it is expected to be realized or intended to be sold or consumed in the Group’s normal operating cycle; it is held primarily for the purpose of trading; it is expected to be realized within 12 months after the reporting period; or the asset is cash or cash equivalent unless restricted from being exchanged or used to settle a liability for at least 12 months after the reporting period. All other assets are classified as non-current.
A liability is current when it is expected to be settled in the Group’s normal operating cycle; it is held primarily for the purpose of trading; it is due to be settled within 12 months after the reporting period; or there is no unconditional right to defer the settlement of the liability for at least 12 months after the reporting period. All other liabilities are classified as non-current. For instances where a liability is based on sales volumes, the payment expected to be realized within 12 months is current based on the underlying estimate of the timing of sales.
Deferred tax assets and liabilities are always classified as non-current.
| 2.8. | Cash and cash equivalents |
For the purpose of presentation in the consolidated statement of cash flows, cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value, and bank overdrafts. Bank overdrafts are shown within borrowings in current liabilities in the consolidated statement of financial position.
| 2.9. | Trade and other receivables |
Trade receivables and other receivables are all classified as financial assets held at amortized cost. Trade receivables are recognized initially at the amount of consideration that is unconditional, unless they contain significant financing components when they are recognized at fair value.
| F-10 |
| a. | Impairment of trade and other receivables |
The collectability of trade and other receivables is reviewed on an ongoing basis. Individual debts which are known to be uncollectible are written off when identified. The Group recognizes an impairment provision based upon anticipated lifetime losses of trade receivables.
The anticipated losses are determined with reference to historical loss experience (when it is available) and are regularly reviewed and updated. They are subsequently measured at amortized cost using the effective interest method, less loss allowance. See note 30.4 for further information about the Group’s accounting for trade receivables and description of the Group’s impairment policies.
| 2.10. | Inventories |
Raw materials and stores, work in progress and finished goods
Raw materials and stores, work in progress and finished goods are stated at the lower of cost and net realizable value. Cost comprises direct materials, direct labor and an appropriate proportion of variable and fixed overhead expenditure, the latter being allocated on the basis of normal operating capacity. Cost includes the reclassification from equity of any gains or losses on qualifying cash flow hedges relating to purchases of raw material but excludes borrowing costs. Costs are assigned to individual items of inventory on the basis of weighted average costs. Costs of purchased inventory are determined after deducting rebates and discounts. Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
| 2.11. | Property, plant and equipment |
All property, plant and equipment is stated at historical cost less accumulated depreciation. Historical cost includes expenditure that is directly attributable to the acquisition of the items. Cost may also include transfer from equity of any gains or losses on qualifying cash flow hedges of foreign currency purchases of property, plant and equipment. Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably. The carrying amount of any component accounted for as a separate asset is derecognized when replaced. All other repairs and maintenance are charged to profit or loss during the reporting period in which they are incurred.
Depreciation is calculated using the straight-line method to allocate the cost, net of the residual values, over the estimated useful lives. The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period. An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
The useful lives of assets are as follows:
| ● | Buildings: 18 years |
| ● | Plant and equipment: 3-5 years |
| ● | Furniture, fittings and equipment: 3-5 years |
| ● | Leased plant and equipment: 3-5 years |
Gains and losses on disposals are determined by comparing proceeds with carrying amount. These are included in profit or loss. When revalued assets are sold, it is Group policy to transfer any amounts included in other reserves in respect of those assets to accumulated losses.
| F-11 |
| 2.12. | Lease liabilities |
Liabilities arising from a lease are initially measured on a present value basis. Lease liabilities include the net present value of the following lease payments:
| ● | fixed payments (including in-substance fixed payments), less any lease incentives receivable |
| ● | variable lease payments that are based on an index or a rate, initially measured using the index or rate as at the commencement date |
| ● | amounts expected to be payable by the Group under residual value guarantees |
| ● | the exercise price of a purchase option if the Group is reasonably certain to exercise that option, and |
| ● | payments of penalties for terminating the lease, if the lease term reflects the Group exercising that option. |
Lease payments to be made under reasonably certain extension options are also included in the measurement of the liability.
Leases are recognized as a right-of-use asset and a corresponding liability at the date at which the leased asset is available for use by the Group. Each lease payment is allocated between the liability and finance cost. The finance cost is charged to profit or loss over the lease period so as to produce a constant periodic rate of interest on the remaining balance of the liability for each period.
| 2.13. | Right-of-use assets |
Right-of-use assets are measured at cost comprising the following:
| ● | the amount of the initial measurement of lease liability |
| ● | any lease payments made at or before the commencement date less any lease incentives received |
| ● | any initial direct costs, and |
| ● | restoration costs. |
Right-of-use assets are depreciated over the shorter of the asset’s useful life and the lease term on a straight-line basis. If the Group is reasonably certain to exercise a purchase option, the right-of-use asset is depreciated over the underlying asset’s useful life.
| 2.14. | Non-current financial assets |
Non-current financial assets held for long-term strategic purposes are classified within non-current assets on the consolidated statement of financial position. The financial impacts related to these financial assets are recorded in other comprehensive income.
Non-current financial assets are initially recorded at fair value on their trade date, which is different from the settlement date when the transaction is ultimately effected. Quoted securities are remeasured at each reporting date to fair value based on current market prices. If the market for a financial asset is not active or no market is available, fair values are established using valuation techniques.
Equity securities held as strategic investments are generally designated at the date of acquisition as financial assets valued at fair value through other comprehensive income with no subsequent recycling through profit or loss. Unrealized gains and losses, including exchange gains and losses, are recorded as a fair value adjustment in the consolidated statement of comprehensive income. They are reclassified to retained earnings when the equity security is sold.
| F-12 |
| 2.15. | Intangible assets |
| a. | Goodwill |
Goodwill on acquisitions of subsidiaries is included in intangible assets. Goodwill is not amortized, but is tested for impairment annually, or more frequently if events or changes in circumstances indicate that it might be impaired and is carried at cost less accumulated impairment losses. Gains and losses on the disposal of an entity include the carrying amount of goodwill relating to the entity sold. Goodwill is allocated to cash-generating units for the purpose of impairment testing. The allocation is made to those cash-generating units or group of cash- generating units that are expected to benefit from the business combination in which the goodwill arose.
| b. | Patents, trademarks, licenses and customer contracts |
Separately acquired trademarks and licenses are shown at historical cost. Trademarks, licenses and customer contracts acquired in a business combination are recognized at fair value at the acquisition date. They have a finite useful life and are subsequently carried at cost less accumulated amortization and impairment losses. The useful life of these intangibles assets is 5 to 20 years.
| c. | Intellectual property |
Intellectual property arising from business combinations is recognized at fair value when separately identifiable from goodwill. Intellectual property is recorded as an indefinite life asset when it is not yet ready for use. At the point the asset is ready for use, the useful life is reassessed as a definite life asset and amortized over a period of 5 to 20 years. Amortization and impairment charges related to currently marketed products are recognized in cost of goods sold.
Assets not available for use are tested annually for impairment. Assets are carried at cost less accumulated impairment losses and/or accumulated amortization. An impairment trigger assessment is performed annually for assets available for use.
| d. | Research and development |
Research expenditure on internal projects is recognized as an expense as incurred. Costs incurred on development projects (relating to the design and testing of new or improved products) are recognized as intangible assets when it is probable that the project will, after considering its commercial and technical feasibility, be completed and generate future economic benefits and its costs can be measured reliably. The expenditure that could be recognized comprises all directly attributable costs, including costs of materials, services, direct labor and an appropriate proportion of overheads.
Other expenditures that do not meet these criteria are recognized as an expense as incurred. As the Group has not met the requirement under the standard to recognize costs in relation to development as intangible assets, these amounts have been expensed within the financial statements.
| 2.16. | Impairment of assets |
Goodwill and intangible assets that have an indefinite useful life are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other assets are tested for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs of disposal and value in use. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash inflows which are largely independent of the cash inflows from other assets or Groups of assets (cash-generating units). Non-financial assets other than goodwill that suffered an impairment are reviewed for possible reversal of the impairment at the end of each reporting period.
| F-13 |
| 2.17. | Trade and other payables |
These amounts represent liabilities for goods and services provided to the Group prior to the reporting date which are unpaid. The amounts are unsecured and are usually paid within 30 days of recognition. Trade and other payables are presented as current liabilities unless payment is not due within 12 months after the reporting period. They are recognized initially at fair value and subsequently measured at amortized cost using the effective interest method.
| 2.18. | Provisions |
Provisions are recognized when the Group has a present (legal or constructive) obligation as a result of a past event, it is probable the Group will be required to settle the obligation, and a reliable estimate can be made of the amount of the obligation. The amount recognized as a provision is the best estimate of the consideration required to settle the present obligation at the reporting date, taking into account the risks and uncertainties surrounding the obligation. If the time value of money is material, provisions are discounted using a current pre-tax rate specific to the liability. The increase in the provision resulting from the passage of time is recognized as a finance cost.
| a. | Decommissioning liability |
The Group has recognized a provision for its obligation to decommission its radiopharmaceutical production facility at the end of its operating life. At the end of a facility’s life, costs are incurred in safely removing certain assets involved in the production of radioactive isotopes. The Group recognizes the full discounted cost of decommissioning as an asset and liability when the obligation to restore sites arises. The decommissioning asset is included within property, plant and equipment with the cost of the related installation. The liability is included within provisions. Revisions to the estimated costs of decommissioning which alter the level of the provisions required are also reflected in adjustments to the decommissioning asset. The amortization of the asset is included in the consolidated statement of comprehensive income or loss and the unwinding of discount of the provision is included within finance costs. Further detail has been provided in note 24.2.
| 2.19. | Contingent consideration |
The contingent consideration liabilities associated with business combinations are measured at fair value which has been calculated with reference to our judgement of the expected probability and timing of the potential future milestone payments, which is then discounted to a present value using appropriate discount rates with reference to the Group’s weighted average cost of capital. Subsequent changes in estimates for contingent consideration liabilities are recognized in Other losses (net). The effect of unwinding the discount over time is recognized in Finance costs.
Contingent consideration in connection with the purchase of individual assets outside of business combinations is recognized as a liability only when a non-contingent obligation arises (i.e. when milestone is met). Where the contingent consideration is payable in shares, or the group has an election to pay in shares, it is accounted for as an equity settled share-based payment. Equity settled share- based payments are recognized at their fair value at the date control of the asset is obtained. The determination of whether the payment should be capitalized or expensed is usually based on the reason for the contingent payment. If the contingent payment is based on regulatory approvals received (i.e. development milestone), it will generally be capitalized as the payment is incidental to the acquisition so the asset may be made available for its intended use. If the contingent payment is based on period volumes sold (i.e. sales related milestone), it will generally be expensed.
| F-14 |
Changes in the fair value of liabilities from contingent consideration will be capitalized or expensed based on the nature of the asset acquired (refer above), except for the effect from unwinding discounts. Interest rate effects from unwinding of discounts are recognized as finance costs. The fair value of equity-settled share-based payments is not re-assessed once the asset has been recognized.
| 2.20. | Employee benefits |
Employee benefits are recognized as an expense, unless the cost qualifies to be capitalized as an asset.
| a. | Short-term obligations |
Liabilities for wages and salaries, including non-monetary benefits and annual leave that is expected to be settled wholly within 12 months after the end of the period in which the employees render the related service are recognized in respect of employees’ services up to the end of the reporting period. These liabilities are measured at the amounts expected to be paid when the liabilities are settled. The liabilities are presented as current employee benefit obligations in the consolidated statement of financial position.
| b. | Other long-term employee benefit obligations |
The liabilities for long service leave are not expected to be settled wholly within 12 months after the end of the period in which the employees render the related service. They are therefore measured as the present value of expected future payments to be made in respect of services provided by employees up to the end of the reporting period using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service. Expected future payments are discounted using market yields at the end of the reporting period of high-quality corporate bonds with terms and currencies that match, as closely as possible, the estimated future cash outflows. Remeasurements as a result of experience adjustments and changes in actuarial assumptions are recognized in profit or loss. The obligations are presented as current liabilities in the consolidated statement of financial position if the entity does not have an unconditional right to defer settlement for at least 12 months after the reporting period, regardless of when the actual settlement is expected to occur.
| c. | Share-based payments |
Equity-settled share-based compensation benefits are provided to certain employees. Equity-settled transactions are awards of shares, options or performance rights over shares, that are provided to employees. The cost of equity-settled transactions is measured at fair value on grant date. Fair value is determined using the Black- Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk-free interest rate for the term of the option and volatility. No account is taken of any other vesting conditions.
If the non-vesting condition is within the control of the consolidated entity or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the consolidated entity or employee and is not satisfied during the vesting period, any remaining expense for the award is recognized over the remaining vesting period, unless the award is forfeited. If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognized immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new awards are treated as if they were a modification.
| d. | Termination benefits |
Termination benefits are payable when employment is terminated by the Group before the normal retirement date, or when an employee accepts voluntary redundancy in exchange for these benefits. The Group recognizes termination benefits at the earlier of the following dates:
| ● | when the Group can no longer withdraw the offer of those benefits, and |
| F-15 |
| ● | when the entity recognizes costs for a restructuring that is within the scope of IAS 37 Provisions, Contingent Liabilities and Contingent Assets and involves the payment of termination |
benefits. In the case of an offer made to encourage voluntary redundancy, the termination benefits are measured based on the number of employees expected to accept the offer. Benefits falling due more than 12 months after the end of the reporting period are discounted to present value.
| 2.21. | Borrowings |
Borrowings are initially recognized at fair value, net of transaction costs incurred. Borrowings are subsequently measured at amortized cost. Any difference between the proceeds (net of transaction costs) and the redemption amount is recognized in profit or loss over the period of the borrowings using the effective interest method. Fees paid on the establishment of loan facilities are recognized as transaction costs of the loan to the extent that it is probable that some or all of the facility will be drawn down. In this case, the fee is deferred until the draw- down occurs. To the extent there is no evidence that it is probable that some or all of the facility will be drawn down, the fee is capitalized as a prepayment for liquidity services and amortized over the period of the facility to which it relates.
Borrowing costs that are directly attributable to the construction of qualifying assets are capitalized as part of the cost of the relevant asset.
Borrowings are removed from the consolidated statement of financial position when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognized in profit or loss as other income or finance costs.
Borrowings are classified as current liabilities unless the Group has an unconditional right to defer settlement of the liability for at least 12 months after the reporting period.
| 2.22. | Revenue |
Revenue is measured at the fair value of the consideration received or receivable. Amounts disclosed as revenue are net of returns, trade allowances, rebates and amounts collected on behalf of third parties.
Revenue is recognized using a five step approach in accordance with IFRS 15 Revenue from Contracts with Customers to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Group expects to be entitled in exchange for those goods or services.
Distinct promises within the contract are identified as performance obligations. The transaction price of the contract is measured based on the amount of consideration the Group expects to be entitled to from the customer in exchange for goods or services. Factors such as requirements around variable consideration, significant financing components, noncash consideration, or amounts payable to customers also determine the transaction price. The transaction is then allocated to separate performance obligations in the contract based on relative standalone selling prices.
Revenue is recognized when, or as, performance obligations are satisfied, which is when control of the promised good or service is transferred to the customer.
Amounts received prior to satisfying the revenue recognition criteria are recorded as contract liabilities. Amounts expected to be recognized as revenue within the 12 months following the consolidated statement of financial position date are classified within current liabilities. Amounts not expected to be recognized as revenue within the 12 months following the consolidated statement of financial position date are classified within non-current liabilities.
| F-16 |
| a. | Sales of goods |
Sales are recognized at a point-in-time when control of the products has transferred, being when the products are delivered to the customer. Further, in determining whether control has transferred, Telix considers if there is a present right to payment and legal title, along with risks and rewards of ownership having transferred to the customer. Revenue from sales is recognized based on the price specified in the contract, net of the estimated volume discounts and government rebates.
Accumulated experience is used to estimate and provide for discounts, using the expected value method, and revenue is recognized to the extent that it is highly probable that a significant reversal will not occur. No element of financing is deemed present as the sales are made with credit terms ranging from 30 to 45 days, which is consistent with market practice.
Where distributors are used to facilitate the supply of a product a distribution fee is charged. This fee represents a cost of satisfying the performance obligation to the customer and expensed within Cost of sales in the Consolidated statement of comprehensive income or loss.
| b. | Licenses of intellectual property |
When licenses of intellectual property are distinct from other goods or services promised in the contract, the transaction price is allocated to the license as revenue upon transfer of control of the license to the customer. All other promised goods or services in the license agreement are evaluated to determine if they are distinct. If they are not distinct, they are combined with other promised goods or services.
The transaction price allocated to the license performance obligation is recognized based on the nature of the license arrangement. The transaction price is recognized over time if the nature of the license is a ‘right to access’ license. This is where the Group performs activities that significantly affect the intellectual property to which the customer has rights, the rights granted by the license directly expose the customer to any positive or negative effects of the Group’s activities, and those activities do not result in the transfer of a good or service to the customer as those activities occur. When licenses do not meet the criteria to be a right to access license, the license is a ‘right to use’ license, and the transaction price is recognized at the point in time when the customer obtains control over the license.
| c. | Research and development services |
Where research and development (R&D) services do not significantly modify or customize the license nor are the license and development services significantly interrelated or interdependent, the provision of R&D services is considered to be distinct. The transaction price is allocated to the R&D services based on a cost-plus margin approach. Revenue is recognized over time based on the costs incurred to date as a percentage of total forecast costs. Reforecasting of total costs is performed at the end of each reporting period to ensure that costs recognized represent the goods or services transferred.
| d. | Financing component |
The existence of a significant financing component in the contract is considered under the five-step method under IFRS 15 Revenue from Contracts with Customers.
If the timing of payments agreed to by the parties to the contract (either explicitly or implicitly) provides the customer or the Group with a significant benefit of financing the transfer of goods or services to the customer, the promised amount of consideration will be adjusted for the effects of the time value of money when determining the transaction price.
| e. | Milestone revenue |
The five-step method under IFRS 15 Revenue from Contracts with Customers is applied to measure and recognize milestone revenue.
| F-17 |
The receipt of milestone payments is often contingent on meeting certain clinical, regulatory or commercial targets, and is therefore considered variable consideration. The transaction price of the contingent milestone is estimated using the most likely amount method. Within the transaction price, some or all of the amount of the contingent milestone is included only to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the contingent milestone is subsequently resolved. Milestone payments that are not within the control of the Group, such as regulatory approvals, are not considered highly probable of being achieved until those approvals are received. Any changes in the transaction price are allocated to all performance obligations in the contract unless the variable consideration relates only to one or more, but not all, of the performance obligations. When consideration for milestones is a sale-based or usage-based royalty that arises from licenses of intellectual property (such as cumulative net sales targets), revenue is recognized at the later of when (or as) the subsequent sale or usage occurs, or when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied).
| f. | Sales-based or usage-based royalties |
Licenses of intellectual property can include royalties that are based on the customer’s usage of the intellectual property or sale of products that contain the intellectual property. The specific exception to the general requirements of variable consideration and the constraint on variable consideration for sales-based or usage-based royalties promised in a license of intellectual property is applied. The exception requires such revenue to be recognized at the later of when (or as) the subsequent sale or usage occurs and the performance obligation to which some or all of the sales-based or usage-based royalty has been allocated has been satisfied (or partially satisfied).
| 2.23. | Government grants |
Income from government grants is recognized at fair value where there is a reasonable assurance that the grant will be received, and the Group will comply with all attached conditions. Income from government grants is recognized in the consolidated statement of comprehensive income or loss on a systematic basis over the periods in which the Group recognizes as an expense the related costs for which the grants are intended to compensate.
| 2.24. | Income tax |
The income tax expense or credit for the period is the tax payable on the current period’s taxable income based on the applicable income tax rate for each jurisdiction adjusted by changes in deferred tax assets and liabilities attributable to temporary differences and to unused tax losses.
Deferred income tax is provided in full, using the liability method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. However, deferred tax liabilities are not recognized if they arise from the initial recognition of goodwill. Deferred income tax is also not accounted for if it arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting nor taxable profit or loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantively enacted by the end of the reporting period and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled. Deferred tax assets are recognized only if it is probable that future taxable amounts will be available to utilize those temporary differences and losses.
Included in income tax expense for the period is the effect of Australian R&D tax credits which may only be offset against Australian taxable income. As such, they are recognized as a component of income tax expense.
| F-18 |
Tax consolidation regime
Telix Pharmaceuticals Limited and its wholly owned Australian resident entities have formed a tax-consolidated group and are therefore taxed as a single entity. The head entity within the tax-consolidated group is Telix Pharmaceuticals Limited. As a consequence, the deferred tax assets and deferred tax liabilities of these entities have been offset in the consolidated financial statements.
| 2.25. | Sales Taxes and Goods and Services Tax (GST) |
Revenues, expenses and assets are recognized net of the amount of associated sales taxes and GST, unless the GST incurred is not recoverable from the taxation authority. In this case it is recognized as part of the cost of acquisition of the asset or as part of the expense.
Cash flows are presented on a gross basis. The GST components of cash flows arising from investing or financing activities which are recoverable from, or payable to the taxation authority, are presented as operating cash flows.
| 2.26. | Earnings per share |
| a. | Basic earnings per share |
Basic earnings per share is calculated by dividing: the profit attributable to owners of the Company, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the financial period, adjusted for bonus elements in ordinary shares issued during the period and excluding treasury shares.
| b. | Diluted earnings per share |
Diluted earnings per share adjusts the figures used in the determination of basic earnings per share to take into account: the after-income tax effect of interest and other financing costs associated with dilutive potential ordinary shares, and the weighted average number of additional ordinary shares that would have been outstanding assuming the conversion of all dilutive potential ordinary shares.
| 2.27. | Fair value measurement |
Certain judgements and estimates are made in determining the fair values of the financial instruments that are recognized and measured at fair value in the financial statements. To provide an indication about the reliability of the inputs used in determining fair value, the Group has classified its financial instruments into the three levels prescribed under the accounting standards. The different levels have been defined as follows:
| ● | Level 1: fair value of financial instruments traded in active markets is based on quoted market prices at the end of the reporting period. The quoted market price used for financial assets is the current bid price. |
| ● | Level 2: fair value of financial instruments that are not traded in an active market is determined using valuation techniques which maximize the use of observable market data and rely as little as possible on entity specific estimates. If all significant inputs required to fair value an instrument are observable, the instrument is included in level 2. |
| ● | Level 3: if one or more of the significant inputs is not based on observable market data, the instrument is included in level 3. |
There were no transfers between level 1, 2 and 3 for recurring fair value measurements during the year. The Group’s policy is to recognize transfers into and transfers out of fair value hierarchy levels at the end of the reporting period. Certain judgements and estimates are made in determining the fair values of the financial instruments that are recognized and measured at fair value in the financial statements.
| F-19 |
| 2.28. | Key judgements and estimates |
In the process of applying the Group’s accounting policies, a number of judgements and estimates of future events are required.
Accrued R&D expenditure
The Group is required to estimate its accrued expenses at each reporting date, which involves reviewing open contracts and purchase orders, communicating with program directors and managers to identify services that have already been performed, estimating the level of services performed with associated costs incurred for the service for which the Group has not yet been invoiced, or otherwise notified of the actual cost. The majority of service providers invoice the Group monthly in arrears for services performed or when contractual milestones are met. The Group estimates accrued expenses at each reporting date based on facts and circumstances known at that time. The Group periodically confirms the accuracy of estimates with the service providers and makes adjustments if necessary. Examples of estimated accrued expenses include fees paid to:
| ● | Contract Research Organizations (CROs) in connection with clinical studies |
| ● | investigative sites in connection with clinical studies |
| ● | vendors in connection with preclinical development activities, and |
| ● | vendors related to product manufacturing, process development and distribution of clinical supplies, all of which are in connection with products for use in clinical trials. |
Impairment assessment – carrying value of goodwill and intangible assets
The assessment of impairment of the goodwill and intangible assets has required estimates and judgements to be made. The inputs for these have been outlined in note 18.
Contingent consideration and decommissioning liabilities
The Group has identified the contingent consideration and decommissioning liabilities as balances requiring estimates and significant judgements. These estimates and judgements have been outlined in note 24 and note 25.
| 3. | Segment reporting |
The Group has operations in the Americas, Asia Pacific, and Europe, Middle East and Africa. During 2022, the Group achieved a major commercial milestone with the launch of its prostate cancer imaging product Illuccix® in the United States (U.S.) and the subsequent receipt of first commercial revenues from sales of Illuccix® in April 2022.
| F-20 |
Reportable segments
The Group operated two reportable segments during the year ended December 31, 2023. The Group’s operating segments are based on the reports reviewed by the Group Chief Executive Officer who is considered to be the chief operating decision maker.
Segment performance is evaluated based on Adjusted earnings before interest, tax depreciation and amortization (Adjusted EBITDA). Adjusted EBITDA excludes the effects of the remeasurement of contingent consideration and government grant liabilities and other income and expenses which may have an impact on the quality of earnings such as impairments where the impairment is the result of an isolated, non-recurring event. Interest income and finance costs are not allocated to segments as this activity is managed centrally by a central treasury function, which manages the cash position of the Group.
Segment assets and liabilities are measured in the same way as in the financial statements. The assets and liabilities are allocated based on the operations of the segment. Finance costs are not allocated to segments, as this type of activity is driven by head office, which manages the cash position of the Group.
| Reportable segment | Principal activities | |
| Commercial operations | Commercial sales of Illuccix® and other products subsequent to obtaining regulatory approvals | |
| Product development | Developing radiopharmaceutical products for commercialization. This segment includes revenue received from license agreements prior to commercialization and research and development services. |
Group and unallocated includes Manufacturing Services and Medical Technologies segments, head office and centrally managed costs (which includes any remeasurements of contingent consideration liabilities).
| Commercial | Product development | Group and unallocated | Group | |||||||||||||
| 2023 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||
| Revenue from contracts with customers | 497,051 | 5,496 | — | 502,547 | ||||||||||||
| Cost of sales | (188,157 | ) | — | — | (188,157 | ) | ||||||||||
| Gross profit | 308,894 | 5,496 | — | 314,390 | ||||||||||||
| Research and development costs | (284 | ) | (128,517 | ) | (43 | ) | (128,844 | ) | ||||||||
| Selling and marketing expenses | (54,437 | ) | — | (430 | ) | (54,867 | ) | |||||||||
| General and administration costs | (36,092 | ) | — | (42,893 | ) | (78,985 | ) | |||||||||
| Other losses (net) | (863 | ) | — | (34,991 | ) | (35,854 | ) | |||||||||
| Operating profit/(loss) | 217,218 | (123,021 | ) | (78,357 | ) | 15,840 | ||||||||||
| F-21 |
| Commercial | Product development | Group and unallocated | Group | |||||||||||||
| 2023 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||
| Finance income | — | — | 1,019 | 1,019 | ||||||||||||
| Finance costs | — | — | (13,772 | ) | (13,772 | ) | ||||||||||
| Profit/(loss) before income tax | 217,218 | (123,021 | ) | (91,110 | ) | 3,087 | ||||||||||
| Income tax benefit | — | — | 2,124 | 2,124 | ||||||||||||
| Profit/(loss) for the year | 217,218 | (123,021 | ) | (88,986 | ) | 5,211 | ||||||||||
| Other losses (net) | 863 | — | 34,991 | 35,854 | ||||||||||||
| Finance income | — | — | (1,019 | ) | (1,019 | ) | ||||||||||
| Finance costs | — | — | 13,772 | 13,772 | ||||||||||||
| Depreciation and amortization | 5,665 | 538 | 540 | 6,743 | ||||||||||||
| Income tax | — | — | (2,124 | ) | (2,124 | ) | ||||||||||
| Adjusted EBITDA | 223,746 | (122,483 | ) | (42,826 | ) | 58,437 | ||||||||||
Operating segment assets and liabilities
| Commercial | Product development | Group and unallocated | Group | |||||||||||||
| 2023 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||
| Total assets | 288,447 | 46,744 | 63,111 | 398,302 | ||||||||||||
| Total liabilities | 86,337 | 40,252 | 122,802 | 249,391 | ||||||||||||
| Additions to non-current assets | 12,025 | 5,116 | 54,296 | 71,437 | ||||||||||||
| F-22 |
| Commercial | Product development | Group and unallocated | Group | |||||||||||||
| 2022 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||
| Revenue from contracts with customers | 156,369 | 3,727 | — | 160,096 | ||||||||||||
| Cost of sales | (65,170 | ) | — | — | (65,170 | ) | ||||||||||
| Gross profit | 91,199 | 3,727 | — | 94,926 | ||||||||||||
| Research and development costs | (704 | ) | (80,304 | ) | — | (81,008 | ) | |||||||||
| Selling and marketing expenses | (37,756 | ) | — | (214 | ) | (37,970 | ) | |||||||||
| General and administration costs | (17,730 | ) | — | (31,398 | ) | (49,128 | ) | |||||||||
| Other losses (net) | (820 | ) | 10 | (17,940 | ) | (18,750 | ) | |||||||||
| Operating profit/(loss) | 34,189 | (76,567 | ) | (49,552 | ) | (91,930 | ) | |||||||||
| Finance income | — | — | 1 | 1 | ||||||||||||
| Finance costs | — | — | (6,693 | ) | (6,693 | ) | ||||||||||
| Profit/(loss) before income tax | 34,189 | (76,567 | ) | (56,244 | ) | (98,622 | ) | |||||||||
| Income tax expense | — | — | (5,457 | ) | (5,457 | ) | ||||||||||
| Profit/(loss) for the year | 34,189 | (76,567 | ) | (61,701 | ) | (104,079 | ) | |||||||||
| Other losses (net) | 820 | (10 | ) | 17,940 | 18,750 | |||||||||||
| Finance income | — | — | (1 | ) | (1 | ) | ||||||||||
| Finance costs | — | — | 6,693 | 6,693 | ||||||||||||
| Depreciation and amortization | 4,694 | 493 | 192 | 5,379 | ||||||||||||
| Income tax expense | — | — | 5,457 | 5,457 | ||||||||||||
| Adjusted EBITDA | 39,703 | (76,084 | ) | (31,420 | ) | (67,801 | ) | |||||||||
Operating segment assets and liabilities
| Commercial | Product development | Group and unallocated | Group | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| Total assets as at December 31, 2022 | 111,619 | 44,275 | 99,459 | 255,353 | ||||||||||||
| Total liabilities as at December 31, 2022 | 60,887 | 19,272 | 95,187 | 175,346 | ||||||||||||
| Additions to non-current assets | 15,789 | 6,823 | — | 22,612 | ||||||||||||
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
Revenue by location of customer | Non-current assets by location of asset | Revenue by location of customer | Non-current assets by location of asset | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| Australia | 1,166 | 21,057 | 149 | 31,815 | ||||||||||||
| Belgium | 458 | 77,469 | 564 | 41,174 | ||||||||||||
| China | 5,291 | — | 3,353 | — | ||||||||||||
| Other countries | 4,669 | — | 3,979 | — | ||||||||||||
| United Kingdom | 1,306 | 50,346 | 2,045 | — | ||||||||||||
| United States | 489,657 | 4,130 | 150,006 | 5,160 | ||||||||||||
| Total | 502,547 | 153,002 | 160,096 | 78,149 | ||||||||||||
The total non-current assets figure above excludes deferred tax assets.
| F-23 |
| 4. | Revenue from contracts with customers |
Disaggregation of revenue from contracts with customers
The Group derives revenue from the sale and transfer of goods and services over time and at a point in time under the following major business activities:
| 2023 | 2022 | |||||||||||
| Recognition | Operating segment | A$’000 | A$’000 | |||||||||
| Sale of goods | At a point in time | Commercial | 496,241 | 155,984 | ||||||||
| Royalty income | At a point in time | Commercial | 392 | 385 | ||||||||
| Provision of services | Over time | Commercial | 418 | — | ||||||||
| Licenses of intellectual property | At a point in time | Product development | 100 | 374 | ||||||||
| Research and development services | Over time | Product development | 5,396 | 3,353 | ||||||||
| Total revenue from continuing operations | 502,547 | 160,096 | ||||||||||
| 5. | Other losses (net) |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Remeasurement of contingent consideration | 34,275 | 16,707 | ||||||
| Remeasurement of provisions | (173 | ) | 1,017 | |||||
| Realized currency (loss)/gain | (2,460 | ) | 668 | |||||
| Impairment of intangible assets | 804 | — | ||||||
| Other income | (20 | ) | (91 | ) | ||||
| Unrealized currency loss | 3,428 | 449 | ||||||
| 35,854 | 18,750 | |||||||
| F-24 |
| 6. | Finance costs |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Unwind of discount | 12,782 | 6,287 | ||||||
| Interest expense on lease liabilities | 636 | 277 | ||||||
| Interest expense | 148 | 46 | ||||||
| Bank fees | 206 | 83 | ||||||
| Finance costs | 13,772 | 6,693 | ||||||
The Group recognized an unwind of discount on contingent consideration liabilities of $11,394,000 (2022: $4,957,000), provisions of $419,000 (2022: $252,000) and contract liabilities of $969,000 (2022: $1,078,000).
| 7. | Income tax (benefit)/expense |
| 7.1. | Income tax (benefit)/expense |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Current tax expense1 | 14,357 | 9,428 | ||||||
| Deferred tax benefit | (16,481 | ) | (3,971 | ) | ||||
| (2,124 | ) | 5,457 | ||||||
| 1. | The current tax expense is attributable to Telix Innovations SA and Telix Pharmaceuticals US Inc and is driven by the individual entity’s taxable profits. |
| 7.2. | Numerical reconciliation of prima facie tax payable to income tax benefit/(expense) |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Profit/(loss) before income tax | 3,087 | (98,622 | ) | |||||
| Prima-facie tax at a rate of 30.0% (2022: 30.0%) | 926 | (29,587 | ) | |||||
| Tax effect of amounts which are not deductible (taxable) in calculating taxable income: | ||||||||
| Net R&D tax incentive credit | (7,408 | ) | (6,688 | ) | ||||
| Remeasurement of provisions | 13,915 | 7,423 | ||||||
| Share-based payments expense | 2,636 | 2,434 | ||||||
| Employee Share Trust payments | (10,776 | ) | (8,073 | ) | ||||
| Sundry items | 569 | 2 | ||||||
| Foreign exchange translation loss | 1,028 | (464 | ) | |||||
| 890 | (34,953 | ) | ||||||
| Current year tax losses not recognized | 35,152 | 46,325 | ||||||
| Prior year tax losses recognized | — | (854 | ) | |||||
| Adjustment for current tax of prior periods | — | 561 | ||||||
| Difference in overseas tax rates | (38,166 | ) | (5,622 | ) | ||||
| Income tax (benefit)/expense | (2,124 | ) | 5,457 | |||||
| F-25 |
| 8. | Earnings per share |
| 8.1. | Basic earnings per share |
| 2023 | 2022 | |||||||
| Cents | Cents | |||||||
| Basic earnings/(loss) per share from continuing operations attributable to the ordinary equity holders of the Company | 1.63 | (33.50 | ) | |||||
| Total basic earnings/(loss) per share attributable to the ordinary equity holders of the Company | 1.63 | (33.50 | ) | |||||
| 8.2. | Diluted earnings per share |
| 2023 | 2022 | |||||||
| Cents | Cents | |||||||
| Diluted earnings/(loss) per share from continuing operations attributable to the ordinary equity holders of the Company | 1.61 | (33.50 | ) | |||||
| Total diluted earnings/(loss) per share attributable to the ordinary equity holders of the Company | 1.61 | (33.50 | ) | |||||
| 8.3. | Weighted average number of shares used as the denominator |
| 2023 | 2022 | |||||||
| Number | Number | |||||||
| ‘000 | ‘000 | |||||||
| Weighted average number of ordinary shares used as the denominator in calculating basic earnings/loss per share1 | 319,181 | 310,644 | ||||||
| Weighted average number of ordinary shares used as the denominator in calculating diluted earnings/loss per share | 323,710 | 310,644 | ||||||
| 1. | There were 4,436,046 options that were not included in the calculation of diluted earnings for the year ended December 31, 2022 as they were antidilutive. |
| F-26 |
| 9. | Employment costs |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Salaries and wages | 82,108 | 47,302 | ||||||
| Short term incentives | 9,413 | 4,025 | ||||||
| Sales commissions | 7,167 | 3,113 | ||||||
| Share based payment charge | 8,786 | 8,114 | ||||||
| Superannuation | 1,798 | 1,270 | ||||||
| Non-Executive Directors’ fees | 577 | 661 | ||||||
| 109,849 | 64,485 | |||||||
Salaries and wages of $1,483,000 (2022: $903,000) are included within the cost of sales in the Consolidated statement of comprehensive income or loss.
| 10. | Depreciation and amortization |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Amortization of intangible assets | 4,344 | 4,098 | ||||||
| Depreciation | 2,399 | 1,281 | ||||||
| 6,743 | 5,379 | |||||||
| 11. | Trade and other receivables |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Trade receivables | 65,310 | 39,354 | ||||||
| Allowance for impairment losses | (533 | ) | — | |||||
| Deposits | 586 | 327 | ||||||
| 65,363 | 39,681 | |||||||
| Current | 64,777 | 39,354 | ||||||
| Non-current | 586 | 327 | ||||||
| Total trade and other receivables | 65,363 | 39,681 | ||||||
| F-27 |
| 12. | Inventories |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Raw materials and stores | 7,700 | 2,422 | ||||||
| Work in progress | 5,961 | 3,773 | ||||||
| Finished goods | 3,649 | 2,282 | ||||||
| Total inventories | 17,310 | 8,477 | ||||||
The amount of inventory recognized as an expense during the year was $22,621,000 (2022: $9,100,000).
| 13. | Other current assets |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Other receivables | 2,363 | 3,675 | ||||||
| GST receivables | 4,739 | 2,890 | ||||||
| Prepayments | 12,422 | 2,508 | ||||||
| Total other current assets | 19,524 | 9,073 | ||||||
14. Financial assets
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Investment in Mauna Kea | 9,497 | — | ||||||
| Investment QSAM Biosciences | 2,763 | — | ||||||
| Total financial assets | 12,260 | — | ||||||
Additions
Mauna Kea
On November 13, 2023 Telix announced a strategic investment in Mauna Kea of $10,130,000 (€6,000,000), to develop new hybrid pharmaceutical-device products through the combination of Telix’s cancer-targeting agents with Mauna Kea’s surgical endomicroscopy platform. Telix’s investment in Mauna Kea will further support the development of advanced imaging techniques for minimally invasive surgery, with a specific focus on urologic oncology.
Under the deal terms, Telix purchased 11,911,852 new ordinary shares of Mauna Kea at €0.5037 per share. Telix owns 19.33% of the share capital and 19.01% of the voting rights of Mauna Kea. The investment was designated at the date of acquisition as a financial asset valued at fair value through other comprehensive income.
| F-28 |
QSAM Biosciences
On November 14, 2023 Telix announced the proposed acquisition of QSAM Biosciences Inc (QSAM). QSAM is a U.S. based clinical stage company developing therapeutic radiopharmaceuticals for primary and metastatic bone cancer.
Telix paid QSAM an upfront Collaboration and Option Fee of $3,025,000 (US$2,000,000) in cash to advance development efforts based on mutually agreed goals and to provide sixty days of exclusivity pending completion of diligence and execution of a definitive acquisition agreement. If the acquisition of QSAM proceeds, upon closing, Telix will pay an upfront purchase price of US$33,100,000 in equity through the issue of fully paid ordinary Telix shares. If the proposed acquisition of QSAM does not close, the Collaboration and Option Fee will be converted to QSAM common stock at US$6.70 per share. The upfront Collaboration and Option Fee has been designated at the date of acquisition as a financial asset valued at fair value through other comprehensive income.
Amounts recognized in other comprehensive income or loss
Fair values have been determined based on the quoted share prices (level 1 inputs) at December 31, 2023, resulting in a loss of $895,000 (2022: $Nil) recognized in other comprehensive income or loss.
| 15. | Deferred tax assets and liabilities |
| 15.1. | Deferred tax assets |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| The balance comprises temporary differences attributable to: | ||||||||
| Tax losses | — | 4,400 | ||||||
| Intangible assets | 8,294 | 2,434 | ||||||
| Employee benefit obligations | 2,791 | 1,052 | ||||||
| Lease liabilities | 1,780 | 803 | ||||||
| Inventories | 10,976 | 363 | ||||||
| Other | 531 | 157 | ||||||
| Total deferred tax assets | 24,372 | 9,209 | ||||||
| Set-off of deferred tax liabilities pursuant to set-off provisions | (3,920 | ) | (5,238 | ) | ||||
| Net deferred tax assets | 20,452 | 3,971 | ||||||
| F-29 |
Tax losses | Intangible assets | Employee benefit obligations | Lease liabilities | Inventories | Other | Total | ||||||||||||||||||||||
| Deferred tax assets movements | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||||||||||
| The balance comprises temporary differences attributable to: | ||||||||||||||||||||||||||||
| Balance at January 1, 2023 | 4,400 | 2,434 | 1,052 | 803 | 363 | 157 | 9,209 | |||||||||||||||||||||
| (Charged)/credited: | ||||||||||||||||||||||||||||
| to profit and loss | (4,400 | ) | 5,860 | 1,739 | 977 | 10,613 | 374 | 15,163 | ||||||||||||||||||||
| Balance at December 31, 2023 | — | 8,294 | 2,791 | 1,780 | 10,976 | 531 | 24,372 | |||||||||||||||||||||
| Balance at January 1, 2022 | 4,692 | — | — | 756 | — | — | 5,448 | |||||||||||||||||||||
| (Charged)/credited: | — | |||||||||||||||||||||||||||
| to profit and loss | (292 | ) | 2,434 | 1,052 | 47 | 363 | 157 | 3,761 | ||||||||||||||||||||
| Balance at December 31, 2022 | 4,400 | 2,434 | 1,052 | 803 | 363 | 157 | 9,209 | |||||||||||||||||||||
15.2. Deferred tax liabilities
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| The balance comprises temporary differences attributable to: | ||||||||
| Intangible assets | 2,376 | 3,634 | ||||||
| Right-of-use assets | 1,544 | 1,604 | ||||||
| Total deferred tax liabilities | 3,920 | 5,238 | ||||||
| Set-off of deferred tax assets pursuant to set-off provisions | (3,920 | ) | (5,238 | ) | ||||
| Net deferred tax liabilities | — | — | ||||||
Intangible assets | Right-of-use assets | Total | ||||||||||
| Deferred tax liabilities movements | A$’000 | A$’000 | A$’000 | |||||||||
| The balance comprises temporary differences attributable to: | ||||||||||||
| Balance at January 1, 2023 | 3,634 | 1,604 | 5,238 | |||||||||
| Charged/(credited): | ||||||||||||
| to profit and loss | (1,258 | ) | (60 | ) | (1,318 | ) | ||||||
| Balance at December 31, 2023 | 2,376 | 1,544 | 3,920 | |||||||||
| Balance at January 1, 2022 | 4,734 | 714 | 5,448 | |||||||||
| Charged/(credited): | ||||||||||||
| to profit and loss | (1,100 | ) | 890 | (210 | ) | |||||||
| Balance at December 31, 2022 | 3,634 | 1,604 | 5,238 | |||||||||
| F-30 |
| 15.3. | Unrecognized deferred tax assets |
The composition of the Group’s unrecognized deferred tax assets is as follows:
| 2023 | 2022 | |||||||
| Unrecognized deferred tax assets | A$’000 | A$’000 | ||||||
| Tax losses and tax credits | 84,412 | 62,833 | ||||||
| Temporary differences in relation to provisions | 212 | 1,600 | ||||||
| Temporary differences in relation to employee benefit obligations | 97 | 898 | ||||||
| Temporary differences in relation to intangible assets | — | 2,127 | ||||||
| Temporary differences in relation to lease liabilities | 211 | 838 | ||||||
| Temporary differences in relation to share based payments | 8,940 | 10,508 | ||||||
| Total unrecognized deferred tax assets | 93,872 | 78,804 |
| 15.4. | Unrecognized tax losses |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Unused tax losses and carried forward tax credits for which no deferred tax asset has been recognized: | ||||||||
| Australia | 82,908 | 61,330 | ||||||
| Other countries | 1,504 | 1,503 | ||||||
| Unrecognized income tax benefit | 84,412 | 62,833 | ||||||
| F-31 |
| 16. | Property, plant and equipment |
Land and buildings | Plant and equipment | Furniture, fittings and equipment | Leasehold improvements | Total | ||||||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||||||
| Balance at January 1, 2023 | 9,611 | 576 | 441 | 1,404 | 12,032 | |||||||||||||||
| Additions | 8,912 | 96 | 168 | 503 | 9,679 | |||||||||||||||
| Acquisition of business | — | 37 | — | — | 37 | |||||||||||||||
| Reclassifications | 2,021 | (12 | ) | 490 | (142 | ) | 2,357 | |||||||||||||
| Depreciation charge | (91 | ) | (207 | ) | (422 | ) | (222 | ) | (942 | ) | ||||||||||
| Exchange differences | (11 | ) | 9 | 3 | 6 | 7 | ||||||||||||||
| Balance at December 31, 2023 | 20,442 | 499 | 680 | 1,549 | 23,170 | |||||||||||||||
| Cost | 20,752 | 895 | 1,600 | 1,908 | 25,155 | |||||||||||||||
| Accumulated depreciation | (310 | ) | (396 | ) | (920 | ) | (359 | ) | (1,985 | ) | ||||||||||
| Net book amount | 20,442 | 499 | 680 | 1,549 | 23,170 | |||||||||||||||
| Balance at January 1, 2022 | 2,203 | 991 | 461 | 296 | 3,951 | |||||||||||||||
| Additions | 6,717 | 152 | 203 | 1,165 | 8,237 | |||||||||||||||
| Acquisition of business | — | 258 | — | — | 258 | |||||||||||||||
| Reclassifications | 766 | (766 | ) | — | — | — | ||||||||||||||
| Depreciation charge | (70 | ) | (63 | ) | (230 | ) | (57 | ) | (420 | ) | ||||||||||
| Exchange differences | (5 | ) | 4 | 7 | — | 6 | ||||||||||||||
| Balance at December 31, 2022 | 9,611 | 576 | 441 | 1,404 | 12,032 | |||||||||||||||
| Cost | 9,830 | 765 | 939 | 1,541 | 13,075 | |||||||||||||||
| Accumulated depreciation | (219 | ) | (189 | ) | (498 | ) | (137 | ) | (1,043 | ) | ||||||||||
| Net book amount | 9,611 | 576 | 441 | 1,404 | 12,032 | |||||||||||||||
| 17. | Right-of-use assets |
| Properties | Motor vehicles | Total | ||||||||||
| A$’000 | A$’000 | A$’000 | ||||||||||
| Balance at January 1, 2023 | 6,327 | 479 | 6,806 | |||||||||
| Additions | 1,188 | 1,158 | 2,346 | |||||||||
| Reclassifications | (336 | ) | — | (336 | ) | |||||||
| Depreciation charge | (1,006 | ) | (451 | ) | (1,457 | ) | ||||||
| Exchange differences | (39 | ) | 3 | (36 | ) | |||||||
| Balance at December 31, 2023 | 6,134 | 1,189 | 7,323 | |||||||||
| Cost | 8,959 | 2,195 | 11,154 | |||||||||
| Accumulated depreciation | (2,825 | ) | (1,006 | ) | (3,831 | ) | ||||||
| Net book amount | 6,134 | 1,189 | 7,323 | |||||||||
| Balance at January 1, 2022 | 2,067 | 311 | 2,378 | |||||||||
| Additions | 5,054 | 384 | 5,438 | |||||||||
| Acquisition of business | 423 | — | 423 | |||||||||
| Depreciation charge | (640 | ) | (221 | ) | (861 | ) | ||||||
| Disposals | (580 | ) | — | (580 | ) | |||||||
| Exchange differences | 3 | 5 | 8 | |||||||||
| Balance at December 31, 2022 | 6,327 | 479 | 6,806 | |||||||||
| Cost | 8,104 | 1,034 | 9,138 | |||||||||
| Accumulated depreciation | (1,777 | ) | (555 | ) | (2,332 | ) | ||||||
| Net book amount | 6,327 | 479 | 6,806 | |||||||||
| F-32 |
The consolidated statement of comprehensive income or loss shows the following amounts relating to right-of- use assets:
| 2023 | 2022 | |||||||
| Depreciation charge on right-of-use assets | A$’000 | A$’000 | ||||||
| Properties | 1,006 | 640 | ||||||
| Motor vehicles | 451 | 221 | ||||||
| 1,457 | 861 | |||||||
| 18. | Intangible assets |
| Goodwill | Intellectual property | Software | Patents | Licenses | Total | |||||||||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||||||||
| Balance at January 1, 2023 | 5,519 | 41,060 | — | 300 | 12,105 | 58,984 | ||||||||||||||||||
| Additions | — | 57,410 | 1,659 | 266 | 77 | 59,412 | ||||||||||||||||||
| Reclassifications | — | — | — | — | (2,021 | ) | (2,021 | ) | ||||||||||||||||
| Amortization charge | — | (4,005 | ) | — | (37 | ) | (302 | ) | (4,344 | ) | ||||||||||||||
| Impairments | — | (804 | ) | — | — | — | (804 | ) | ||||||||||||||||
| Changes in provisions | (672 | ) | 489 | — | — | 282 | 99 | |||||||||||||||||
| Exchange differences | — | (1,933 | ) | (37 | ) | — | 307 | (1,663 | ) | |||||||||||||||
| Balance at December 31, 2023 | 4,847 | 92,217 | 1,622 | 529 | 10,448 | 109,663 | ||||||||||||||||||
| Cost | 4,847 | 114,048 | 1,622 | 949 | 11,604 | 133,070 | ||||||||||||||||||
| Accumulated amortization | — | (21,831 | ) | — | (420 | ) | (1,156 | ) | (23,407 | ) | ||||||||||||||
| Net book amount | 4,847 | 92,217 | 1,622 | 529 | 10,448 | 109,663 | ||||||||||||||||||
| Balance at January 1, 2022 | 4,097 | 44,486 | — | 337 | 6,809 | 55,729 | ||||||||||||||||||
| Acquisition of business | 1,433 | — | — | — | — | 1,433 | ||||||||||||||||||
| Additions | — | — | — | — | 6,823 | 6,823 | ||||||||||||||||||
| Amortization charge | — | (3,742 | ) | — | (34 | ) | (322 | ) | (4,098 | ) | ||||||||||||||
| Changes in provisions | — | 256 | — | — | (1,120 | ) | (864 | ) | ||||||||||||||||
| Exchange differences | (11 | ) | 60 | — | (3 | ) | (85 | ) | (39 | ) | ||||||||||||||
| Balance at December 31, 2022 | 5,519 | 41,060 | — | 300 | 12,105 | 58,984 | ||||||||||||||||||
| Cost | 5,519 | 58,875 | — | 675 | 12,835 | 77,904 | ||||||||||||||||||
| Accumulated amortization | — | (17,815 | ) | — | (375 | ) | (730 | ) | (18,920 | ) | ||||||||||||||
| Net book amount | 5,519 | 41,060 | — | 300 | 12,105 | 58,984 | ||||||||||||||||||
| F-33 |
Cash generating units
The allocation of intangible assets to each cash-generating unit (CGU) is summarized below:
| 2023 | 2022 | |||||||||||
| CGU | Useful life | Status | A$’000 | A$’000 | ||||||||
| TLX591-CDx (Illuccix®) | Definite | Commercial | 10,876 | 14,709 | ||||||||
| TLX591 | Indefinite | Product development | 17,912 | 12,796 | ||||||||
| TLX101 | Definite | Product development | 1,613 | 1,676 | ||||||||
| TLX66 | Indefinite | Product development | 15,569 | 15,080 | ||||||||
| TLX66-CDx | Definite | Commercial | — | 898 | ||||||||
| TLX300 | Indefinite | Product development | 6,823 | 6,823 | ||||||||
| Manufacturing services | Definite | Product development | 4,298 | 6,702 | ||||||||
| Medical technologies | Indefinite | Product development | 52,043 | — | ||||||||
| Patents | Definite | Product development | 529 | 300 | ||||||||
| 109,663 | 58,984 | |||||||||||
Impairment test for goodwill and indefinite life intangible assets
Goodwill and indefinite life intangible assets are tested annually for impairment. At December 31, 2023, the Directors used a fair value less costs to sell approach to assess the carrying value of goodwill and indefinite life intangible assets. No impairment was recognized by the Group.
| F-34 |
Key assumptions used for the fair value less costs to sell approach
The Group has identified the estimate of the recoverable amount as a significant judgement for the year ended December 31, 2023. In determining the recoverable amount of goodwill and indefinite life intangible assets, the Group has used discounted cash flow forecasts and the following key assumptions (classified as level 3 inputs in the fair value hierarchy):
| ● | discounted expected future cash flows of each program which span 10 years from marketing authorization, reflecting the anticipated product life cycle, and include cash inflows and outflows determined using further assumptions below | |
| ● | risk adjusted post-tax discount rate – 15.0% (2022: 15.0%) | |
| ● | regulatory/marketing authorization approval dates, these are re-assessed in conjunction with Senior Management and Commercial teams | |
| ● | expected sales volumes, these are determined by applying a target market share to cancer incidence rates across countries within Americas, European and APAC regions, sourced from data provided by the World Health Organization’s International Agency for Research on Cancer | |
| ● | net sales price per unit, for commercialized products forecast average selling price is used and for products in development a target sales price is used | |
| ● | approval for marketing authorization probability success factor, this varies depending on the clinical trial stage of each program | |
| ● | in relation to cash outflows consideration has been given to cost of sales, selling and marketing expenses, general and administration costs and the anticipated research and development costs to reach commercialization. Associated expenses such as royalties, milestone payments and license fees are included, and | |
| ● | costs of disposal were assumed to be immaterial at December 31, 2023. |
Impact of possible changes in key assumptions
The Group has considered reasonable possible changes in the key assumptions and has not identified any instances that could cause the carrying amounts of the intangible assets at December 31, 2023 to exceed their recoverable amounts.
Whilst there is no impairment, the key sensitivities in the valuation remain the continued successful development and commercialization of core programs. If the Group is unable to successfully develop each product, this may result in an impairment of the carrying amount of our intangible assets.
Impairment triggers for definite life intangible assets
TLX66CDx (Scintimun) manufacturing uses Triton X-100, which can no longer be used in Europe without authorization from the Regulation on the registration, evaluation, authorization and restriction of chemicals (REACH). In December 2023, REACH declined an application from the Group for exemption for the use of Triton X-100 in the manufacturing of TLX66CDx. These adverse events indicated that the carrying amount of TLX66-CDx of $898,000 may not be recoverable at December 31, 2023 and the intangible asset was impaired.
Management is currently exploring whether Scintimun could be used for dosimetry to support the TLX66 program, subject to clinical testing. Improvements to the manufacturing process in response to these events could also result in a significant increase in productivity and a reduction in manufacturing costs, which could benefit both Scintimun and TLX66 in the future.
| F-35 |
Other than the impairment trigger on TLX66-CDx, there were no other internal or external factors identified that could result in an impairment of definite life intangible assets at December 31, 2023.
| 19. | Acquisitions |
Dedicaid GmbH
The Group completed the acquisition of Vienna-based Dedicaid GmbH on April 27, 2023. The acquisition does not meet the definition of a business in IFRS 3 Business Combinations and the transaction has been recognized as an asset acquisition. The fair values of identifiable assets on acquisition are outlined below:
| 2023 | ||||
| Consideration | A$’000 | |||
| Equity issued | 1,829 | |||
| Total consideration | 1,829 | |||
| Recognized amounts of identifiable assets acquired and liabilities assumed | ||||
| Trade and other receivables | 111 | |||
| Software | 1,659 | |||
| Cash and cash equivalents | 123 | |||
| Trade and other payables | (64 | ) | ||
| Total identifiable assets | 1,829 | |||
Lightpoint Medical
The Group completed the acquisition of Lightpoint Medical’s RGS business, assets and operations, through the purchase of Lightpoint Medical Limited’s wholly owned subsidiary, Lightpoint Surgical Limited on November 1, 2023. Lightpoint Medical – a technology leader in precision-guided robotic cancer surgery – develops and markets miniaturized imaging and sensing tools for advanced intra-operative cancer detection. The acquisition will support and expand Telix’s late- stage urologic pipeline and, together with its complementary AI technologies, will strengthen Telix’s capabilities in deploying molecular imaging in the surgical setting.
The upfront consideration was $31,522,000 (US$20,000,000) of which $30,895,000 (US$19,600,000) has been paid to Lightpoint Medical in equity through the issue of 3,298,073 fully paid ordinary Telix shares at $9.3659 per share, with the balance paid in cash. A further $23,624,000 (US$15,000,000) is payable via an earn-out in the form of rights (Performance Rights). Performance Rights will be settled in cash or equity (at Telix’s election) upon achievement of certain milestones (Milestone Events) relating to the ongoing development and commercialization of the SENSEI® probe and amounts have been recognized based on the probability of achieving the milestones.
The Group has determined that substantially all of the fair value of the gross assets acquired is concentrated in a single asset or a group of similar assets. The Group has applied the optional concentration of fair value test in IFRS 3 Business Combinations and concluded that the components acquired will be treated as an asset acquisition.
The Performance Rights have been recognized as an equity settled share based payment at a fair value of $21,278,000, which has been included in the fair value of intellectual property. Each milestone has a fixed dollar amount which can be settled either in cash of shares. The fair value of the Performance Rights was determined based on management’s assessment of the likelihood of each milestone being reached against the fixed dollar amount for that milestone. The likelihood of the milestones being attained are considered non-vesting conditions as there are no further services or obligations of the counterparty, thus being reflected in the fair value.
The fair values of identifiable assets on acquisition are outlined below:
| 2023 | ||||
| Consideration | A$’000 | |||
| Cash paid | 627 | |||
| Equity issued | 30,895 | |||
| Performance Rights issued | 21,278 | |||
| Total consideration | 52,800 | |||
| Recognized amounts of identifiable assets acquired and liabilities assumed | ||||
| Intellectual property | 52,294 | |||
| 2023 | ||||
| Consideration | A$’000 | |||
| Inventory | 406 | |||
| Patents | 266 | |||
| Property, plant and equipment | 37 | |||
| Other current assets | 32 | |||
| Trade payables | (235 | ) | ||
| Total identifiable assets | 52,800 | |||
| 20. | Trade and other payables |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Trade creditors | 32,837 | 16,806 | ||||||
| Accruals | 37,895 | 22,325 | ||||||
| Other creditors | 6,738 | 3,148 | ||||||
| Accrued royalties | 3,205 | 1,919 | ||||||
| Payroll liabilities | 899 | 972 | ||||||
| Government rebates payable | 130 | 4,349 | ||||||
| Total trade and other payables | 81,704 | 49,519 | ||||||
| F-36 |
| 21. | Borrowings |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Current | 964 | — | ||||||
| Non-current | 8,209 | 3,312 | ||||||
| Total borrowings | 9,173 | 3,312 | ||||||
All borrowings outstanding at December 31, 2023 are in relation to the build-out of the Brussels South radiopharmaceutical production facility. Telix Pharmaceuticals (Belgium) SPRL (a wholly owned subsidiary of Telix) entered into two loan agreements, one with BNP Paribas and IMBC Group totaling €10,100,000 on a 10-year term, and a second loan with BNP Paribas totaling €2,000,000 on a two-year extendable term. All loans have a two-year repayment holiday period, with repayments due to commence from March 2024. The loans are secured by a fixed charged over the facility.
The loan agreements entitle BNP Paribas and IMBC Group to suspend or terminate all or part of the undrawn portion of the loan facilities with immediate effect and without prior notice. At December 31, 2023, the undrawn portion under the agreements was €6,455,000 ($10,488,000). As at the reporting date Telix has not received any notice to this effect.
The loan agreements require Telix Pharmaceuticals (Belgium) SPRL to comply with various covenants relating to the conduct of the business, including non-payment of required repayments, specified cross-defaults (in the event of the use of trade bills) and ensuring cumulative losses of Telix Pharmaceuticals (Belgium) SPRL do not exceed 25% of its capital and reserves. Upon the occurrence of an event of default and in the event of a change of control, BNP Paribas and IMBC Group may accelerate payments due under the loan agreements or terminate the loan agreements. There were no events of default or changes of control during the year.
2023
| Lenders | Loan balance | Due < 1 year | Due > 1 year | Maturity date | ||||||||||
| A$’000 | A$’000 | A$’000 | ||||||||||||
| BNP Paribas | 9,173 | 964 | 8,209 | 29-Feb-32 | ||||||||||
| Total | 9,173 | 964 | 8,209 | |||||||||||
2022
| Lenders | Loan balance | Due < 1 year | Due > 1 year | Maturity date | ||||||||||
| A$’000 | A$’000 | A$’000 | ||||||||||||
| BNP Paribas | 3,312 | — | 3,312 | 29-Feb-32 | ||||||||||
| Total | 3,312 | — | 3,312 | |||||||||||
| F-37 |
Fair value: For all borrowings, the fair values are not materially different to their carrying amounts, since the interest payable on those borrowings is either close to current market rates or the borrowings are of a short-term nature.
Capital risk management: Capital is defined as the combination of shareholders’ equity, reserves and net debt. The key objective of the Group when managing its capital is to safeguard its ability to continue as a going concern, so that the Group can continue to provide benefits for stakeholders and maintain an optimal capital and funding structure. The aim of the Group’s capital management framework is to maintain, monitor and secure access to future funding arrangements to finance the necessary research and development activities being performed by the Group. Consistent with others in the industry, the Group monitors capital on the basis of the following gearing ratio: Debt as divided by Equity. At December 31, 2023 the Group’s on-balance sheet gearing and leverage ratio was less than 1% (2022: less than 1%).
Reconciliation of liabilities arising from financing activities:
Opening balance | Net cash inflow/ (outflow) | Other non- cash movements | Closing balance | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| For the year ended December 31, 2023 | ||||||||||||||||
| Borrowings | 3,312 | 5,756 | 105 | 9,173 | ||||||||||||
| Lease liabilities | 7,134 | (2,858 | ) | 3,996 | 8,272 | |||||||||||
| 10,446 | 2,898 | 4,101 | 17,445 | |||||||||||||
| For the year ended December 31, 2022 | ||||||||||||||||
| Borrowings | 19 | 3,293 | — | 3,312 | ||||||||||||
| Lease liabilities | 2,520 | (1,541 | ) | 6,155 | 7,134 | |||||||||||
| 2,539 | 1,752 | 6,155 | 10,446 |
Other non-cash movements include new leases entered into during the year, leases acquired via acquisitions of a business, disposal of leases and exchange differences.
| 22. | Contract liabilities |
The Group has recognized the following liabilities related to contracts with customers in licensing arrangements and non-reimbursable government grants received:
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Balance at January 1 | 27,462 | 29,199 | ||||||
| Consideration received | — | 537 | ||||||
| Revenue recognized | (5,291 | ) | (3,352 | ) | ||||
| Exchange differences | 17 | — | ||||||
| Unwind of discount | 969 | 1,078 | ||||||
| Balance at December 31 | 23,157 | 27,462 | ||||||
| Current | 10,995 | 4,940 | ||||||
| Non-current | 12,162 | 22,522 | ||||||
| Total contract liabilities | 23,157 | 27,462 | ||||||
| F-38 |
Grand Pharma strategic partnership
On November 2, 2020, the Group entered into a strategic commercial partnership with Grand Pharmaceutical Group Limited (Grand Pharma or GP, formerly known as China Grand Pharma or CGP) for the Group’s portfolio of targeted radiation products. A non-refundable upfront payment of US$25,000,000 was received upon signing of the contract with GP. The strategic partnership with GP is accounted for as a revenue contract comprising the grant of a sublicense of the Group’s existing intellectual property and the provision of research and development services. The Group has measured its contractual liability to undertake the identified future performance obligations relating to research and development services using a cost plus margin approach. As the performance obligation relating to research and development services is expected to be completed over several years from execution, a financing component has been recognized within Finance costs in profit or loss on an effective interest basis.
Walloon Region non-reimbursable grant
On August 29, 2022, Telix Innovations SA received a non-reimbursable government grant to support research efforts associated with 211At-TLX591/TLX592. The first installment received was for €365,000, this amount will be released to the Consolidated statement of comprehensive income or loss as the associated expenditure is incurred.
| 23. | Lease liabilities |
The consolidated statement of financial position shows the following amounts relating to leases:
| Lease liabilities | 2023 | 2022 | ||||||
| A$’000 | A$’000 | |||||||
| Current | 595 | 641 | ||||||
| Non-current | 7,677 | 6,493 | ||||||
| Total lease liabilities | 8,272 | 7,134 | ||||||
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Balance at January 1 | 7,134 | 2,520 | ||||||
| Additions | 3,436 | 6,164 | ||||||
| Acquisition of business | — | 423 | ||||||
| Interest expense | 636 | 277 | ||||||
| Lease payments (principal and interest) | (2,858 | ) | (1,541 | ) | ||||
| Disposals | — | (633 | ) | |||||
| Exchange differences | (76 | ) | (76 | ) | ||||
| Balance at December 31 | 8,272 | 7,134 | ||||||
| F-39 |
The consolidated statement of comprehensive income shows the following amounts relating to leases:
| Interest expense relating to leases | 2023 | 2022 | ||||||
| A$’000 | A$’000 | |||||||
| Properties | 604 | 244 | ||||||
| Motor vehicles | 32 | 33 | ||||||
| Total lease interest | 636 | 277 | ||||||
The total cash outflow for leases in 2023 comprises $2,222,000 (2022: $1,264,000) principal and $636,000 (2022:$277,000) interest payments.
| 24. | Provisions |
Government grant liability | Decommissioning liability | Total | ||||||||||
| A$’000 | A$’000 | A$’000 | ||||||||||
| Balance at January 1, 2023 | 2,551 | 5,333 | 7,884 | |||||||||
| Remeasurement of provisions | (173 | ) | — | (173 | ) | |||||||
| Unwind of discount | 238 | 181 | 419 | |||||||||
| Charged to profit or loss | 65 | 181 | 246 | |||||||||
| Exchange differences | 48 | 173 | 221 | |||||||||
| Amounts adjusted to intangible assets | — | 286 | 286 | |||||||||
| Provision utilized | — | (56 | ) | (56 | ) | |||||||
| Balance at December 31, 2023 | 2,664 | 5,917 | 8,581 | |||||||||
| Current | 577 | — | 577 | |||||||||
| Non-current | 2,087 | 5,917 | 8,004 | |||||||||
| Total provisions | 2,664 | 5,917 | 8,581 | |||||||||
| Balance at January 1, 2022 | 1,539 | 8,532 | 10,071 | |||||||||
| Remeasurement of provisions | 1,017 | — | 1,017 | |||||||||
| Unwind of discount | 115 | 137 | 252 | |||||||||
| Charged to profit or loss | 1,132 | 137 | 1,269 | |||||||||
| Exchange differences | (59 | ) | (73 | ) | (132 | ) | ||||||
| Acquisition of business | — | — | — | |||||||||
| Amounts adjusted to intangible assets | — | (1,100 | ) | (1,100 | ) | |||||||
| Provision utilized | (61 | ) | (2,163 | ) | (2,224 | ) | ||||||
| Balance at December 31, 2022 | 2,551 | 5,333 | 7,884 | |||||||||
| Current | 402 | — | 402 | |||||||||
| Non-current | 2,149 | 5,333 | 7,482 | |||||||||
| Total provisions | 2,551 | 5,333 | 7,884 | |||||||||
| 24.1. | Government grant liability |
Telix Innovations has received grants from the Walloon regional government in Belgium. These grants meet the definition of a financial liability as defined in IFRS 9 Financial Instruments and were designated to be measured at fair value through profit and loss.
The grants are repayable to the Walloon government based on a split between fixed and variable repayments. The fixed proportion is based on contractual cash flows agreed with the Walloon government. The variable cash flows are based on a fixed percentage of future sales and are capped at an agreed upon level.
The Group has estimated that the full variable repayments will be made up to the pre-agreed capped amount. The key inputs into this calculation are the risk adjusted discount rate of 3.3% (2022: 3.2%), the expected sales volumes and the net sales price per unit. The expected sales volumes and net sales price per unit assumptions are consistent with those utilized by the Group in the calculation of the contingent consideration liability and intellectual property valuation.
| 24.2. | Decommissioning liability |
Telix purchased the radiopharmaceutical production facility in Belgium on April 27, 2020. The site had cyclotrons installed in concrete shielded vaults which also contained some nuclear contamination associated with past manufacturing activities. As part of this transaction, Telix assumed the obligation to remove the cyclotrons and restore the site.
The Group removed the cyclotrons from the site during 2022. Other decommissioning activities not required to upgrade the production facility have been deferred to the end of the operating life of the facility in 2041. The decommissioning costs expected to be incurred in 2041 of €6,021,000 (2022: €6,021,000) have been discounted using the Belgium risk-free rate of 3.3% (2022: 3.2%) and translated to Australian dollars at the exchange rate at December 31, 2023.
The provision represents the best estimate of the expenditures required to settle the present obligation at December 31, 2023. While the Group has made its best estimate in establishing its decommissioning liability, because of potential changes in technology as well as safety and environmental requirements, plus the actual timescale to complete decommissioning, the ultimate provision requirements could vary from the Group’s current estimates. Any subsequent changes in estimate which alter the level of the provision required are also reflected in adjustments to the intangible license asset. Each year, the provision is increased to reflect the unwind of discount and to accrue an estimate for the effects of inflation, with the charges being presented in the consolidated statement of comprehensive income or loss. Actual payments for commencement of decommissioning activity are disclosed as provision utilized in the above table.
| F-40 |
| 25. | Contingent consideration |
| ANMI | TheraPharm | Optimal Tracers | Contingent
consideration | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| Balance at January 1, 2023 | 62,541 | 1,690 | 718 | 64,949 | ||||||||||||
| Remeasurement of contingent consideration | 34,275 | — | — | 34,275 | ||||||||||||
| Unwind of discount | 11,033 | 278 | 83 | 11,394 | ||||||||||||
| Charged to profit or loss | 45,308 | 278 | 83 | 45,669 | ||||||||||||
| Exchange differences | 410 | (279 | ) | (46 | ) | 85 | ||||||||||
| Amounts adjusted to intangible assets | — | 489 | (672 | ) | (183 | ) | ||||||||||
| Payments for contingent consideration | (17,766 | ) | — | — | (17,766 | ) | ||||||||||
| Balance at December 31, 2023 | 90,493 | 2,178 | 83 | 92,754 | ||||||||||||
| Current | 37,070 | — | 83 | 37,153 | ||||||||||||
| Non-current | 53,423 | 2,178 | — | 55,601 | ||||||||||||
| Total contingent consideration | 90,493 | 2,178 | 83 | 92,754 | ||||||||||||
| Balance at January 1, 2022 | 40,635 | 1,275 | — | 41,910 | ||||||||||||
| Remeasurement of contingent consideration | 16,707 | — | — | 16,707 | ||||||||||||
| Unwind of discount | 4,798 | 159 | — | 4,957 | ||||||||||||
| Charged to profit or loss | 21,505 | 159 | — | 21,664 | ||||||||||||
| Exchange differences | 401 | — | — | 401 | ||||||||||||
| Acquisition of business | — | — | 718 | 718 | ||||||||||||
| Amounts adjusted to intangible assets | — | 256 | — | 256 | ||||||||||||
| Balance at December 31, 2022 | 62,541 | 1,690 | 718 | 64,949 | ||||||||||||
| Current | 14,811 | — | 372 | 15,183 | ||||||||||||
| Non-current | 47,730 | 1,690 | 346 | 49,766 | ||||||||||||
| Total contingent consideration | 62,541 | 1,690 | 718 | 64,949 | ||||||||||||
Telix Innovations (formerly ANMI)
The Group acquired ANMI on December 24, 2018. The Group is liable for future variable payments which are calculated based on the percentage of net sales for five years following the achievement of marketing authorization of the product. The percentage of net sales varies depending on the net sales achieved in the U.S. and the rest of the world. The Group also holds an option to buy-out the remaining future variable payments in the third year following the achievement of marketing authorization, if specified sales thresholds are met.
As at consolidated statement of financial position date, the Group has remeasured the contingent consideration to its fair value. The remeasurement is as a result of changes to the key assumptions such as risk adjusted post-tax discount rate, expected sales volumes and net sales price per unit.
| F-41 |
The contingent consideration liability has been valued using a discounted cash flow model that utilizes certain unobservable level 3 inputs. These key assumptions include risk adjusted post-tax discount rate 15.0% (2022: 15.0%), expected sales volumes over the forecast period and net sales price per unit.
The following table summarizes the quantitative information about these assumptions, including the impact of sensitivities from reasonably possible changes where applicable:
Contingent consideration valuation
Unobservable input |
Methodology | December 31, 2023 | ||
| Risk adjusted post-tax discount rate | The post-tax discount rate used in the valuation has been determined based on required rates of returns of listed companies in the biotechnology industry (having regards to their stage of development, size and risk adjustments). | A 0.5% increase in the post-tax discount rate would decrease the contingent consideration by 0.4% and a 0.5% decrease in the post-tax discount rate would increase the contingent consideration by 0.4%. | ||
| Expected sales volumes | This is determined using actual sales volumes for 2023 and forecasting sales volumes for 2024 and beyond for each region. | A 10% increase in sales volumes across all regions would increase the contingent consideration by 5.5% and a 10% decrease in sales volumes would decrease the contingent consideration by 5.5% | ||
| Net sales price per unit | This is determined using actual sales prices for 2023 and forecasting sales prices for 2024 and beyond for each region. | A 10% increase in net sales price per unit across all regions would increase the contingent consideration by 5.6% and a 10% decrease in sales prices would decrease the contingent consideration by 5.6%. |
Telix Switzerland (formerly TheraPharm)
Telix acquired TheraPharm on December 14, 2020. Part of the consideration for the acquisition was in the form of future payments contingent on certain milestones. These are:
| ● | €5,000,000 cash payment upon successful completion of a Phase III pivotal registration trial | |
| ● | €5,000,000 cash payment upon achievement of marketing authorization in the Europe or the U.S., whichever approval comes first, and | |
| ● | 5% of net sales for the first three years following marketing authorization in the Europe or the U.S., whichever approval comes first. |
The valuation of the contingent consideration has been performed utilizing a discounted cash flow model that uses certain unobservable assumptions. These key assumptions include risk adjusted post-tax discount rate of 15.0% (2022: 15.0%), marketing authorization date, expected sales volumes over the forecast period, net sales price per unit and approval for marketing authorization probability success factor.
| F-42 |
The following table summarizes the quantitative information about these assumptions, including the impact of sensitivities from reasonably possible changes where applicable:
Contingent consideration valuation
Unobservable input |
Methodology | December 31, 2023 | ||
| Risk adjusted post-tax discount rate | The post-tax discount rate used in the valuation has been determined based on required rates of returns of listed companies in the biotechnology industry (having regards to their stage of development, size and risk adjustments). | A 0.5% increase in the post-tax discount rate would decrease the contingent consideration by 2.0% and a decrease in the post-tax discount rate by 0.5% would increase the contingent consideration by 2.0%. | ||
| Expected sales volumes | This is determined through assumptions on target market population, penetration and growth rates in the U.S. and Europe. | A 10% increase in the sales volumes would increase the contingent consideration by 0.7% and a 10% decrease in sales volumes would decrease the contingent consideration by 0.7%. | ||
| Net sales price per unit | The net sales price per unit is estimated based on comparable products currently in the market. | A 10% increase in the net sales price per unit would increase the contingent consideration by 1.6% and a 10% decrease in net sales price per unit would decrease the contingent consideration by 1.6%. | ||
| Approval for marketing authorization probability success factor | This assumption is based on management’s estimate for achieving regulatory approval and is determined through benchmarking of historic approval rates. | An increase in the probability of success factor by 10% would increase the contingent consideration by 50.0% and a 10% decrease in the probability of success factor would decrease the contingent consideration to nil. |
Telix Optimal Tracers
The Group acquired the assets of Optimal Tracers on December 31, 2022. The consideration includes two contingent payments based on a percentage of revenue from existing customers for the years ending December 31, 2023 and 2024.
| F-43 |
The valuation of the contingent consideration has been performed utilizing a discounted cash flow model that uses certain unobservable assumptions. These key assumptions include risk adjusted post-tax discount rate of 15.0% and expected revenue from existing customers over the next year.
The following table summarizes the quantitative information about these assumptions, including the impact of sensitivities from reasonably possible changes where applicable:
Contingent consideration valuation
Unobservable input |
Methodology | December 31, 2023 | ||
| Risk adjusted post-tax discount rate | The post-tax discount rate used in the valuation has been determined based on required rates of returns of listed companies in the biotechnology industry (having regards to their stage of development, size and risk adjustments). | A 0.5% increase in the post-tax discount rate would decrease the contingent consideration by 0.6% and a 0.5% decrease in the post-tax discount rate would increase the contingent consideration by 0.6%. | ||
| Expected revenue | This is determined using actual revenue for 2023 and forecasting revenue for 2024. | A 10% increase in revenue would increase the contingent consideration by 10.0% and a 10% decrease in revenue would decrease the contingent consideration by 10.0% |
| 26. | Employee benefit obligations |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Bonus | 10,630 | 5,101 | ||||||
| Annual leave | 3,282 | 2,450 | ||||||
| Long service leave | 330 | 215 | ||||||
| Balance at December 31 | 14,242 | 7,766 | ||||||
| Current | 13,912 | 7,551 | ||||||
| Non-current | 330 | 215 | ||||||
| Total employee benefit obligations | 14,242 | 7,766 | ||||||
| F-44 |
| 27. | Equity |
| 27.1. | Share capital |
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
| Number ‘000 | A$’000 | Number ‘000 | A$’000 | |||||||||||||
| Balance at January 1 | 316,343 | 370,972 | 285,073 | 170,840 | ||||||||||||
| Shares issued through the exercise of share options and warrants1 | 3,879 | 42,572 | 8,543 | 32,948 | ||||||||||||
| Contributions of equity2 | — | — | 22,727 | 175,000 | ||||||||||||
| Shares issued for Dedicaid GmbH3 | 207 | 1,829 | — | — | ||||||||||||
| Shares issued for Lightpoint transaction4 | 3,298 | 30,895 | — | — | ||||||||||||
| Transaction costs arising on new share issues | — | — | — | (7,816 | ) | |||||||||||
| Balance at December 31 | 323,727 | 446,268 | 316,343 | 370,972 | ||||||||||||
| 1. | Options exercised during the year through the employee Equity Incentive Plan resulted in 3,879,000 (2022: 8,543,000) shares being issued of total value of $42,572,000 (2022: $32,948,000). | |
| 2. | On January 27, 2022, the Group completed a $175,000,000 institutional placement of 22,727,000 new, fully paid ordinary shares at a price of $7.70 per share. As part of this placement, the Group also incurred $7,816,000 of associated transaction costs. | |
| 3. | On April 27, 2023, the Group completed the acquisition of Dedicaid GmbH. The consideration for the acquisition comprised 207,000 in Telix shares at a 10-day volume weighted average price of shares on the execution date of $8.73 per share. | |
| 4. | On November 1, 2023, the Group completed the acquisition of Lightpoint through the issue of 3,298,000 fully paid ordinary Telix shares at $9.3659 per share. |
The weighted average ordinary shares for the period January 1, 2023 to December 31, 2023 is 319,180,783 (2022: 310,644,169). The Company does not have a limited amount of authorized capital under Australian law.
Rights applying to securities:
| 1. | Ordinary shares: Ordinary shares entitle the holder to participate in dividends, and to share in the proceeds of winding up the Company in proportion to the number of and amounts paid on the shares held. | |
| 2. | Options and rights: Holders of Options and rights have no voting rights. Information relating to the Company’s Employee Incentive Plan (EIP), including details of Options issued, exercised and lapsed during the financial year, is set out in note 28. |
| 27.2. | Share capital reserve |
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
| Number ‘000 | A$’000 | Number ‘000 | A$’000 | |||||||||||||
| Balance at January 1 | — | (26,909 | ) | — | — | |||||||||||
| Treasury shares acquired | 3,877 | (35,920 | ) | 4,054 | (26,909 | ) | ||||||||||
| Shares allocated to employees | (3,877 | ) | — | (4,054 | ) | — | ||||||||||
| Balance at December 31 | — | (62,829 | ) | — | (26,909 | ) | ||||||||||
| F-45 |
Ordinary shares in the Company were purchased by the Telix Pharmaceuticals Employee Share Trust for the purpose of issuing shares under the Equity Incentive Plan, these shares are allocated to employees and are not held within the Employee Share Trust (see note 28 for further information).
| 27.3. | Share-based payments reserve |
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
| Number ‘000 | A$’000 | Number ‘000 | A$’000 | |||||||||||||
| Balance at January 1 | 11,736 | 9,321 | 17,148 | 5,942 | ||||||||||||
| EIP options issued | 6,689 | 8,786 | 4,436 | 8,114 | ||||||||||||
| Performance Rights issued1 | 2,524 | 21,278 | — | — | ||||||||||||
| Options exercised | (4,524 | ) | (3,939 | ) | (8,843 | ) | (4,735 | ) | ||||||||
| Options lapsed | (1,824 | ) | — | (1,005 | ) | — | ||||||||||
| Balance at December 31 | 14,601 | 35,446 | 11,736 | 9,321 | ||||||||||||
| 1. | Relates to the acquisition of Lightpoint. |
| 27.4. | Financial assets at FVOCI reserve |
The group has elected to recognize changes in the fair value of certain investments in equity securities in Other comprehensive income (OCI), as explained in note 14. These changes are accumulated within the FVOCI reserve within equity.
The table below shows how the FVOCI reserve relates to equity securities:
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Balance at January 1 | — | — | ||||||
| Revaluation - gross | (895 | ) | — | |||||
| Deferred tax | — | — | ||||||
| Balance at December 31 | (895 | ) | — | |||||
| 28. | Share based payments |
Equity Incentive Plan and Options
The Equity Incentive Plan (EIP) was established to allow the Board of Telix to make offers to Eligible Employees to acquire securities in the Company and to otherwise incentivize employees. ‘Eligible Employees’ includes full time, part time or casual employees of a Group Company, a Non-Executive Director of a Group Company, a Contractor, or any other person who is declared by the Board to be eligible.
| F-46 |
The Board may, from time to time and in its absolute discretion, invite Eligible Employees to participate in a grant of Incentive Securities, which may comprise Rights (including Performance Share Appreciation Rights), Options, and/or Restricted Shares. Vesting of Incentive Securities under the EIP is subject to any vesting or performance conditions determined by the Board. Incentive Securities are normally granted under the EIP for no consideration and carry no dividend or voting rights. When exercised, each Incentive Security is convertible into one Share.
Non-Executive Directors are able to participate in the Equity Incentive Plan, under which equity may be issued subject to Shareholder approval. Options are however normally issued to Non-Executive Directors not as an ‘incentive’ under the EIP but as a means of cost-effective consideration for agreeing to join the Board. The details of Incentive Securities on issue to individual Directors can be found in the Remuneration report for the year ended December 31, 2023. For the purposes of this table and to illustrate the total number of Incentive Securities on issue under the rules of the EIP, all Incentive Securities issued to Non-Executive Directors, Executive Directors, employees and contractors are included.
Incentive Securities contain a cashless exercise clause that allows employees to exercise the securities for an exercise price of $0.00 in exchange for forfeiting a portion of their vested securities.
| 2023 | 2023 | 2022 | 2022 | |||||||||||||
| Number | Number | |||||||||||||||
| ‘000 | WAEP1 | ‘000 | WAEP1 | |||||||||||||
| Balance at January 1 | 11,736 | 3.62 | 17,148 | 2.03 | ||||||||||||
| Granted during the year | 6,689 | 6.64 | 4,436 | 5.10 | ||||||||||||
| Exercised during the year | (4,524 | ) | 2.68 | (8,843 | ) | 1.25 | ||||||||||
| Lapsed/forfeited during the year | (1,824 | ) | 4.00 | (1,005 | ) | 3.80 | ||||||||||
| Balance at December 31 | 12,077 | 5.59 | 11,736 | 3.62 | ||||||||||||
| Vested and exercisable at December 31 | 2,221 | 3.73 | 3,199 | 3.93 | ||||||||||||
| 1. | WAEP - weighted average exercise price |
Expense arising from share based payments transactions:
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Options issued under EIP | 8,786 | 8,114 | ||||||
| Total | 8,786 | 8,114 | ||||||
Equity Incentive Plan and Options
| F-47 |
Details of the number of options issued under the EIP outstanding at the end of the year:
| Grant date | Vesting date | Expiry date | Exercise price | Options on issue at January 1, 2023 | Issued during the year | Vested during the year | Exercised during the year | Lapsed during the year | Options on issue at December 31, 2023 | |||||||||||||||||||||||||||
| ‘000 | ‘000 | ‘000 | ‘000 | ‘000 | ‘000 | |||||||||||||||||||||||||||||||
| 11-Jun-18 | 11-Jun-20 | 11-Jun-22 | 0.85 | — | — | — | — | — | — | |||||||||||||||||||||||||||
| 11-Jun-18 | 11-Jun-21 | 11-Jun-22 | 0.85 | — | — | — | — | — | — | |||||||||||||||||||||||||||
| 24-Jan-19 | 24-Jan-22 | 24-Jan-23 | 1.09 | 450 | — | — | (200 | ) | (250 | ) | — | |||||||||||||||||||||||||
| 4-Nov-19 | 4-Nov-22 | 3-Nov-23 | 2.30 | 430 | — | — | (330 | ) | — | 100 | ||||||||||||||||||||||||||
| 13-Jan-20 | 13-Jan-23 | 12-Jan-24 | 2.23 | 3,080 | — | 3,080 | (2,210 | ) | (135 | ) | 735 | |||||||||||||||||||||||||
| 1-Jul-20 | 1-Jul-23 | 30-Jun-24 | 1.83 | 1,300 | — | 1,300 | (762 | ) | (450 | ) | 88 | |||||||||||||||||||||||||
| 27-Jan-21 | 28-Oct-22 | 26-Jan-26 | 4.38 | 1,386 | — | — | (674 | ) | — | 712 | ||||||||||||||||||||||||||
| 27-Jul-21 | 28-Oct-22 | 27-Jul-26 | 5.37 | 933 | — | — | (348 | ) | — | 585 | ||||||||||||||||||||||||||
| 27-Jul-21 | 27-Jul-25 | 27-Jul-26 | 0.00 | 100 | — | — | — | — | 100 | |||||||||||||||||||||||||||
| 5-Apr-22 | 31-Dec-24 | 4-Apr-27 | 4.95 | 2,452 | — | — | — | (374 | ) | 2,078 | ||||||||||||||||||||||||||
| 5-Apr-22 | 31-Dec-24 | 4-Apr-27 | 0.00 | 205 | — | — | — | (55 | ) | 150 | ||||||||||||||||||||||||||
| 24-Oct-22 | 31-Dec-24 | 24-Oct-27 | 6.15 | 1,400 | — | — | — | (141 | ) | 1,259 | ||||||||||||||||||||||||||
| 2-May-23 | 31-Dec-25 | 27-Mar-28 | 6.90 | — | 3,362 | — | — | (286 | ) | 3,076 | ||||||||||||||||||||||||||
| 6-Jul-23 | 31-Dec-25 | 16-May-28 | 10.04 | — | 817 | — | — | (38 | ) | 779 | ||||||||||||||||||||||||||
| 6-Jul-23 | 31-Mar-25 or 31-Dec-25 | 15-Jun-25, 15-Jun-28 | 0.00 | — | 260 | — | — | (15 | ) | 245 | ||||||||||||||||||||||||||
| 18-Oct-23 | 30-Jun-26 | 20-Sep-28 | 11.37 | — | 508 | — | — | (42 | ) | 466 | ||||||||||||||||||||||||||
| 31-Oct-23 | 31-Dec-26 | 1-Nov-28 | 0.00 | — | 466 | — | — | — | 466 | |||||||||||||||||||||||||||
| 31-Oct-23 | 31-Dec-27 | 1-Nov-29 | 0.00 | — | 466 | — | — | — | 466 | |||||||||||||||||||||||||||
| 30-Nov-23 | 30-Jun-26 | 14-Nov-28 | 8.91 | — | 810 | — | — | (38 | ) | 772 | ||||||||||||||||||||||||||
| 11,736 | 6,689 | 4,380 | (4,524 | ) | (1,824 | ) | 12,077 | |||||||||||||||||||||||||||||
The assessed fair value of recent tranches of options granted are outlined below. The fair value at grant date is independently determined using the Black Scholes Model. The model inputs for options granted during the year ended December 31, 2023 and December 31, 2022 are included below.
| F-48 |
| Apr-22 | Oct-22 | May-23 | Jul-23 | Oct-23 | Nov-23 | |||||||||||||||||||
| Fair value | $ | 2.43 | $ | 3.08 | $ | 3.79 | $ | 6.44 | $ | 6.33 | $ | 5.21 | ||||||||||||
| Consideration | $NIL | $NIL | $NIL | $NIL | $NIL | $NIL | ||||||||||||||||||
| Exercise price | $ | 4.95 | $ | 6.15 | $ | 6.90 | $ | 10.04 | $ | 11.37 | $ | 8.91 | ||||||||||||
| Grant date | 5-Apr-22 | 24-Oct-22 | 2-May-23 | 6-Jul-23 | 18-Oct-23 | 30-Nov-23 | ||||||||||||||||||
| Expiry date | 4-Apr-27 | 24-Oct-27 | 27-Mar-28 | 16-May-28 | 20-Sep-28 | 14-Nov-28 | ||||||||||||||||||
| Term | 5 years | 5 years | 5 years | 5 years | 6 years | 7 years | ||||||||||||||||||
| Share price at grant date | $ | 4.53 | $ | 6.97 | $ | 7.03 | $ | 11.36 | $ | 11.50 | $ | 9.28 | ||||||||||||
| Volatility | 60 | % | 60 | % | 60 | % | 60 | % | 60 | % | 60 | % | ||||||||||||
| Dividend yield | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | 0.00 | % | ||||||||||||
| Risk-free rate | 2.62 | % | 3.52 | % | 2.91 | % | 3.15 | % | 3.98 | % | 4.36 | % | ||||||||||||
| 29. | Cash flow information |
| 29.1. | Reconciliation of profit/(loss) after income tax to net cash from/(used in) operating activities |
| 2023 | 2022 | |||||||
| A$’000 | A$’000 | |||||||
| Profit/(loss) before income tax | 3,087 | (98,622 | ) | |||||
| Adjustments for | ||||||||
| Depreciation and amortization | 6,743 | 5,379 | ||||||
| Impairment of intangible assets | 804 | — | ||||||
| Fair value remeasurement of contingent consideration | 34,275 | 16,707 | ||||||
| Fair value remeasurement of provisions | (173 | ) | 1,017 | |||||
| Unwind of discount | 12,782 | 6,287 | ||||||
| Share based payments | 8,786 | 8,114 | ||||||
| Foreign exchange losses | 1,339 | 433 | ||||||
| Income taxes paid | (10,253 | ) | (2,278 | ) | ||||
| Change in assets and liabilities | ||||||||
| (Increase) in trade and other receivables | (27,382 | ) | (19,934 | ) | ||||
| (Increase) in inventory | (9,636 | ) | (5,023 | ) | ||||
| (Increase)/decrease in other current assets | (10,451 | ) | (6,441 | ) | ||||
| (Increase) in other non-current assets | (259 | ) | (115 | ) | ||||
| Increase in trade creditors | 33,704 | 30,451 | ||||||
| Deduct trade and other payables capitalized to intangible assets | (4,385 | ) | — | |||||
| Contingent consideration payments classified as operating | (16,282 | ) | — | |||||
| Increase in employee benefit obligations | 6,476 | 2,870 | ||||||
| (Decrease) in contract liabilities | (5,291 | ) | (2,815 | ) | ||||
| Net cash from/(used in) operating activities | 23,884 | (63,970 | ) | |||||
| F-49 |
| 30. | Financial risk management |
The Group’s activities expose it to a variety of financial risks: market risk, credit risk and liquidity risk. The overall risk management program focuses on the unpredictability of markets and seeks to minimize potential adverse effects on the financial performance of the Group. The Group uses different methods to measure different types of risk to which it is exposed.
| 30.1. | Interest rate risk |
The Group’s borrowings that have been drawn down at December 31, 2023 have fixed interest rates, and therefore the Group is not exposed to any significant interest rate risk.
| 30.2. | Price risk |
The Group is not exposed to any significant price risk as contracts are in place to meet current estimated material requirements.
| 30.3. | Foreign currency risk |
Foreign currency risk is the risk of fluctuation in fair value or future cash flows of a financial instrument as a result of changes in foreign exchange rates. The Group operates internationally and is exposed to foreign exchange risk, primarily the US dollar and Euro. Foreign exchange risk arises from commercial activities in the U.S. and research and development activities in Europe and the U.S.
The Group’s treasury risk management policy is to settle all US dollar denominated expenditure with US dollar denominated receipts from sales of Illuccix® in the U.S. The Group also manages currency risk by making decisions as to the levels of cash to hold in each currency by assessing its future activities which will likely be incurred in those currencies. Any remaining foreign currency exposure has therefore not been hedged.
The Group has both foreign currency receivables and payables, predominantly denominated in US dollar and Euro. The Group had a surplus of foreign currency receivables over payables of $26,488,000 at December 31, 2023 (2022: $24,176,000).
The Group’s exposure to the risk of changes in foreign exchange rates also relates to the Group’s net investments in foreign subsidiaries, which predominantly include denominations in Euro and US dollar, however given the level of current investments in foreign subsidiaries, the impact is limited.
As at December 31, 2023, the Group held 6.7% (2022: 44.5%) of its cash in Australian dollars, 77.5% (2022: 52.1%) in US dollars, 15.4% (2022: 3.2%) in EUR, 0.1% (2022: 0.1%) in Japanese Yen (JPY) and 0.3% (2022: 0.1%) in Swiss Francs (CHF).
| F-50 |
Exposure
The balances held at December 31, 2023 that give rise to currency risk exposure are presented in Australian dollars below:
| USD | EUR | CHF | JPY | SGD | GBP | CAD | ||||||||||||||||||||||
| $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | ||||||||||||||||||||||
| Cash and cash equivalents | 95,543 | 18,953 | 315 | 134 | — | — | 72 | |||||||||||||||||||||
| Trade receivables | 63,634 | 403 | — | — | — | — | — | |||||||||||||||||||||
| Financial assets | 2,763 | 9,497 | — | — | — | — | — | |||||||||||||||||||||
| Trade payables | (37,843 | ) | (11,765 | ) | (192 | ) | (12 | ) | — | 3 | — | |||||||||||||||||
| Government grant liability | — | (2,663 | ) | — | — | — | — | — | ||||||||||||||||||||
| Decommissioning liability | — | (5,917 | ) | — | — | — | — | — | ||||||||||||||||||||
| Contingent consideration liability | (72,314 | ) | (17,100 | ) | — | — | — | — | — | |||||||||||||||||||
| Borrowings | — | (9,173 | ) | — | — | — | — | — | ||||||||||||||||||||
The balances held at December 31, 2022 that give rise to currency risk exposure are presented in Australian dollars below:
| USD | EUR | CHF | JPY | SGD | GBP | CAD | ||||||||||||||||||||||
| $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | ||||||||||||||||||||||
| Cash and cash equivalents | 60,659 | 3,678 | 118 | 133 | — | — | — | |||||||||||||||||||||
| Trade receivables | 37,131 | 1,168 | — | — | — | — | — | |||||||||||||||||||||
| Trade payables | (9,224 | ) | (4,721 | ) | — | (8 | ) | — | (162 | ) | (8 | ) | ||||||||||||||||
| Government grant liability | — | (2,550 | ) | — | — | — | — | — | ||||||||||||||||||||
| Decommissioning liability | — | (5,333 | ) | — | — | — | — | — | ||||||||||||||||||||
| Contingent consideration liability | — | (64,231 | ) | — | — | — | — | — | ||||||||||||||||||||
| Borrowings | — | (3,312 | ) | — | — | — | — | — | ||||||||||||||||||||
Sensitivity
Outlined below is a sensitivity analysis which assesses the impact that a change of +/- 10% in the exchange rates as at each reporting date would have on the Group’s reported profit/(loss) after income tax and/or equity balance.
| Impact on post-tax profit/(loss) | ||||||||||||||||||||||||||||||||
| 2023 | 2023 | 2023 | 2023 | 2022 | 2022 | 2022 | 2022 | |||||||||||||||||||||||||
+10% Profit/ (loss) | -10% Profit/ (loss) | +10% Equity | -10% Equity | +10% Profit/ (loss) | -10% Profit/ (loss) | +10% Equity | -10% Equity | |||||||||||||||||||||||||
| $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | $’000 | |||||||||||||||||||||||||
| USD | 1,699 | (2,076 | ) | (7,860 | ) | 9,606 | (2,036 | ) | 2,488 | (6,016 | ) | 7,352 | ||||||||||||||||||||
| EUR | 1,496 | (1,828 | ) | (231 | ) | 283 | 5,837 | (7,134 | ) | 1,009 | (1,233 | ) | ||||||||||||||||||||
| CHF | — | — | (29 | ) | 35 | (11 | ) | 13 | — | — | ||||||||||||||||||||||
| JPY | — | — | (12 | ) | 14 | (11 | ) | 14 | — | — | ||||||||||||||||||||||
| SGD | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| GBP | — | 1 | — | — | 15 | (18 | ) | — | — | |||||||||||||||||||||||
| CAD | — | — | (7 | ) | 8 | 1 | (1 | ) | — | — | ||||||||||||||||||||||
| Total | 3,195 | (3,903 | ) | (8,139 | ) | 9,946 | 3,795 | (4,638 | ) | (5,007 | ) | 6,119 | ||||||||||||||||||||
| F-51 |
| 30.4. | Credit risk |
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Group. Credit risk arises from cash and cash equivalents and credit exposures to customers, including outstanding receivables.
Credit risk is managed on a group basis. If customers are independently rated, these ratings are used. Otherwise, if there is no independent rating, the Group assesses the credit quality of the customer, taking into account its financial position, past experience and other factors. Individual risk limits are set based on internal or external ratings. The compliance with credit limits by customers is regularly monitored. The Group obtains guarantees where appropriate to mitigate credit risk.
The Group applies the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance for all trade receivables.
To measure the expected credit losses, trade receivables have been grouped based on shared credit risk characteristics and the days past due. The expected loss rates are based on historical payment profiles of sales and the corresponding historical credit losses experienced. The historical loss rates are adjusted to reflect current and forward-looking information on macroeconomic factors affecting the ability of the customers to settle the receivables.
Trade receivables are written off where there is no reasonable expectation of recovery. Indicators that there is no reasonable expectation of recovery include, amongst others, the failure of a debtor to engage in a repayment plan with the Group, and the failure to make contractual payments for a period of greater than 120 days past due.
Impairment losses on trade receivables are presented within selling, general and administration costs within profit or loss. Subsequent recoveries of amounts previously written off are credited against the same line item.
As at December 31, 2023, the expected credit losses are $533,000 (2022: $Nil). The following tables sets out the ageing of trade receivables, according to their due date:
Aged trade receivables
| Expected credit losses | Gross carrying amount | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| Not past due: | — | — | 57,576 | 37,145 | ||||||||||||
| Past due: | ||||||||||||||||
| 30 days | — | — | 4,298 | 1,599 | ||||||||||||
| F-52 |
| Expected credit losses | Gross carrying amount | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| A$’000 | A$’000 | A$’000 | A$’000 | |||||||||||||
| 60 days | (1 | ) | — | 381 | 121 | |||||||||||
| 90 days | (4 | ) | — | 932 | 34 | |||||||||||
| 120 days | (528 | ) | — | 2,123 | 455 | |||||||||||
| Total | (533 | ) | — | 65,310 | 39,354 | |||||||||||
Credit risk concentration profile
The Group has a significant credit risk exposure to three distributors of 81% (2022: 89% to three distributors). The Group defines major credit risk as exposure to a concentration exceeding 10% of a total class of such asset.
| 30.5. | Liquidity risk |
The Group is exposed to liquidity and funding risk from operations and from external borrowings, where the risk is that the Group may not be able to refinance debt obligations or meet other cash outflow obligations when required. Vigilant liquidity risk management requires the Group to maintain sufficient liquid assets (mainly cash and cash equivalents). The Group manages liquidity risk by maintaining adequate cash reserves by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities.
Remaining contractual maturities:
The following tables detail the Group’s remaining contractual maturity for its financial instrument liabilities. The tables have been drawn up based on the undiscounted cash flows of financial liabilities based on the earliest date on which the financial liabilities are required to be paid. The tables include both interest and principal cash flows disclosed as remaining contractual maturities and therefore these totals may differ from their carrying amount in the consolidated statement of financial position.
| 1-6 months | 6-12 months | 1-5 years | Over 5 years | Total contractual cash flows | Carrying amount of liabilities | |||||||||||||||||||
| As at December 31, 2023 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||||||||
| Non-derivatives | ||||||||||||||||||||||||
| Trade and other payables | 81,704 | — | — | — | 81,704 | 81,704 | ||||||||||||||||||
| Borrowings | 1,105 | 1,105 | 8,839 | 6,859 | 17,908 | 9,173 | ||||||||||||||||||
| Lease liabilities | 1,044 | 1,057 | 6,744 | 1,264 | 10,109 | 8,272 | ||||||||||||||||||
| Government grant liability | 376 | 577 | 3,169 | 593 | 4,715 | 2,664 | ||||||||||||||||||
| Decommissioning liability | — | — | — | 9,782 | 9,782 | 5,917 | ||||||||||||||||||
| Contingent consideration | — | 38,382 | 65,229 | 2,352 | 105,963 | 92,754 | ||||||||||||||||||
| Total financial liabilities | 84,229 | 41,121 | 83,981 | 20,850 | 230,181 | 200,484 | ||||||||||||||||||
| F-53 |
| 1-6 months | 6-12 months | 1-5 years | Over 5 years | Total contractual cash flows | Carrying amount of liabilities | |||||||||||||||||||
| As at December 31, 2022 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | A$’000 | ||||||||||||||||||
| Non-derivatives | ||||||||||||||||||||||||
| Trade and other payables | 49,519 | — | — | — | 49,519 | 49,519 | ||||||||||||||||||
| Borrowings | 58 | 58 | 5,080 | 1,800 | 6,996 | 3,312 | ||||||||||||||||||
| Lease liabilities | 815 | 802 | 6,419 | 1,862 | 9,898 | 7,134 | ||||||||||||||||||
| Government grant liability | 330 | 550 | 1,490 | 368 | 2,738 | 2,551 | ||||||||||||||||||
| Decommissioning liability | — | — | — | 9,468 | 9,468 | 5,333 | ||||||||||||||||||
| Contingent consideration | 15,331 | — | 63,793 | 2,130 | 81,254 | 64,949 | ||||||||||||||||||
| Total financial liabilities | 66,053 | 1,410 | 76,782 | 15,628 | 159,873 | 132,798 | ||||||||||||||||||
| 30.6. | Fair value |
| 30.6.1. | Financial assets |
Financial assets are categorized as level 1 financial assets and remeasured at each reporting date with movements recognized in other comprehensive income. The inputs used in the fair value calculations are with reference to published price quotations for the associated equity instruments in an active market.
Sensitivity of level 1 financial assets
An increase/(decrease) of 10% in the share price of each financial asset while holding all other variables constant will increase/(decrease) other comprehensive income by $1,178,000 (2022: $nil).
| 30.6.2. | Financial liabilities |
Contingent consideration liabilities are categorized as level 3 financial liabilities and remeasured at each reporting date with movements recognized in profit or loss, except in instances where changes are permitted to be added to/reduce an associated asset. The inputs used in fair value calculations are determined by Management.
The carrying amount of financial liabilities measured at fair value is principally calculated based on inputs other than quoted prices that are observable for these financial liabilities, either directly (i.e. as unquoted prices) or indirectly (i.e. derived from prices). Where no price information is available from a quoted market source, alternative market mechanisms or recent comparable transactions, fair value is estimated based on the management’s views on relevant future prices, net of valuation allowances to accommodate liquidity, modelling and other risks implicit in such estimates.
| F-54 |
Sensitivity of level 3 financial liabilities
The potential effect of using reasonably possible alternative assumptions in valuation models, based on a change in the most significant input, such as sales volumes, by an increase/(decrease) of 10% while holding all other variables constant will increase/(decrease) profit before tax by $5,061,000 (2022: $4,510,000).
Valuation processes
The finance team of the Group performs the valuation of contingent consideration liabilities required for financial reporting purposes, including level 3 fair values. This team reports directly to the Chief Financial Officer (CFO). Discussions of valuation processes and results are held between the CFO and Board at least once every six months, in line with the Group’s half-yearly reporting periods.
The main level 3 inputs used by the Group in measuring the fair value of contingent consideration liabilities are derived and evaluated as follows:
| ● | discount rates are determined by an independent third party using a weighted average cost of capital model to calculate a post-tax rate that reflects current market assessments of the time value of money and the risk specific to the asset | |
| ● | regulatory/marketing authorization approval dates and approval for marketing authorization probability risk factors are derived in consultation with the Group’s regulatory team | |
| ● | expected sales volumes and net sales price per unit are estimated based on market information on annual incidence rates and information for similar products and expected market penetration, and | |
| ● | contingent consideration cash flows are estimated based on the terms of the sale contract. Changes in fair values are analyzed at the end of each reporting period during the half-yearly valuation discussion between the CFO and Board. As part of this discussion the CFO presents a report that explains the reason for the fair value movement. |
| 31. | Contingent liabilities |
The Group has entered into collaboration arrangements, including in-licensing arrangements with various companies. Such collaboration agreements may require the Group to make payments on achievement of stages of development, launch or revenue milestones and may include variable payments that are based on unit sales or profit (e.g. royalty and profit share payments). The amount of variable payments under the arrangements are inherently uncertain and difficult to predict, given the direct link to future sales, profit levels and the range of outcomes.
The Group also has certain take or pay arrangements with contract manufacturers or service providers which serve as commercial manufacturers and suppliers for certain products. To the extent a commitment is determined to be onerous, these are provided for within provisions in the consolidated statement of financial position.
On March 18, 2021 the Group entered into a non-exclusive global clinical and commercial supply agreement with Garching-based ITM Isotopen Technologien München AG (ITM) for the supply of highly pure no-carrier-added lutetium-177, a therapeutic isotope. ITM will supply the product for use in the Group’s investigational programs in prostate and kidney cancer therapy and subject to approval of the Group’s drug candidates for therapeutic use, also provide the product for scale-up and commercialization. At December 31, 2023 there is a possible obligation for the Group to pay€1,000,000 to ITM on the approval of the product for therapeutic use by the relevant regulatory authority in either U.S., France, Germany, Spain, Italy or the UK and €1,000,000 when the Group makes a commercial arms-length sale of the product. The existence of the obligation will be confirmed only by the occurrence of one or more uncertain future events not wholly within the control of the Group.
On December 19, 2023 the Group submitted its Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for its investigational PET imaging agent TLX250-CDx in clear cell renal cell carcinoma (ccRCC). As at December 31, 2023, there are potential milestone payments of US$1,850,000 to a licensor should the Group be successful in obtaining regulatory approval and commercialization in the U.S.
| F-55 |
| 32. | Commitments |
At December 31, 2023 and at the date of these financial statements, the Group had commitments against existing R&D and capital commitments relating to the purchase of Ytterbium-176 isotopes from a vendor over a three year period. R&D commitments in future years are estimated based on the contractual obligations included within agreements entered into by the Group.
| Due < 1 year | Due > 1 year | |||||||
| A$’000 | A$’000 | |||||||
| At December 31, 2023 | ||||||||
| Capital commitments1 | 16,572 | 40,000 | ||||||
| R&D commitments | 28,112 | 20,403 | ||||||
| 44,684 | 60,403 | |||||||
| December 31, 2022 | ||||||||
| Capital commitments2 | 6,764 | — | ||||||
| R&D commitments | 15,583 | 2,293 | ||||||
| 22,347 | 2,293 | |||||||
1. Includes the three year supply of Ytterbium-176 isotope.
2. Restated to exclude Brussels South radiopharmaceutical production facility buildout costs incurred to December 31, 2022.
| 33. | Related party transactions |
| 33.1. | Key management personnel compensation |
| 2023 | 2022 | |||||||
| A$ | A$ | |||||||
| Short-term employee benefits | 3,092,881 | 2,146,954 | ||||||
| Superannuation entitlements | 159,017 | 116,922 | ||||||
| Share-based payments | 1,167,650 | 542,456 | ||||||
| 4,419,548 | 2,806,332 | |||||||
| F-56 |
| 33.2. | Transactions with other related parties |
| 2023 | 2022 | |||||||
| A$ | A$ | |||||||
| Purchases of various goods and services from entities controlled by key management personnel1 | 1,256,490 | 3,685,543 | ||||||
| 1. | Non-Executive Director, Dr Andreas Kluge, is the principal owner and Geschäftsführer (Managing Director) of ABX- CRO, a clinical research organization (CRO) that specializes in radiopharmaceutical product development. |
Telix entered into a master services agreement with ABX-CRO in 2018 for the provision of project management, clinical and analytical services for its ZIRCON clinical trial. During 2023, ABX-CRO were engaged to perform close out activities relating to the Phase III Zircon trial for TLX250-CDx, including delivery of dosimetry, PK evaluation, and the imaging report.
During the year ended December 31, 2023, the total amount paid was $1,256,490 (2022: $3,411,019) and the amount payable to ABX-CRO at December 31, 2023 was $nil (2022: $274,524) respectively. ABX-CRO’s fees and charges for activities undertaken in 2023 were on an arm’s length basis and competitive with quotes obtained from other CRO’s for similar services.
| 33.3. | Interests in other entities |
The Group’s principal subsidiaries at December 31, 2023 are set out below. Unless otherwise stated, they have share capital consisting solely of ordinary shares that are held directly by the Group, and the proportion of ownership interests held equals the voting rights held by the Group. The country of incorporation or registration is also the principal place of business.
| F-57 |
| Name of entity | Place of business/ country of incorporation |
Ownership interest held by the Group (%) |
Principal activities | |||
| Telix Pharmaceuticals (EST) Pty Ltd | Australia | 100 | Dormant | |||
| Telix Pharmaceuticals (Innovations) Pty Limited (formerly Telix International Pty Ltd)1 | Australia | 100 | Manufacturing and development | |||
| Telix Pharmaceuticals Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals International Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals Australia Holdings Pty Limited1 | Australia | 100 | Holding company | |||
| Telix Pharmaceuticals (ANZ) Pty Ltd1 | Australia | 100 | Commercial operations | |||
| Telix Pharmaceuticals (Corporate) Pty Limited1 | Australia | 100 | Commercial operations | |||
| Telix Pharmaceuticals (Belgium) SRL | Belgium | 100 | Manufacturing and development | |||
| Telix Innovations SA | Belgium | 100 | Commercial operations | |||
| Telix Pharmaceuticals (Canada) Inc. | Canada | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (France) SAS | France | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Germany) GmbH (formerly Telix Pharmaceuticals Holdings (Germany) GmbH) | Germany | 100 | Clinical R&D | |||
| Rhine Pharma GmbH (formerly Telix Pharmaceuticals (Germany) GmbH) | Germany | 100 | Clinical R&D | |||
| Therapeia GmbH & Co. KG | Germany | 100 | Clinical R&D | |||
| Dedicaid GmbH | Austria | 100 | Software | |||
| Telix Pharma Japan KK | Japan | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (NZ) Limited | New Zealand | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Singapore) Pte Ltd | Singapore | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (Switzerland) GmbH | Switzerland | 100 | Clinical R&D | |||
| Telix Pharmaceuticals (UK) Ltd (formerly Telix Life Sciences (UK) Ltd) | United Kingdom | 100 | Clinical R&D | |||
| Lightpoint Surgical Ltd | United Kingdom | 100 | Medical devices | |||
| Lightpoint Medical Espana SLU | Spain | 100 | Medical devices | |||
| Telix Pharmaceuticals (US) Inc. | USA | 100 | Commercial operations | |||
| Telix Optimal Tracers, LLC | USA | 100 | Manufacturing and development |
| 1. | Denotes an entity that is a party to a deed of cross guarantee |
TheraPharm Deutschland GmbH was wound up during the financial year.
| 34. | Remuneration of auditor |
| Auditors of the Group - PricewaterhouseCoopers Australia and related network firms | 2023 | 2022 | ||||||
| A$ | A$ | |||||||
| Audit or review of financial statements | 1,380,000 | 367,200 | ||||||
| Other assurance services | 170,000 | — | ||||||
| Other advisory services | 291,861 | 156,857 | ||||||
| 1,841,861 | 524,057 | |||||||
| F-58 |
| Other auditors and their related network firms | 2023 | 2022 | ||||||
| A$ | A$ | |||||||
| Audit or review of financial statements | 52,538 | 89,621 | ||||||
| Other advisory services | — | 9,435 | ||||||
| 52,538 | 99,056 | |||||||
| 35. | Events occurring after the reporting period |
On January 5, 2024 Telix announced that it is considering an initial public offering (IPO) of American Depositary Shares (ADSs) representing its ordinary shares in the U.S. and listing on the Nasdaq Global Market (Nasdaq). Telix’s ordinary shares will remain listed on the Australian Securities Exchange. The number of ADSs that may be offered, the number of underlying ordinary shares that may be issued, the price for such instruments and the timing of the offering have not yet been finalized. No final decision has been made in respect of the offering or Nasdaq listing and there can be no assurance as to the occurrence, timing, pricing and/or completion of such an offering or listing.
On February 8, 2024 Telix entered into an agreement to acquire QSAM Biosciences, Inc. (QSAM), a U.S. based company developing therapeutic radiopharmaceuticals for primary and metastatic bone cancer. The purchase price comprises $50,800,000 (US$33,100,000) upfront, which is payable in the form of 4,369,914 Telix ordinary shares (subject to certain adjustments at completion) and performance rights, that represent the right of the holders to receive contingent payments up to $138,000,000 (US$90,000,000) in aggregate. The contingent payments are payable in cash and/or in ordinary shares, upon achievement of certain clinical and commercial milestones.
On February 27, 2024, Telix entered into an agreement to acquire IsoTherapeutics Group LLC (IsoTherapeutics). IsoTherapeutics is a privately held, commercial-stage company based in Texas, U.S. that provides radiochemistry and bioconjugation development and contract manufacturing services to the radiopharmaceutical industry. The purchase price comprises A$3.0 million (US$2 million) upfront consideration payable in cash and A$9.2 million (US$6.0 million) upfront consideration payable in shares, A$7.6 million (US$5 million) in performance-related milestone payments payable in cash, and a two-year revenue share based on actual revenue earned from existing customers of IsoTherapeutics payable in cash of approximately A$0.9 million (US$0.6 million). The cash upfront consideration is subject to customary working capital, debt and transaction expense adjustments.
On March 5, 2024 Telix entered into an agreement to acquire radioisotope production technology firm ARTMS Inc. (ARTMS), its advanced cyclotron-based isotope production platform, manufacturing plant and stockpile of ultra-pure rare metals required for consumable target production. ARTMS is a privately held company based in British Columbia, Canada. The purchase price comprises A$65.3 million (US$42.5 million) upfront consideration payable in shares, A$23.0 million (US$15.0 million) upfront consideration payable in cash, AU$37.6 million (US$24.5 million) in contingent future earn out payments payable in cash following achievement of certain clinical or commercial milestones and cash earn-outs representing low single to low double-digit percentage of net sales of ARTMS products or Telix products prepared using ARTMS products for defined periods depending on the product location where the sale occurs. All earn-outs which have not otherwise expired will terminate on the 10 year anniversary following completion of the ARTMS acquisition. The cash upfront consideration is subject to customary working capital, debt and transaction expense adjustments.
The closing of these acquisitions is still subject to customary conditions, including regulatory approvals. The initial accounting for these acquisitions was incomplete at the time the financial statements were authorized for issue, as an assessment of acquisition-date fair values of assets and liabilities was not yet undertaken.
There were no other subsequent events that required adjustment to or disclosure in the Financial statements of the Company for the year ended December 31, 2023.
| F-59 |
AGREEMENT AND PLAN OF MERGER
dated as of
February 7, 2024
by and among
Telix Pharmaceuticals Limited,
CYCLONE Merger Sub I, Inc.,
CYCLONE Merger Sub II, Inc.,
QSAM BIOSCIENCES, INC.
and
DAVID H. CLARKE
TABLE OF CONTENTS
| Page | |||
| Article I. | CERTAIN DEFINITIONS | 2 | |
| 1.1 | Definitions | 2 | |
| 1.2 | Construction | 17 | |
| Article II. | THE MERGER; CLOSING | 18 | |
| 2.1 | First Merger and Second Merger | 18 | |
| 2.2 | Effects of the Merger | 18 | |
| 2.3 | Closing; First Effective Time and Second Effective Time | 19 | |
| 2.4 | Certificate of Incorporation and Bylaws | 19 | |
| 2.5 | Directors and Officers | 20 | |
| Article III. | EFFECTS OF THE MERGER ON THE CAPITAL STOCK AND EQUITY AWARDS | 21 | |
| 3.1 | Conversion of Capital Stock | 21 | |
| 3.2 | Treatment of Company Options | 22 | |
| 3.3 | Certain Adjustments | 22 | |
| 3.4 | Closing Payment Certificate | 22 | |
| 3.5 | Closing Date Payments; Holdback | 23 | |
| 3.6 | Closing Date Allocation Schedule | 23 | |
| 3.7 | Exchange Procedures | 24 | |
| 3.8 | Post-Closing Adjustment | 25 | |
| 3.9 | Company Stockholder Representative | 27 | |
| 3.10 | Dissenting Shares | 31 | |
| 3.11 | Withholding | 32 | |
| 3.12 | Transfer Restrictions on Share Consideration | 32 | |
| 3.13 | No Fractional Shares | 32 | |
| 3.14 | Non-Accredited Investors | 32 | |
| Article IV. | REPRESENTATIONS AND WARRANTIES OF THE COMPANY | 32 | |
| 4.1 | Corporate Organization of the Company | 33 | |
| 4.2 | Subsidiaries | 33 | |
| 4.3 | Due Authorization | 33 | |
| 4.4 | No Conflict | 34 | |
| 4.5 | Governmental Consents | 34 | |
| 4.6 | Capitalization of the Company; Preliminary Allocation Schedule | 34 | |
| 4.7 | SEC Filings; Financial Statements | 36 | |
| 4.8 | Undisclosed Liabilities | 37 | |
| 4.9 | Litigation and Proceedings | 38 | |
| 4.10 | Compliance with Laws | 38 | |
| 4.11 | FDA Matters | 39 | |
| - i - |
| 4.12 | Contracts; No Defaults | 42 | |
| 4.13 | Company Benefit Plans | 44 | |
| 4.14 | Employment and Labor Relations | 46 | |
| 4.15 | Taxes | 48 | |
| 4.16 | Brokers’ Fees | 51 | |
| 4.17 | Insurance | 51 | |
| 4.18 | Licenses, Permits and Authorizations | 51 | |
| 4.19 | Real Property | 51 | |
| 4.20 | Intellectual Property | 51 | |
| 4.21 | Environmental Matters | 54 | |
| 4.22 | Data Privacy | 54 | |
| 4.23 | Absence of Changes | 54 | |
| 4.24 | Affiliate Matters | 55 | |
| 4.25 | Accredited Investors | 55 | |
| 4.26 | No Additional Representations or Warranties | 55 | |
| Article V. | REPRESENTATIONS AND WARRANTIES OF BUYER AND MERGER SUBS | 56 | |
| 5.1 | Corporate Organization | 56 | |
| 5.2 | Due Authorization | 56 | |
| 5.3 | No Conflict | 57 | |
| 5.4 | Governmental Consents | 57 | |
| 5.5 | Litigation and Proceedings | 57 | |
| 5.6 | Issuance of Buyer Ordinary Shares | 58 | |
| 5.7 | No Additional Representations or Warranties | 58 | |
| Article VI. | COVENANTS OF THE COMPANY | 59 | |
| 6.1 | Conduct of Business | 59 | |
| 6.2 | Inspection | 61 | |
| 6.3 | Information Statement | 62 | |
| 6.4 | Director & Officer Tail Policy | 63 | |
| 6.5 | Exclusivity | 63 | |
| 6.6 | Reverse Split | 63 | |
| Article VII. | COVENANTS OF BUYER | 64 | |
| 7.1 | Appendix 3B | 64 | |
| 7.2 | Director & Officer Indemnification and Insurance | 64 | |
| Article VIII. | JOINT COVENANTS | 65 | |
| 8.1 | Support of Transaction | 65 | |
| 8.2 | Stockholder Approval | 65 | |
| 8.3 | Further Assurances | 65 | |
| 8.4 | Tax Matters | 65 | |
| 8.5 | Private Placement | 67 | |
| 8.6 | CVR Agreement | 67 | |
| - ii - |
| Article IX. | CONDITIONS TO OBLIGATIONS | 67 | |
| 9.1 | Conditions to the Obligations of Buyer and Merger Subs | 67 | |
| 9.2 | Conditions to the Obligations of the Company | 69 | |
| 9.3 | Waiver of Conditions; Frustration of Conditions | 69 | |
| Article X. | TERMINATION/EFFECTIVENESS | 69 | |
| 10.1 | Termination | 69 | |
| 10.2 | Effect of Termination | 70 | |
| Article XI. | INDEMNIFICATION | 71 | |
| 11.1 | Survival of Representations, Warranties and Covenants | 71 | |
| 11.2 | Indemnification | 71 | |
| 11.3 | Indemnification Claim Procedures | 73 | |
| 11.4 | Limitations on Indemnification Liability | 74 | |
| 11.5 | Offset | 75 | |
| 11.6 | Indemnification Sole and Exclusive Remedy | 75 | |
| 11.7 | Tax Treatment | 76 | |
| Article XII. | MISCELLANEOUS | 76 | |
| 12.1 | Waiver | 76 | |
| 12.2 | Notices | 76 | |
| 12.3 | Assignment | 77 | |
| 12.4 | Rights of Third Parties | 78 | |
| 12.5 | Expenses | 78 | |
| 12.6 | Governing Law | 78 | |
| 12.7 | Captions; Counterparts | 78 | |
| 12.8 | Schedules and Annexes | 78 | |
| 12.9 | Entire Agreement | 79 | |
| 12.10 | Amendments | 79 | |
| 12.11 | Publicity | 79 | |
| 12.12 | Severability | 79 | |
| 12.13 | Jurisdiction; Waiver of Jury Trial | 80 | |
| 12.14 | Enforcement | 80 | |
| 12.15 | Tax Advice | 80 | |
| - iii - |
Annexes
Annex A – Form of Lock-up Agreement
Annex B – Form of CVR Agreement
Annex C – Preliminary Allocation Schedule
Annex D – Form of Written Consent
Annex E-1 – Form of First Certificate of Merger
Annex E-2 – Form of Second Certificate of Merger
Annex F – Form of Option Acknowledgement Agreement
Annex G – Form of Letter of Transmittal
Annex H – Form of Investor Questionnaire
Schedules
Schedule A – Lock-up Parties
Company Disclosure Schedule
| - iv - |
AGREEMENT AND PLAN OF MERGER
This Agreement and Plan of Merger (this “Agreement”), dated as of February 7, 2024, is entered into by and among Telix Pharmaceuticals Limited ACN 616 620 369, a public limited company registered under the Laws of the Commonwealth of Australia (“Buyer”), Cyclone Merger Sub I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Buyer (“Merger Sub I”), Cyclone Merger Sub II, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Buyer (“Merger Sub II”, and together with Merger Sub I, “Merger Subs”), QSAM Biosciences, Inc., a Delaware corporation (the “Company”), and David H. Clarke, solely in his capacity as the Company Stockholder Representative hereunder.
RECITALS
WHEREAS, the respective Boards of Directors of Buyer, Merger Sub I and the Company have approved and declared advisable the First Merger upon the terms and subject to the conditions of this Agreement and in accordance with the Delaware General Corporation Law (the “DGCL”) and have determined that the First Merger (as defined below) is in furtherance of and consistent with their respective business strategies and is fair to, and in the best interest of, their respective stockholders;
WHEREAS, the Board of Directors of the Company has determined that an amendment to the Company Charter to effect the Reverse Split is advisable and in the best interests of the Company and its stockholders and determined to recommend that the Company Stockholders vote to approve an amendment to the Company Charter to effect the Reverse Split, to become effective prior to the First Effective Time;
WHEREAS, Buyer, the Merger Subs and the Company intend to effect a reorganization in which, as steps in a single, integrated transaction, (a) Merger Sub I will merge with and into the Company, Merger Sub I will cease to exist, and the Company will survive as a direct, wholly owned subsidiary of Buyer (the “First Merger”), and (b) as part of the same overall transaction, the Company will merge with and into Merger Sub II, the Company will cease to exist, and Merger Sub II will survive as a direct, wholly owned subsidiary of Buyer (the “Second Merger” and, collectively or ad seriatim with the First Merger, as appropriate, the “Merger”);
WHEREAS, the parties intend that the Merger qualify as a “reorganization” within the meaning of Section 368(a) of the Code, and that this Agreement be a “plan of reorganization” for purposes of Sections 354 and 361 of the Code and within the meaning of Section 1.368-2(g) of the Treasury Regulations;
WHEREAS, immediately after the execution and delivery of this Agreement, the Company will obtain and deliver to Buyer a true, correct and complete copy of an irrevocable written consent of stockholders of the Company in sufficient number to evidence the approval of this Agreement, the First Merger and the other transactions contemplated hereby in accordance with the DGCL;
WHEREAS, concurrently with the execution of this Agreement, and as a condition of the willingness of Buyer to enter into this Agreement, the Company Employees and Company Stockholders listed on Schedule A are entering into Lock-Up Agreements with Buyer, the form of which is attached as Annex A hereto (each, a “Lock-Up Agreement”);
WHEREAS, subject to the terms and conditions of this Agreement, at or prior to the Closing, Buyer and a rights agent mutually agreeable to Buyer and the Company (the “Rights Agent”) will enter into a Contingent Value Rights Agreement in substantially the form attached hereto as Annex B, subject to any revisions to the CVR Agreement that are reasonably requested by such Rights Agent or are required by applicable Law (the “CVR Agreement”); and
WHEREAS, for certain limited purposes, and subject to the terms set forth herein, the Company Stockholder Representative shall serve as a representative of the Pre-Reverse Split Company Stockholders and the Company Stockholders.
NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth in this Agreement and intending to be legally bound hereby, Buyer, Merger Subs, the Company and, solely in his capacity as such, the Company Stockholder Representative, agree as follows:
Article
I.
CERTAIN DEFINITIONS
1.1 Definitions. As used herein, the following terms shall have the following meanings:
“Accredited Investor” means an “accredited investor” as defined in Rule 501(a) of Regulation D promulgated under the Securities Act.
“Acquisition Proposal” has the meaning specified in Section 6.5(a).
“Action” means any claim, action, demand, complaint, suit, audit, assessment, arbitration, inquiry, hearing, proceeding or investigation, in each case, by or before any Governmental Authority.
“Adjustment Amount” means the sum of (a) the Closing Indebtedness Amount, plus (b) the Closing Transaction Expenses.
“Affiliate” means, with respect to any specified Person, any Person that, directly or indirectly, controls, is controlled by, or is under common control with, such specified Person, through one or more intermediaries or otherwise. For the avoidance of doubt, following the Closing, (i) the Company shall constitute an Affiliate of Buyer and (ii) neither Buyer nor any of its Subsidiaries (including the Company) shall constitute an Affiliate of any Company Stockholder.
“Aggregate Non-CVR Closing Consideration Amount” means an amount equal to (a) Aggregate Non-CVR Consideration Amount, minus (b) the Reverse Split Fractional Share Cashout Amount.
“Aggregate Non-CVR Consideration Amount” means an amount equal to (a) the Base Purchase Price, minus (b) the Adjustment Amount.
“Agreement” has the meaning specified in the preamble hereto.
| - 2 - |
“Anti-Bribery Laws” has the meaning specified in Section 4.10(b).
“ASX” means ASX Limited ACN 008 624 691 and the securities exchange operated by it (as the case applies).
“ASX Listing Rules” means the official listing rules of the ASX.
“Base Purchase Price” means $33,100,000.
“Basket Amount” has the meaning specified in Section 11.4(b).
“Business Day” means any day that is not a Saturday, a Sunday or other day on which the commercial banking institutions in New York, New York or Melbourne, Australia are authorized to close for business.
“Buyer” has the meaning specified in the preamble hereto.
“Buyer Closing Certificate” has the meaning specified in Section 9.2(c).
“Buyer Cure Period” has the meaning specified in Section 10.1(c)(i).
“Buyer Financial Reports” means all ASX announcements, annual reports, financial reports and presentations and corporate governance documents disclosed or otherwise made available by Buyer at https://telixpharma.com/investor-centre/ as of on or after January 1, 2021.
“Buyer Indemnified Parties” has the meaning specified in Section 11.2(a).
“Buyer Ordinary Shares” means the ordinary shares of Buyer.
“Buyer Share Price” means $7.57452, representing the volume weighted average price at which Buyer Ordinary Shares traded on the ASX (excluding special crossings and overnight sales) over the ten (10) trading-day period ending on the Business Day prior to the date hereof, as converted from AUD to USD at the exchange rate published in the Wall Street Journal as of the Business Day prior to the date hereof.
“Cancelled Shares” has the meaning specified in Section 3.1(a).
“CERCLA” means the federal Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended.
“Certificates” has the meaning specified in Section 3.7(b).
“Change in Control Payments” means any amounts payable by the Company, the Final Surviving Corporation or their Subsidiaries at or at any time after the Closing (or, to the extent such amounts are unpaid as of immediately prior to the Closing, at any time prior to the Closing) as a result of the execution and delivery of this Agreement or the consummation of the First Merger (whether or not conditioned upon a related or concurrent or subsequent termination of employment or the occurrence of any other event), plus the employer’s share of Taxes payable with respect to all such amounts.
| - 3 - |
“Closing” has the meaning specified in Section 2.3.
“Closing Adjustment Schedule” means a schedule, prepared by the Company, setting forth, in reasonable detail, the Company’s good faith calculations of the Adjustment Amount, including calculations of the Closing Indebtedness Amount and the Closing Transaction Expenses, prepared in accordance with GAAP and certified by the Company’s chief executive officer and chief financial officer.
“Closing Certificate” has the meaning specified in Section 9.1(c).
“Closing Date” has the meaning specified in Section 2.3.
“Closing Date Allocation Schedule” means a schedule, prepared by the Company in the format of the Preliminary Allocation Schedule and dated as of the date on which the Closing Payment Certificate is delivered to Buyer setting forth: (a) for each Pre-Reverse Split Company Stockholder who is a stockholder of record or a non-objecting beneficial owner of shares of Company Stock held in street name: (i) such Person’s name and address, or other identifying information reasonably requested by Buyer to the extent that the name and address are not available; (ii) the number of shares of Company Capital Stock held or beneficially owned, as applicable, as of the Measurement Date by such Person; (iii) the aggregate Pre-Reverse Split Pro Rata Share and Pro Rata Share attributable to such Person’s Company Capital Stock, assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing; (iv) the amounts of Buyer Ordinary Shares, CVRs and cash payable to such Person pursuant to the Reverse Split, assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in clause (a)(ii) of this definition as of the Reverse Split; (v) the amounts of Buyer Ordinary Shares (rounded to the nearest whole share in accordance with Section 3.12) and CVRs payable to such Person at Closing pursuant to Section 3.1(a), assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing; (vi) the number of Holdback Shares to be withheld from such Person’s portion of the Share Consideration at Closing (in accordance with their respective Pro Rata Shares) pursuant to Section 3.5(b), assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing and (vii) whether such Person has provided a valid and signed Investor Questionnaire and, if so, whether such signed Investor Questionnaire indicates that such Person is an Accredited Investor and (b) the information described in clause (a) of this definition for each Pre-Reverse Split Company Stockholder who is an objecting beneficial owner of shares of Company Stock held in street name, to the extent known or obtained by the Company. As used in this definition, the term “Measurement Date” means (1) with respect to information regarding Pre-Reverse Split Company Stockholders of record, the Business Day prior to the date on which the Closing Payment Certificate is delivered to Buyer and (2) with respect to Pre-Reverse Split Company Stockholders who are the beneficial owners of Shares held in street name, the date of a NOBO list and OBO share range report as provided from Broadridge which shall be no earlier than five (5) Business Days prior to the date that the Closing Payment Certificate is delivered to Buyer pursuant to Section 3.4(a).
| - 4 - |
“Closing Indebtedness” means all Indebtedness and payables of the Company as of immediately prior to the First Effective Time, except for the Indebtedness and payables set forth on Section 1.1(a) of the Company Disclosure Schedule (which schedule may be updated from time to time after the date hereof at the mutual written agreement of Buyer and the Company), calculated in accordance with GAAP applied in a manner consistent with the principles applied in connection with the preparation of the most recent audited balance sheet included in the Financial Statements, in each case to the extent such Closing Indebtedness is unpaid as of the Closing.
“Closing Indebtedness Amount” means the amount of all Closing Indebtedness.
“Closing Payment Certificate” means a certificate, signed by an executive officer of the Company on behalf of the Company, which (a) sets forth (i) the amounts and payees of any Closing Indebtedness, (ii) the amounts of any Transaction Expenses and the payees to whom such amounts are owed, and whether such payments are payable in cash or in Buyer Ordinary Shares, (iii) the applicable wire (or issuance) instructions for the account or accounts of such payees and (iv) the aggregate estimated Reverse Split Fractional Share Cashout Amount in respect of all fractional shares of Company Common Stock resulting from the Reverse Split and (b) attaches the Closing Date Allocation Schedule as a schedule thereto.
“Closing Transaction Expenses” means the Excess Transaction Expenses and the Specified Transaction Expenses.
“Code” means the United States Internal Revenue Code of 1986, as amended.
“Company” has the meaning specified in the preamble hereto.
“Company Balance Sheet” means the balance sheet of the Company as of September 30, 2023 contained in the Company SEC Reports.
“Company Benefit Plans” has the meaning specified in Section 4.13(a).
“Company Bylaws” means the bylaws of the Company, as amended.
“Company Capital Stock” means the Company Common Stock and the Company Preferred Stock.
“Company Charter” means the Amended and Restated Certificate of Incorporation of the Company, as amended.
“Company Common Stock” means the common stock, par value $0.0001 per share, of the Company.
“Company Cure Period” has the meaning specified in Section 10.1(c)(i).
“Company Disclosure Schedule” means the Company Disclosure Schedule delivered by the Company to Buyer on the date hereof.
| - 5 - |
“Company Employee” means each current and former employee of the Company and its Subsidiaries.
“Company Equity Plans” means any stock incentive or equity-related agreement or plan of the Company.
“Company Intellectual Property” means the Company Owned Intellectual Property and the Company Licensed Intellectual Property.
“Company’s Knowledge,” “Knowledge of the Company” and words of similar effect means the knowledge of each of the individuals identified in Section 1.1(b) of the Company Disclosure Schedule, in each case after due and reasonable inquiry.
“Company Licensed Intellectual Property” means all Intellectual Property that is, or is purported to be, licensed to the Company or any of its Subsidiaries, or with respect to which the Company or any of its Subsidiaries has been given a covenant not to assert, by any third party.
“Company Option” means each option to purchase shares of Company Common Stock granted pursuant to any Company Equity Plan.
“Company Owned Intellectual Property” means all Intellectual Property owned or purported to be owned by the Company and its Subsidiaries, solely or jointly with any other Person.
“Company Permits” has the meaning specified in Section 4.18.
“Company Registered IP” has the meaning specified in Section 4.20(a).
“Company Regulated Product” has the meaning specified in Section 4.11(a).
“Company Preferred Stock” means the Company Series A Preferred Stock and the Company Series B Preferred Stock.
“Company SEC Reports” has the meaning specified in Section 4.7(a),
“Company Series A Preferred Stock” means the Company’s Series A Preferred Stock, par value $0.0001 per share.
“Company Series B Preferred Stock” means the Company’s Series B Preferred Stock, par value $0.0001 per share.
“Company Stockholder” means each Person who holds one or more Shares immediately prior to the First Effective Time (after giving effect to the Reverse Split).
“Company Stockholder Representative” means a representative designated by the parties to act on behalf of the Pre-Reverse Split Company Stockholders and the Company Stockholders, as the exclusive agent and attorney-in-fact for and on behalf of such Persons, for certain limited purposes, as specified herein. Company Stockholder Representative shall initially be David H. Clarke.
| - 6 - |
“Company Stockholder Representative Expense Amount” has the meaning specified in Section 3.9(c).
“Company Stockholder Representative Expense Fund” has the meaning specified in Section 3.9(c).
“Confidentiality Agreement” has the meaning specified in Section 12.9.
“Contract” means any contract, covenant, plan, undertaking, concession, agreement, agreement in principle, franchise, instrument, license, sublicense, lease, sublease, note, bond, indenture, deed of trust, mortgage, Lien, loan agreement, instrument of Indebtedness or other understanding, commitment or arrangement, whether written or oral.
“Corporations Act” means the Corporations Act 2001 (Cth) of Australia,
“CVR” has the meaning specified in Section 3.1(a).
“CVR Agreement” has the meaning specified in the Recitals.
“DGCL” has the meaning specified in the Recitals.
“Dispute Notice” has the meaning specified in Section 3.8(a).
“Dissenting Share” has the meaning specified in Section 3.1(a).
“D&O Tail Policy” has the meaning specified in Section 6.4.
“Environmental Law” means any Law relating to the environment, occupational health and safety, or exposure of persons or property to Materials of Environmental Concern, including any statute, regulation, administrative decision or order pertaining to: (a) the presence of or the treatment, storage, disposal, generation, transportation, handling, distribution, manufacture, processing, use, import, export, labeling, recycling, registration, investigation or remediation of Materials of Environmental Concern or documentation related to the foregoing; (b) air, water and noise pollution; (c) groundwater and soil contamination; (d) the release, threatened release, or accidental release into the environment, the workplace or other areas of Materials of Environmental Concern, including emissions, discharges, injections, spills, escapes or dumping of Materials of Environmental Concern; (e) transfer of interests in or control of real property which may be contaminated; (f) community or worker right-to-know disclosures with respect to Materials of Environmental Concern; (g) the protection of wild life, marine life and wetlands, and endangered and threatened species; (h) storage tanks, vessels, containers, abandoned or discarded barrels and other closed receptacles; and (i) health and safety of employees and other persons. As used above, the term “release” shall have the meaning specified in CERCLA.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate” means any entity which is, or at any applicable time was, a member of (a) a controlled group of corporations (as defined in Section 414(b) of the Code), (b) a group of trades or businesses under common control (as defined in Section 414(c) of the Code), or (c) an affiliated service group (as defined under Section 414(m) of the Code or the regulations under Section 414(o) of the Code), any of which includes or included the Company, or otherwise would be treated as a single employer with the Company for purposes of Title IV of ERISA.
| - 7 - |
“Excess Transaction Expenses” means (without duplication) any and all Transaction Expenses, other than the fees, costs and expenses designated as “Assumed/Paid by Telix” on Section 1.1(a) of the Company Disclosure Schedule (which schedule may be updated from time to time after the date hereof at the mutual written agreement of Buyer and the Company) to the extent unpaid as of Closing.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Exchange Agent” has the meaning specified in Section 3.7(a).
“Existing In-License Agreements” has the meaning specified in Section 4.20(b).
“Exploitation” means the act of making, having made, importing, using, selling, offering for sale, otherwise disposing of, researching, developing, registering, modifying, enhancing, improving, manufacturing, having manufactured, licensing, storing, formulating, optimizing, exporting, transporting, distributing, commercializing, promoting, marketing, having sold or otherwise having. disposed of.
“FDA” means the United States Food and Drug Administration.
“FDCA” means the United States Federal Food, Drug and Cosmetic Act, as amended, and the regulations promulgated thereunder.
“Final Surviving Corporation” has the meaning specified in Section 2.1(d).
“Financial Statements” has the meaning specified in Section 4.7(b).
“First Certificate of Merger” has the meaning specified in Section 2.1(a).
“First Effective Time” has the meaning specified in Section 2.3.
“First Merger” has the meaning specified in the Recitals.
“First Merger Constituent Corporations” has the meaning specified in Section 2.1(a).
“First Step Surviving Corporation” has the meaning specified in Section 2.1(b).
“Fully Diluted Shares” means a number of shares of Company Capital Stock equal to (a) the aggregate number of shares of Company Common Stock outstanding as of immediately prior to the First Effective Time (other than the Cancelled Shares), plus (b) the aggregate number of shares of Company Common Stock issuable upon conversion of the Company Preferred Stock outstanding immediately prior to the First Effective Time in accordance with the Company Charter, in each case after giving effect to the Reverse Split. Fully Diluted Shares shall be deemed to be held by a Company Stockholder to the extent the corresponding shares of Company Capital Stock are held by such Company Stockholder as of immediately prior to the First Effective Time, after giving effect to the Reverse Split.
| - 8 - |
“Fundamental Representations” means the representations and warranties of the Company in Sections 4.1, 4.2, 4.3, 4.4(b), 4.6, 4.15, 4.16 and 4.25 and the representations and warranties of Buyer and Merger Subs in Sections 5.1, 5.2, 5.3(b) and 5.6.
“GAAP” means United States generally accepted accounting principles, consistently applied.
“Governmental Authority” means any U.S. or foreign federal, state, local or municipal government or any agency, instrumentality, commission, office, legislative body, court, arbitrational tribunal, mediator, securities exchange, administrative agency, government authority or other governmental or quasi-governmental regulatory authority or body.
“Grant Date” has the meaning specified in Section 4.6(d).
“HIPAA” has the meaning specified in Section 4.11(h).
“Holdback Amount” means $500,000.
“Holdback Shares” means 66,011 Buyer Ordinary Shares, representing the Holdback Amount divided by the Buyer Share Price.
“Indebtedness” with respect to any Person means (a) any indebtedness or other obligation for borrowed money, including indebtedness evidenced by notes, bonds, mortgages, debentures or similar instruments; (b) any obligation incurred for all or any part of the purchase price of property or other assets (including earnout, milestone, royalty, seller note, installment payment, contingency payments and similar obligations) or for the cost of property or other assets constructed or of improvements thereto, other than accounts payable included in current liabilities and incurred in respect of property purchased in the ordinary course of business; (c) the face amount of all letters of credit issued for the account of such Person; (d) obligations (whether or not such Person has assumed or become liable for the payment of such obligation) secured by Liens; (e) capitalized lease obligations and any off-balance sheet financing; (f) all guarantees and similar obligations of such Person; (g) the amount of any unpaid Taxes of such Person with respect to a Pre-Closing Tax Period and any Transfer Taxes allocated to the Company Stockholders pursuant to Section 8.4(e); (h) Liabilities for any commissions earned but not yet paid; (i) Liabilities for any earned but unpaid compensation (including salary, bonuses and paid time off); (j) Liabilities for any unpaid severance arising from any terminations prior to the Closing (whether or not accrued); (k) Liabilities with respect to any bonuses accrued with respect to the period commencing on the first day of the Company’s current fiscal year and ending on the Closing Date; (l) Liabilities for the employer portion of Taxes arising in connection any of clauses (h), (i), (j) or (k); (m) all accrued interest, fees and charges in respect of any indebtedness; (n) obligations arising out of hedging, interest rate and currency swap arrangements, collar agreements and any other arrangements designed to provide protection against fluctuations in interest or currency rates, in each case, to the extent payable if such agreements are terminated at the Closing; (o) obligations pursuant to conditional sale or other title retention agreements; (p) all bankers acceptances and overdrafts; (q) all Liabilities of the type described in the foregoing clauses (a) through (p) of this definition of any other Person for which such first Person is responsible or liable, as obligor, guarantor, surety or otherwise, including any guarantee of such obligations; and (r) all interest, prepayment premiums and penalties, and any other fees, expenses, indemnities and other amounts payable as a result of the prepayment or discharge of any of the foregoing.
| - 9 - |
“Indemnified Persons” has the meaning specified in Section 7.1.
“Indemnitor” means the party required to provide indemnification pursuant to Section 11.2; provided, however, that solely for the purposes of Sections 11.3 and 11.4, the Company Stockholder Representative shall be considered the Indemnitor with respect to claims for indemnification pursuant to Section 11.2(a) (it being understood that such status as an Indemnitor is solely for the purpose of providing the Company Stockholder Representative with the right (i) to control the defense and settlement of any Action giving rise to a claim for indemnification pursuant to Section 11.2(a) and (ii) to engage in discussions, negotiations, and other dispute resolution with the applicable Buyer Indemnified Party regarding the claim for indemnification, and such status shall not obligate the Company Stockholder Representative, in such capacity, to provide any indemnification or otherwise impose any liability on the Company Stockholder Representative).
“Independent Auditor” has the meaning specified in Section 3.8(b).
“Information Statement” has the meaning specified in Section 6.3(b).
“Intellectual Property” means any of the following: (i) patents and patent applications (including provisional patent applications) and other governmental grants for the protection of inventions, including any substitutions, divisionals, continuations, continuations-in-part, reissues, renewals, registrations, re-examinations, extensions, supplementary protection certificates and the like (collectively, “Patent Rights”); (ii) registered and unregistered trademarks, service marks and trade names, pending trademark and service mark registration applications, and intent-to-use registrations or similar reservations of marks, and all goodwill in the foregoing; (iii) registered and unregistered copyrights, moral rights of authors and applications for registration of copyright; (iv) internet domain names; and (v) trade secrets, inventions, invention disclosures, data, technology, processes and know-how.
“Intended Tax Treatment” has the meaning specified in Section 8.4(f).
“Investor Questionnaire” has the meaning specified in Section 4.25.
“IRB” has the meaning specified in Section 4.11(d).
“IRS” means the United States Internal Revenue Service.
“Key Employees” has the meaning specified in the Recitals.
“Last Exercise Date” has the meaning specified in Section 3.2(a).
“Law” means any United States federal, state, municipal, or local or foreign law, common law, constitution, treaty, statute, standard, ordinance, code, rule, regulation, resolution, guidance or promulgation, or any decree, order, injunction, rule, judgment, consent of or by any Governmental Authority, or any Permit or similar right granted under any of the foregoing, or any similar provision having the force or effect of law.
| - 10 - |
“Leased Real Property” means all real property leased by the Company or any of its Subsidiaries.
“Liability” means any debt, loss, damage, claim, Tax, fine, penalty, expense, liability or obligation (whether direct or indirect, known or unknown, asserted or unasserted, absolute or contingent, accrued or unaccrued, matured or unmatured, determined or determinable, liquidated or unliquidated, or due or to become due, and whether in contract, tort, strict liability or otherwise), and including all costs and expenses relating thereto including all fees, disbursements and expenses of legal counsel, experts, engineers and consultants and costs of investigation.
“Lien” means any mortgage, deed of trust, pledge, hypothecation, encumbrance, security interest or other lien of any kind.
“Lock-Up Agreement” has the meaning specified in the Recitals.
“Losses” means any and all claims, debts, losses, obligations and other Liabilities (whether absolute, accrued, contingent, fixed, or whether known or unknown, or due or to become due or otherwise), monetary damages (including (a) direct damages, (b) consequential or incidental damages in each case to the extent reasonably foreseeable and (c) subject to, and in accordance with, Section 11.4(e), special, exemplary or punitive damages), fines, fees, penalties, interest obligations, deficiencies, losses and expenses (including amounts paid in settlement, interest, court costs, costs of investigators, reasonable fees and expenses of attorneys, accountants, financial advisors and other experts, and other expenses of litigation, arbitration or other dispute resolution procedures).
“Material Adverse Effect” means, (i) with respect to the Company, any event, occurrence, fact, condition or change that, individually or in the aggregate, (x) has had or would reasonably be expected to have a material adverse effect on the business, assets, Liabilities, results of operations or condition (financial or otherwise) of the Company and its Subsidiaries, taken as a whole; provided, however, that, for purposes of this clause (x), in no event will any of the following (or the effect of any of the following), alone or in combination, be deemed to constitute, or be taken into account in determining whether there has been or will be, a “Material Adverse Effect” on or in respect of the Company, in each case to the extent first arising after the date hereof: (A) any change in Law, regulatory policies, accounting standards or principles (including GAAP) or any guidance relating thereto or interpretation thereof, (B) any change in interest rates or economic, political, business or financial market conditions generally (including any changes in credit, financial, commodities, securities or banking markets), (C) any change generally affecting any of the industries in which the Company operates or the economy as a whole, (D) any natural disaster, (E) any acts of terrorism, sabotage, war, the outbreak or escalation of hostilities, weather conditions, change in geopolitical conditions or other force majeure events, or (F) any failure of the Company to meet any projections or forecasts, provided that this clause (F) shall not prevent a determination that any change or effect underlying such failure to meet projections or forecasts has resulted in a Material Adverse Effect (to the extent such change or effect is not otherwise excluded from this definition of Material Adverse Effect); except, in the case of clauses (A), (B), (C), (D) and (E) above, to the extent that any such change, condition, event or effect has a materially disproportionate and adverse effect on the business of the Company relative to other businesses in the industries in which the Company operates or (y) would or would reasonably be expected to prevent or materially delay or impair the Company from consummating the transactions contemplated by this Agreement or from performing its material obligations under this Agreement; and (ii) with respect to Buyer or Merger Subs, any event, occurrence, fact, condition or change that would or would reasonably be expected to prevent or materially delay or impair Buyer from consummating the transactions contemplated by this Agreement or from performing its material obligations under this Agreement.
| - 11 - |
“Materials of Environmental Concern” means any: pollutants, contaminants or hazardous substances (as such terms are defined under CERCLA), pesticides (as such term is defined under the Federal Insecticide, Fungicide and Rodenticide Act), solid wastes and hazardous wastes (as such terms are defined under the Resource Conservation and Recovery Act), chemicals, other hazardous, radioactive or toxic materials, oil, petroleum and petroleum products (and fractions thereof), or any other material (or article containing such material) listed or subject to regulation under any Law due to its potential, directly or indirectly, to harm the environment or the health of humans or other living beings.
“Merger” has the meaning specified in the Recitals.
“Merger Consent” has the meaning specified in Section 8.2.
“Merger Consideration” means the Share Consideration and the CVRs, including any amounts that become payable to the holders of CVRs pursuant to the CVR Agreement.
“Merger Sub I” has the meaning specified in the preamble hereto.
“Merger Sub II” has the meaning specified in the preamble hereto.
“Merger Subs” has the meaning specified in the preamble hereto.
“Necessary Company IP” has the meaning specified in Section 4.20(d).
“Outside Date” has the meaning specified in Section 10.1(b)(ii).
“Patent Rights” has the meaning specified in the definition of Intellectual Property.
“Pay-Off Letter” has the meaning specified in Section 3.4(a).
“Permits” means all permits, licenses, registrations, certificates, orders, approvals, consents, franchises, variances and similar rights issued by or obtained from any Governmental Authority (including those issued or required under Environmental Laws and those relating to the occupancy or use of owned or leased real property).
“Permitted Liens” means (a) mechanic’s, material men’s and similar liens, the existence of which would not constitute an event of default under, or a breach of, a lease and the Liabilities of the Company or any Subsidiary in respect of which are not overdue or otherwise in default, (b) liens arising under worker’s compensation, unemployment insurance, social security, retirement and similar legislation, and (c) liens on goods in transit incurred pursuant to documentary letters of credit, in each case arising in the ordinary course of business of the Company and the Subsidiaries and not material to the Company and the Subsidiaries, taken as a whole.
| - 12 - |
“Person” means any natural person, firm, limited liability company, general or limited partnership, association, corporation, unincorporated organization, company, joint venture, trust, Governmental Authority or other entity.
“Personal Information” has the meaning specified in Section 4.22.
“Post-Closing Adjustment Schedule” has the meaning set forth in Section 3.8(a).
“Pre-Closing Taxes” means (i) any Taxes for, or allocated in accordance with Section 8.4(b) to, any Pre-Closing Tax Period due and payable by the Company or any of its Subsidiaries; (ii) any Taxes for which the Company or any of its Subsidiaries has any liability under Treasury Regulations Section 1.1502-6 or under any comparable or similar provision of state, local or foreign Laws as a result of being a member of an affiliated, consolidated, combined, unitary or similar group on or prior to the Closing Date; (iii) any Taxes for which the Company or any of its Subsidiaries has any liability as a transferee or successor, pursuant to any contractual obligation or otherwise, which Tax is attributable to the operations of the Company or any of its Subsidiaries on or prior to the Closing Date or an event or transaction occurring before the Closing; and (iv) any Transfer Taxes.
“Pre-Closing Tax Period” means any Tax period ending on or before the Closing Date and the portion of any Straddle Period ending on and including the Closing Date.
“Pre-Reverse Split Company Stockholder” means each holder of Company Capital Stock as of immediately prior to the Reverse Split.
“Pre-Reverse Split Fully Diluted Shares” means a number of shares of Company Capital Stock equal to (a) the aggregate number of shares of Company Common Stock outstanding as of immediately prior to the Reverse Split, plus (b) the aggregate number of shares of Company Common Stock issuable upon conversion of the Company Preferred Stock outstanding as of immediately prior to the Reverse Split in accordance with the Company Charter. Fully Diluted Shares shall be deemed to be held by a Company Stockholder to the extent the corresponding shares of Company Capital Stock are held by such Company Stockholder as of immediately prior to the Reverse Split.
“Pre-Reverse Split Pro Rata Share” means, with respect to any shares of Company Capital Stock (or the shares of Company Capital Stock held by any Company Stockholder, as applicable), a fraction, (a) the numerator of which is the number of shares of Company Common Stock represented thereby or subject thereto (as applicable) as of immediately prior to the Reverse Split (it being understood that the number of shares of Company Common Stock represented by a share of Company Preferred Stock shall be the number of shares of Company Common Stock issuable upon conversion thereof pursuant to the Company Charter), and (b) the denominator of which is the number of Pre-Reverse Split Fully Diluted Shares.
| - 13 - |
“Preliminary Allocation Schedule” means the schedule attached hereto as Annex C and dated the date hereof, setting forth: (a) for each Pre-Reverse Split Company Stockholder who is a stockholder of record or a non-objecting beneficial owner of shares of Company Stock held in street name: (i) such Person’s name and address, or other identifying information reasonably requested by Buyer to the extent that the name and address are not available; (ii) the number of shares of Company Capital Stock held or beneficially owned, as applicable, as of the Measurement Date by such Person; (iii) the aggregate Pre-Reverse Split Pro Rata Share and Pro Rata Share attributable to such Person’s Company Capital Stock, assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing; (iv) the amounts of Buyer Ordinary Shares, CVRs and cash payable to such Person pursuant to the Reverse Split, assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in clause (a)(ii) of this definition as of the Reverse Split; (v) the amounts of Buyer Ordinary Shares (rounded to the nearest whole share in accordance with Section 3.12) and CVRs payable to such Person at Closing pursuant to Section 3.1(a), assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing; (vi) the number of Holdback Shares to be withheld from such Person’s portion of the Share Consideration at Closing (in accordance with their respective Pro Rata Shares) pursuant to Section 3.5(b), assuming such Person will hold or beneficially own, as applicable, the number of shares of Company Capital Stock set forth in (a)(ii) above as of the Reverse Split and will hold or beneficially own, as applicable, all shares received by such Person in the Reverse Split as of the Closing and (vii) whether such Person has provided a valid and signed Investor Questionnaire and, if so, whether such signed Investor Questionnaire indicates that such Person is an Accredited Investor and (b) the information described in clause (a) of this definition for each Pre-Reverse Split Company Stockholder who is an objecting beneficial owner of shares of Company Stock held in street name, to the extent known or obtained by the Company. As used in this definition, the term “Measurement Date” means (1) with respect to information regarding Pre-Reverse Split Company Stockholders of record, January 26, 2024 and (2) with respect to Pre-Reverse Split Company Stockholders who are the beneficial owners of Shares held in street name, January 26, 2024.
“Preliminary Information Statement” has the meaning specified in Section 6.3(a).
“Preliminary Stockholder Materials” has the meaning specified in Section 6.3(a).
“Property Taxes” means all real property Taxes, personal property Taxes and similar ad valorem Taxes.
“Pro Rata Share” means, with respect to any shares of Company Capital Stock (or the shares of Company Capital Stock held by any Company Stockholder, as applicable), a fraction, (a) the numerator of which is the number of shares of Company Common Stock represented thereby or subject thereto (as applicable) as of immediately prior to the First Effective Time (it being understood that the number of shares of Company Common Stock represented by a share of Company Preferred Stock shall be the number of shares of Company Common Stock issuable upon conversion thereof pursuant to the Company Charter), and (b) the denominator of which is the number of Fully Diluted Shares.
| - 14 - |
“Remedies Exception” has the meaning specified in Section 4.3.
“Response Date” has the meaning specified in Section 3.8(a).
“Reverse Split” means a reverse stock split of all outstanding shares of Company Common Stock, in a ratio approved by the Company Stockholders and as recommended by the Company’s Board and within the range set forth on Section 1.1(c) of the Company Disclosure Schedule (and in any event, such finally determined ratio shall be subject to the consent of Buyer, such consent not to be unreasonably withheld, conditioned or delayed), that is effected by the Company prior to the First Effective Time pursuant to which, among other things, any remaining fractional shares of Company Common Stock held by a holder of Company Common Stock (determined after determining the whole number of shares of Company Common Stock held by such holder, if any) after giving effect to the Reverse Split will be automatically exchanged for (a) such holder’s Pre-Reverse Split Pro Rata Share of the Reverse Split Fractional Share Cashout Amount (to be paid to the holders of such fractional shares after the Closing) and (b) the right to receive, upon execution of the CVR Agreement at Closing, one (1) CVR for each share of Company Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares held by the applicable holder) pursuant to the Reverse Split.
“Reverse Split Fractional Share Cashout Amount” means an amount of cash equal to the aggregate amount of the Pre-Reverse Split Pro Rata Share of the Aggregate Non-CVR Consideration Amount in respect of all fractional shares resulting from the Reverse Split (after determining the amount of any whole numbers of Company Common Stock held by any Company Stockholder after aggregating all as-converted post-Reverse Split shares of Company Common Stock held by such Company Stockholder).
“Rights Agent” has the meaning specified in the Recitals.
“SEC” means the United States Securities and Exchange Commission.
“Second Certificate of Merger” has the meaning specified in Section 2.1(c).
“Second Effective Time” has the meaning specified in Section 2.3.
“Second Merger” has the meaning specified in the Recitals.
“Second Merger Constituent Corporations” has the meaning specified in Section 2.1(c).
“Securities Act” means the Securities Act of 1933, as amended.
“Security Incident” has the meaning specified in Section 4.22.
“Share Consideration” means the aggregate Buyer Ordinary Shares issuable to the Company Stockholders pursuant to Section 3.1(a).
“Shares” has the meaning specified in Section 3.1(a).
| - 15 - |
“Specified Transaction Expenses” means the amounts designated on Section 1.1(a) of the Company Disclosure Schedule as “Purchase Price Adjustments” (which schedule may be updated from time to time after the date hereof at the mutual written agreement of Buyer and the Company).
“Stockholder Materials” has the meaning specified in Section 6.3(b).
“Straddle Period” means any Tax period that begins on or before, and ends after, the Closing Date.
“Subsidiary” means, with respect to a Person, a corporation or other entity of which more than 50% of the voting power of the equity securities or equity interests is owned, directly or indirectly, by such Person.
“Survival Expiration Date” has the meaning specified in Section 11.1.
“Tax Authority” means any Governmental Authority having or purporting to exercise jurisdiction with respect to any Tax.
“Tax Returns” means any and all reports, returns (including information returns), declarations, or statements relating to Taxes, including any schedule or attachment thereto and any amendment thereof, filed with or submitted to, or required to be filed with or submitted to, any Governmental Authority in connection with the determination, assessment, collection or payment of Taxes or in connection with the administration, implementation or enforcement of or compliance with any legal requirement relating to any Tax.
“Taxes” means all federal, state, local, foreign or other tax, charge, fee, duty, contribution, levy or other similar assessment or Liability in the nature of a tax, including all income, gross receipts, corporation, net worth, capital gains, insurance, business license, business organization, license, payroll, employment, excise, severance, stamp, occupation, premium, windfall profits, environmental, customs duties, capital stock, ad valorem, value added, inventory, franchise, escheat, profits, withholding, social security (or similar), national insurance, workers compensation, unemployment, disability, real property, personal property, sales, use, lease, service, service use, transfer, registration, documentary, recapture, recording, alternative or add-on minimum, or estimated tax and other taxes of any kind whatsoever imposed by the United States of America or any state, local or foreign government, or any agency or political subdivision thereof, and any interest, fine, penalty or addition thereto.
“Term Sheet” means the term sheet, dated November 14, 2023, between the Company and Telix Pharmaceuticals (US) Inc.
“Third-Party Claim” has the meaning specified in Section 11.3(a).
“Transaction Expenses” means (without duplication) any and all (a) legal, accounting, investment banking, consulting and other out-of-pocket fees, costs or expenses incurred by or on behalf of the Company or any of its Subsidiaries in connection with the transactions contemplated by this Agreement and (b) all Change in Control Payments, in each case to the extent unpaid as of the Closing.
| - 16 - |
“Transfer Taxes” means any transfer, sales, use, stamp, documentary, registration, conveyance, recording, value-added or other similar non-income Tax or governmental fee (including, without limitation, notary fees) arising in connection with the consummation of the transactions contemplated by this Agreement.
“Treasury Regulations” means the United States Treasury regulations promulgated under the Code.
“Written Consent” means a written consent of the stockholders of the Company in the form attached hereto as Annex D.
1.2 Construction.
(a) Unless the context of this Agreement otherwise requires, (i) words of any gender include each other gender; (ii) words using the singular or plural number also include the plural or singular number, respectively; (iii) the terms “hereof,” “herein,” “hereby,” “hereto” and derivative or similar words refer to this entire Agreement; (iv) the terms “Article,” “Section,” “Schedule” or “Annex” refer to the specified Article or Section of, or Schedule or Annex to, this Agreement; (v) the word “including” shall mean “including, without limitation,” and (vi) the word “or” shall be disjunctive but not exclusive.
(b) Unless the context of this Agreement otherwise requires, references to Contracts and other documents shall be deemed to include all subsequent amendments and other modifications thereto.
(c) Unless the context of this Agreement otherwise requires, references to statutes shall include all rules and regulations promulgated thereunder.
(d) The language used in this Agreement shall be deemed to be the language chosen jointly by the parties to express their mutual intent and no rule of strict construction shall be applied against any party.
(e) Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified.
(f) The phrase “to the extent” shall mean the degree to which a subject or other thing extends, and such phrase shall not mean simply “if.”
(g) All accounting terms used herein and not expressly defined herein shall have the meanings given to them under GAAP.
(h) All amounts payable pursuant to this Agreement shall be paid in U.S. dollars, and all references to “$” or “dollars” shall mean the lawful currency of the United States of America and all references to “A$” and “AUD” shall mean Australian Dollars, being the lawful currency of the Commonwealth of Australia.
(i) All references in this Agreement to a list or a copy will be deemed to mean a complete and accurate list and copy.
| - 17 - |
(j) All references to “ordinary course of business” will be deemed to mean “ordinary course of business consistent with past practice”.
(k) When reference is made in this Agreement to information that has been “made available” to Buyer, that shall consist of only the information that was contained in the Company’s electronic data room no later than 5:00 p.m., Eastern time, on the second (2nd) Business Day prior to the date of this Agreement.
Article
II.
THE MERGER; CLOSING
2.1 First Merger and Second Merger.
(a) Upon the terms and subject to the conditions set forth in this Agreement, and in accordance with the applicable provisions of the DGCL, Buyer, Merger Sub I and the Company (Merger Sub I and the Company sometimes being referred to herein as the “First Merger Constituent Corporations”) shall cause the First Merger to be consummated. The First Merger shall be consummated at the First Effective Time in accordance with this Agreement and evidenced by a certificate of merger relating to the First Merger in substantially the form of Annex E-1 (the “First Certificate of Merger”).
(b) Upon consummation of the First Merger, the separate corporate existence of Merger Sub I shall cease and the Company, as the surviving corporation of the First Merger (hereinafter referred to for the periods at and after the First Effective Time as the “First Step Surviving Corporation”), shall continue its corporate existence under the DGCL as a wholly owned subsidiary of Buyer.
(c) Upon the terms and subject to the conditions set forth in this Agreement, and in accordance with the applicable provisions of the DGCL, Buyer, Merger Sub II and the First Step Surviving Corporation (Merger Sub II and the First Step Surviving Corporation sometimes being referred to herein as the “Second Merger Constituent Corporations”) shall cause the Second Merger to be consummated. The Second Merger shall be consummated at the Second Effective Time in accordance with this Agreement and evidenced by a certificate of merger relating to the Second Merger in substantially the form of Annex E-2 (the “Second Certificate of Merger”).
(d) Upon consummation of the Second Merger, the separate corporate existence of the First Step Surviving Corporation shall cease and Merger Sub II, as the surviving corporation of the Second Merger (hereinafter referred to for the periods at and after the Second Effective Time as the “Final Surviving Corporation”), shall continue its corporate existence under the DGCL as a wholly owned subsidiary of Buyer.
2.2 Effects of the Merger.
(a) At and after the First Effective Time, the effect of the First Merger shall be as provided in this Agreement and the applicable provisions of the DGCL. Without limiting the foregoing, the First Step Surviving Corporation shall thereupon and thereafter possess all of the rights, property, privileges, powers and franchises, of a public as well as a private nature, of the First Merger Constituent Corporations, and shall become subject to all the restrictions, disabilities and duties of each of the First Merger Constituent Corporations.
| - 18 - |
(b) At and after the Second Effective Time, the effect of the Second Merger shall be as provided in this Agreement and the applicable provisions of the DGCL. Without limiting the foregoing, the Final Surviving Corporation shall thereupon and thereafter possess all of the rights, property, privileges, powers and franchises, of a public as well as a private nature, of the Second Merger Constituent Corporations, and shall become subject to all the restrictions, disabilities and duties of each of the Second Merger Constituent Corporations.
2.3 Closing; First Effective Time and Second Effective Time. Subject to the terms and conditions of this Agreement, the closing of the First Merger (the “Closing”) shall take place by the electronic exchange of executed counterpart documents as soon as practicable on or after the execution and delivery of this Agreement, but in any event no later than the date which is two (2) Business Days after the date on which all conditions set forth in Article IX shall have been satisfied or waived (other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of such conditions) or such other time and place as Buyer and the Company may mutually agree in writing. The date on which the Closing actually occurs is referred to in this Agreement as the “Closing Date.” Subject to the satisfaction or waiver of all of the conditions set forth in Article IX, and provided that this Agreement has not theretofore been terminated pursuant to its terms, at the Closing, Buyer, Merger Sub I and the Company shall cause the First Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of the State of Delaware as provided in Section 251 of the DGCL. The First Merger shall become effective at the time when the First Certificate of Merger has been duly filed with the Secretary of State of the State of Delaware or at such later time as may be agreed by Buyer and the Company in writing and specified in the First Certificate of Merger (the “First Effective Time”). Promptly following the First Effective Time, but in no event later than two (2) Business Days thereafter, Buyer, the First Step Surviving Corporation and Merger Sub II shall cause the Second Certificate of Merger to be filed with the Secretary of State of Delaware (the “Second Effective Time”).
2.4 Certificate of Incorporation and Bylaws.
(a) At the First Effective Time, the Company Charter shall be amended as of the First Effective Time to read in its entirety as the certificate of incorporation of Merger Sub I reads as in effect immediately prior to the First Effective Time, provided, that such certificate of incorporation shall reflect, as of the First Effective Time, “Telix QSAM, Inc.” as the name of the First Step Surviving Corporation, and, as so amended, shall become the certificate of incorporation of the First Step Surviving Corporation until thereafter amended in accordance with the applicable provisions of the DGCL and such certificate of incorporation.
(b) The parties hereto shall take all actions necessary so that the Company Bylaws shall, from and after the First Effective Time, be amended in their entirety in the form of the bylaws of Merger Sub I as in effect immediately prior to the First Effective Time (except that all references to the name of Merger Sub I shall be changed to refer to the name of the First Step Surviving Corporation as set forth in Section 2.4(a)), until thereafter amended in accordance with the applicable provisions of the DGCL, the certificate of incorporation of the First Step Surviving Corporation and such bylaws.
| - 19 - |
(c) At the Second Effective Time, the certificate of incorporation of the First Step Surviving Corporation shall be amended as of the Second Effective Time to read in its entirety as the certificate of incorporation of Merger Sub II reads as in effect immediately prior to the Second Effective Time, provided, that such certificate of incorporation shall reflect, as of the Second Effective Time, “Telix QSAM, Inc.” as the name of the Final Surviving Corporation, and, as so amended, shall become the certificate of incorporation of the Final Surviving Corporation until thereafter amended in accordance with the applicable provisions of the DGCL and such certificate of incorporation.
(d) The parties hereto shall take all actions necessary so that the bylaws of the Final Surviving Corporation shall, from and after the Second Effective Time, be amended in their entirety in the form of the bylaws of Merger Sub II as in effect immediately prior to the Second Effective Time (except that all references to the name of Merger Sub II shall be changed to refer to the name of the Final Surviving Corporation as set forth in Section 2.4(c)), until thereafter amended in accordance with the applicable provisions of the DGCL, the certificate of incorporation of the Final Surviving Corporation and such bylaws.
2.5 Directors and Officers.
(a) The directors of Merger Sub I immediately prior to the First Effective Time shall be the directors of the First Step Surviving Corporation immediately after the First Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the First Step Surviving Corporation until their respective successors are duly elected or appointed and qualified or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the First Step Surviving Corporation.
(b) The officers of Merger Sub I immediately prior to the First Effective Time shall be the officers of the Final Surviving Corporation immediately after the First Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation until their respective successors are duly appointed or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation.
(c) The directors of Merger Sub II immediately prior to the Second Effective Time shall be the directors of the Final Surviving Corporation immediately after the Second Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation until their respective successors are duly elected or appointed and qualified or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation.
(d) The officers of Merger Sub II immediately prior to the Second Effective Time shall be the officers of the Final Surviving Corporation immediately after the Second Effective Time, each to hold office in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation until their respective successors are duly appointed or until their earlier death, resignation or removal in accordance with the certificate of incorporation and bylaws of the Final Surviving Corporation.
| - 20 - |
Article III.
EFFECTS OF THE MERGER ON THE CAPITAL STOCK AND EQUITY AWARDS
3.1 Conversion of Capital Stock.
(a) At the First Effective Time, by virtue of the First Merger and without any further action on the part of any stockholder of the Company, Buyer or Merger Sub I, each share of Company Capital Stock held by Buyer, Merger Subs or the Company in treasury or otherwise, shall be cancelled and retired and shall cease to exist, and no consideration shall be delivered or receivable in exchange therefor (such shares, “Cancelled Shares”). At the First Effective Time, by virtue of the First Merger and without any action on the part of any Company Stockholder (other than compliance with Section 3.7(b) by the applicable holder), each share of Company Capital Stock (a “Share”) that is issued and outstanding immediately prior to the First Effective Time (which, for clarity, shall exclude any non-whole number shares of Company Capital Stock, which shall have been automatically cancelled in exchange for the right to receive cash in the Reverse Split, and shall be determined after giving effect to the conversion of all shares of Company Preferred Stock into Company Common Stock), other than (A) Cancelled Shares and (B) shares of Company Capital Stock held by Persons who object to the First Merger and comply with the provisions of the DGCL concerning the rights of holders of Company Capital Stock to dissent from the First Merger and require appraisal of their shares of Company Capital Stock (each such share, a “Dissenting Share”), shall thereupon be cancelled and converted into and become the right to receive, in each case as set forth on the Closing Date Allocation Schedule: (i) subject to Sections 3.8(c)(ii) and 3.14, a number of Buyer Ordinary Shares equal to (x) such Share’s Pro Rata Share of the Aggregate Non-CVR Closing Consideration Amount, divided by (y) the Buyer Share Price, plus (ii) a number of CVRs equal to the denominator in the Reverse Split, in each case, upon the terms and subject to the conditions of the CVR Agreement, without interest (each, a “CVR”), and upon the terms and subject to the conditions of this Agreement.
(b) At the First Effective Time, by virtue of the First Merger and without any action on the part of Buyer or Merger Sub I, each share of common stock, par value $0.0001 per share, of Merger Sub I issued and outstanding immediately prior to the First Effective Time shall be cancelled and, in exchange for the cancellation of such shares of Merger Sub I common stock and the payment of the Merger Consideration by Buyer, the First Step Surviving Corporation shall issue an equivalent number of shares of common stock, par value $0.0001 per share, all of which shares shall be held by Buyer, and which shall constitute the only outstanding shares of common stock of the First Step Surviving Corporation immediately following the First Effective Time.
(c) From and after the First Effective Time, (i) the Company Stockholders shall cease to have any rights as stockholders of the Company and (ii) the consideration paid to each Company Stockholder pursuant to this Article III upon the completion by such Company Stockholder of the exchange procedures set forth in Section 3.7 shall be deemed to have been paid in full satisfaction of all rights pertaining to the Shares, subject to the continuing rights of the Company Stockholders under this Agreement. At the First Effective Time, the transfer books of the Company shall be closed and no transfer of Shares shall be made thereafter.
| - 21 - |
(d) At the Second Effective Time, by virtue of the Second Merger and without any action on the part of Buyer or Merger Sub II, each share of common stock, par value $0.0001 per share, of the First Step Surviving Corporation issued and outstanding immediately prior to the Second Effective Time shall be cancelled and, in exchange for the cancellation of such shares of First Step Surviving Corporation common stock, the Final Surviving Corporation shall issue an equivalent number of shares of common stock, par value $0.0001 per share, all of which shares shall be held by Buyer, and which shall constitute the only outstanding shares of common stock of the Final Surviving Corporation immediately following the Second Effective Time.
3.2 Treatment of Company Options.
(a) Effective as of the date of the filing of the definitive Information Statement, each then-outstanding and unexercised Company Option shall vest in full and become exercisable up to and through the close of regular trading on seventh Business Day after the date the definitive Information Statement is filed (such date, the “Last Exercise Date”) in accordance with the terms and conditions of such Company Option in effect on the date hereof, and such Company Option shall terminate for no consideration and be of no further force or effect as of immediately prior to Closing if not exercised by the holder on or prior to the close of regular trading on the Last Exercise Date.
(b) As soon as practicable following the execution of this Agreement, the Company shall mail to each Person who is a holder of outstanding Company Options an Option Acknowledgement Agreement in the form attached hereto as Annex F (each, an “Option Acknowledgement Agreement”) describing the treatment of the Company Options under the terms of this Agreement, which the option holder shall be required to execute and return to the Company. Prior to the First Effective Time, the Company, the Board of Directors of the Company and/or the Compensation Committee of the Board of Directors of the Company, as applicable, shall adopt any resolutions and take any actions which are necessary to effectuate the provisions of this Section 3.2 and to terminate each Company Equity Plan, in each case after consultation with, and subject to the reasonable approval of, Buyer.
3.3 Certain Adjustments. In the event of any share split, combination, reclassification, bonus issue of shares or similar capitalization change with respect to Buyer Ordinary Shares prior to Closing and/or before the Holdback Shares, if payable, are paid, or if a record date with respect to the foregoing is fixed, appropriate and proportionate adjustments shall be made to the unissued Share Consideration and the Closing Date Allocation Schedule.
3.4 Closing Payment Certificate.
(a) No later than five (5) Business Days prior to the Closing Date, the Company shall deliver to Buyer: (i) the Closing Payment Certificate (with the Closing Date Allocation Schedule and Closing Adjustment Schedule attached as annexes thereto); (ii) a pay-off letter in form and substance reasonably satisfactory to Buyer duly executed by each Person to whom any Closing Indebtedness (other than Taxes included in Closing Indebtedness) is (or at the Closing will be) owed by the Company, the Final Surviving Corporation or any Subsidiary of the Company, which shall include a complete release of the Company, the Final Surviving Corporation and each Subsidiary of the Company from all Liens and Liabilities with respect to such Closing Indebtedness, effective upon the discharge of such Closing Indebtedness at the Closing, and authorization of Buyer or the Company to prepare and file all related Lien release documentation (each, a “Pay-Off Letter”); and (iii) final invoices submitted by each Person to whom any Transaction Expenses (other than any Taxes included in Transaction Expenses) are (or at the Closing will be) owed, which shall state that the amount invoiced thereby represents all Transaction Expenses payable to such Person with respect to the period through the Closing.
| - 22 - |
(b) Between the date of delivery of the Closing Payment Certificate and until the Closing, the Company shall make available its accountants and/or counsel, the work papers and back-up materials used or useful in preparing the Closing Payment Certificate to Buyer, as reasonably requested by Buyer, and shall cause the relevant personnel of the Company to cooperate with Buyer in connection with its review.
(c) The Company will review any comments to the Closing Payment Certificate, the Closing Date Allocation Schedule, the Closing Adjustment Schedule and the Adjustment Amount provided by Buyer and consider, in good faith, any changes proposed by Buyer, and shall accept any reasonable comments proposed by Buyer. If any information contained in the Closing Payment Certificate, including the Closing Date Allocation Schedule and/or the Closing Adjustment Schedule, is determined to be inaccurate or incomplete, the Company shall deliver an updated Closing Payment Certificate, Closing Date Allocation Schedule and Closing Adjustment Schedule no later than the next Business Day after the need for such update is determined or identified.
3.5 Closing Date Payments; Holdback; Specified Transaction Expenses.
(a) On the Closing Date, Buyer shall make (or cause to be made) the following payments, in each case in the respective amounts set forth in the Closing Payment Certificate:
(i) to each Person specified in the Closing Payment Certificate as a recipient of payments in respect of the Closing Indebtedness who has delivered a Pay-Off Letter, by wire transfer of immediately available funds, the amount payable to such Person as specified in the Closing Payment Certificate; and
(ii) to each Person specified in the Closing Payment Certificate as a recipient of payments in respect of Excess Transaction Expenses, by wire transfer of immediately available funds, the amount payable to such Person as specified in the Closing Payment Certificate.
(b) For clarity, at the Closing, Buyer shall hold back, and not deliver, the Holdback Shares from the Share Consideration due to the Company Stockholders as partial security in respect of the Company Stockholders’ obligations set forth in Section 3.8(c)(ii).
(c) Buyer shall make (or cause to be made), in each case in the respective amounts set forth in the Closing Payment Certificate (subject to Section 3.11), all payments of Buyer Ordinary Shares in respect of Specified Transaction Expenses on or promptly following the date on which Buyer issues and delivers the Share Consideration to the Exchange Agent.
3.6 Closing Date Allocation Schedule.
(a) Upon the Company’s transfer agent’s determination of the Reverse Split Fractional Share Cashout Amount, the Company Stockholder Representative shall make appropriate updates to the Closing Date Allocation Schedule and deliver such updated Closing Date Allocation Schedule to Buyer. The Company Stockholder Representative will review any comments to the updated Closing Date Allocation Schedule provided by Buyer and consider, in good faith, any changes proposed by Buyer, and shall accept any reasonable comments proposed by Buyer.
| - 23 - |
(b) From time to time after the Second Effective Time (but without limiting Buyer’s rights under Article XI), the Company Stockholder Representative may, with the prior written agreement of Buyer, update, correct or otherwise amend or modify the Closing Date Allocation Schedule in any manner that is consistent with the express provisions of Article I and this Article III. Buyer shall be entitled to rely conclusively on the Closing Date Allocation Schedule as in effect from time to time, and, as between any or all Company Stockholders, on the one hand, and Buyer and the Final Surviving Corporation, on the other hand, any amounts delivered by Buyer to any Company Stockholder in accordance with the Closing Date Allocation Schedule in effect from time to time shall be deemed for all purposes to have been delivered to the applicable Company Stockholder in full satisfaction of the obligations of Buyer, the First Surviving Corporation and the Final Surviving Corporation under this Article III.
3.7 Exchange Procedures.
(a) As soon as practicable following the determination of the Reverse Split Fractional Share Cashout Amount and the delivery of the updated Closing Date Allocation Schedule pursuant to Section 3.6(a), in consideration of the First Merger being consummated, Buyer shall cause to be issued and delivered to Equiniti Trust Company, LLC, as exchange agent (the “Exchange Agent”) the Share Consideration (less, for avoidance of doubt, the aggregate number of Holdback Shares to be withheld from the Share Consideration at Closing, unless and until such Holdback Shares become payable to the Company Stockholders pursuant to this Agreement) for distribution to the Company Stockholders (in respect of their Shares) who have complied with Section 3.7(b).
(b) After the First Effective Time, each Company Stockholder, upon surrender of any outstanding certificate or certificates for shares of Company Capital Stock (collectively, the “Certificates”), or receipt by the Exchange Agent of an “agent’s message” with respect to shares of Company Capital Stock in book entry form, as applicable, delivery of a letter of transmittal in the form attached hereto as Annex G (“Letter of Transmittal”) (which shall include, among other things, an executed consent to the indemnification obligations contemplated by Article XI and the appointment of the Company Stockholder Representative) and, to the extent not delivered by such Company Stockholder to the Company prior to the Closing, delivery of an Investor Questionnaire, in each case to the Exchange Agent or Buyer, shall be entitled to receive from the Exchange Agent or Buyer in exchange therefor the consideration specified in Section 3.1(a), less the number of Holdback Shares to be withheld from such Company Stockholder’s consideration as set forth in the Closing Date Allocation Schedule (unless and until such Holdback Shares become payable to such Company Stockholder pursuant to Section 3.8(c)). Upon delivery of Buyer Ordinary Shares to the applicable Company Stockholders pursuant to this Section 3.7(b), the Exchange Agent shall request from Buyer’s share registry, and promptly provide to the applicable Stockholder upon receipt, a holding statement in respect of such Buyer Ordinary Shares.
| - 24 - |
(c) Any amount of the Merger Consideration that remains undistributed to the Company Stockholders until twelve (12) months after the Closing Date shall be delivered to Buyer or its nominee (subject to abandoned property, escheat or similar Law). If any Company Stockholder shall not have completed the exchange procedures contemplated by Section 3.7(b) prior to the date that is twelve (12) months after the Closing Date, any such Merger Consideration in respect thereof shall, to the extent permitted by applicable Law, become the property of Buyer or its nominee, free and clear of all claims or interest of any Person previously entitled thereto. To the extent permitted by applicable Law, none of the Buyer, Merger Subs, the Company, the First Surviving Corporation, the Final Surviving Corporation or the Exchange Agent shall be liable to any Company Stockholder for any amount delivered to a public official pursuant to any applicable abandoned property, escheat or similar Law.
(d) In the event any Certificate has been lost, stolen or destroyed, upon the making of an affidavit of that fact and a customary indemnification of the Company and Buyer in a form reasonably satisfactory to the Exchange Agent and Buyer by the Person claiming such Certificate to be lost, stolen or destroyed, the Exchange Agent shall deliver in exchange for such lost, stolen or destroyed Certificate the Share Consideration deliverable in respect thereof as determined in accordance with this Article III.
(e) On or promptly after delivery of the Share Consideration to the Exchange Agent, Buyer shall do all such acts, matters and things that are necessary to procure the official quotation of such Buyer Ordinary Shares, including: (i) apply for official quotation of such Buyer Ordinary Shares on ASX by lodging an Appendix 2A; (ii) lodge with ASX a cleansing notice in accordance with section 708A(5)(e) of the Corporations Act in respect of such Buyer Ordinary Shares; and (iii) cause Buyer’s share registry to enter such Buyer Ordinary Shares in the share register of Buyer.
3.8 Post-Closing Adjustment.
(a) As soon as reasonably practicable following the Closing Date, and in any event within sixty (60) calendar days thereof, Buyer shall prepare and deliver to the Company Stockholder Representative a schedule setting forth, in reasonable detail, Buyer’s good faith calculations of the Adjustment Amount, including calculations of the Closing Indebtedness Amount and the Closing Transaction Expenses, prepared in accordance with GAAP (the “Post-Closing Adjustment Schedule”). If the Company Stockholder Representative shall disagree with any calculations in the Post-Closing Adjustment Schedule, it shall notify Buyer of such disagreement in writing within five (5) Business Days of the date Buyer delivers the Post-Closing Adjustment Schedule (the last day of such period, the “Response Date”), setting forth in reasonable detail the particulars of such disagreement (such notice, a “Dispute Notice”). In the event that the Company Stockholder Representative does not provide a Dispute Notice on or prior to 5:00pm Eastern Time on the Response Date, the Post-Closing Adjustment Schedule as delivered by Buyer, including Buyer’s calculation of the Adjustment Amount and the components thereof, shall be final, binding and conclusive for all purposes hereunder. In the event any Dispute Notice is timely provided, Buyer and the Company shall promptly meet and attempt in good faith to resolve the disputed item(s) and negotiate an agreed-upon determination of the items relating to such dispute, and any such agreed-upon items shall be deemed to have been finally determined for all purposes of this Agreement.
| - 25 - |
(b) In the event that any disputed items set forth in a Dispute Notice remain unresolved after thirty (30) calendar days of the delivery of the Dispute Notice, such remaining disagreements shall be resolved by an independent accounting or financial consulting firm of recognized national standing to be mutually selected (neither party to unreasonably withhold, condition or delay their selection) by Buyer and the Company Stockholder Representative (such firm, the “Independent Auditor”). Each of Buyer and the Company Stockholder Representative shall promptly provide their respective assertions regarding the Adjustment Amount, the Closing Indebtedness Amount and/or the Closing Transaction Expenses, as applicable, in writing to the Independent Auditor and to each other as promptly as possible after the engagement of the Independent Auditor. The Independent Auditor shall be instructed to render its determination with respect to such disagreements as soon as reasonably possible (which the parties hereto agree should not be later than thirty (30) days following the day on which the disagreement is referred to the Independent Auditor). The Independent Auditor shall base its determination solely on (i) the written submissions of the parties and shall not conduct an independent investigation and (ii) the extent (if any) to which the any of the Adjustment Amount, the Closing Indebtedness Amount and/or the Closing Transaction Expenses requires adjustment (only with respect to the remaining disagreements submitted to the Independent Auditor) in order to be determined. In resolving any disputed item, the Independent Auditor may not assign a value to any item greater than the greatest value for such item claimed by either party or less than the smallest value for such item claimed by either party and shall act as an expert, not an arbitrator. Absent manifest error or fraud, the determination of such disputed items by Independent Auditor shall be final, conclusive and binding on the parties, and the Adjustment Amount as calculated by the Independent Auditor shall conclusive, final and binding on the parties hereto for all purposes hereunder. All fees and expenses of the Independent Auditor relating to the work, if any, to be performed by the Independent Auditor hereunder shall be borne pro rata as between Buyer, on the one hand, and the Company Stockholder Representative (subject to the Company Stockholder Representative’s right to be indemnified by the Pre-Reverse Split Company Stockholders and the Company Stockholders pursuant to Section 3.9(d), if such expenses exceed the Representative Expense Fund), on the other hand, in proportion to the allocation of the dollar value of the amounts in dispute as between Buyer and the Company Stockholder Representative (set forth in the written submissions to the Independent Auditor) made by the Independent Auditor such that the party prevailing on the greater dollar value of such disputes pays the lesser proportion of the fees and expenses. For example, if Buyer challenges the calculation of any items underlying the calculation of Closing Transaction Expenses in the net amount of $1,000,000, and the Independent Auditor determines that the Company has a valid claim that $400,000 of the $1,000,000 claimed by Buyer do not constitute Closing Transaction Expenses, the Company Stockholder Representative shall bear 60% of the fees and expenses of the Independent Auditor (subject to the Company Stockholder Representative’s right to be indemnified by the Pre-Reverse Split Company Stockholders and the Company Stockholders pursuant to Section 3.9(d), if such expenses exceed the Representative Expense Fund) and Buyer shall bear the remaining 40% of the fees and expenses of the Independent Auditor.
| - 26 - |
(c) The “True-up Amount” means an amount equal to (i) the Adjustment Amount as finally determined pursuant to this Section 3.8, minus (ii) the Adjustment Amount as reflected in the final Closing Adjustment Schedule. For the avoidance of doubt the True-up Amount may be a positive or negative number.
(i) If the True-up Amount is less than or equal to zero (0), then Buyer shall, subject to Section 3.14, issue and shall deliver (or cause to be delivered) the Holdback Shares to the Company Stockholders, in accordance with the allocations set forth in the Closing Date Allocation Schedule, to the extent they have completed the exchange procedures set forth in Section 3.7(b).
(ii) If the True-up Amount is greater than zero (0) and less than or equal to the Holdback Amount, then Buyer (i) shall be entitled to retain, and the Company Stockholders shall forfeit any right to receive, a number of Holdback Shares equal to (A) the True-up Amount divided by (B) the Buyer Share Price and (ii) Buyer shall, subject to Section 3.14, issue and shall deliver (or cause to be delivered) the remaining Holdback Shares to the Company Stockholders (if any), in accordance with the allocations set forth in the Closing Date Allocation Schedule, to the extent they have completed the exchange procedures set forth in Section 3.7(b).
(iii) If the True-up Amount is greater than the Holdback Amount, then Buyer (i) shall be entitled to retain, and the Company Stockholders shall forfeit any right to receive, all of the Holdback Shares and (ii) shall be entitled to indemnification (including rights to offset or set off against amounts that are or may become payable pursuant to the CVR Agreement) pursuant to Section 11.2(a)(iv) for the portion of the True-up Amount in excess of the Holdback Amount.
(d) All amounts paid or forfeited pursuant to this Section 3.8 shall be treated by the parties hereto for all Tax purposes as adjustments to the Merger Consideration to the greatest extent permitted by applicable Law, and shall be reported as such by the parties hereto on their Tax Returns.
3.9 Company Stockholder Representative.
(a) By their execution of the Letter of Transmittal, approval of the Merger and adoption of this Agreement and/or their acceptance of any consideration pursuant to this Agreement or the CVR Agreement, the Pre-Reverse Split Company Stockholders and the Company Stockholders hereby irrevocably (subject only to Section 3.9(e)) appoint the Company Stockholder Representative as the representative, attorney-in-fact and agent of the Pre-Reverse Split Company Stockholders and the Company Stockholders for all purposes in connection with the transactions contemplated by this Agreement and any other agreements ancillary hereto and in any litigation or arbitration involving this Agreement. In connection therewith, the Company Stockholder Representative is authorized to do or refrain from doing all further acts and things, and to execute all such documents as the Company Stockholder Representative shall deem necessary or appropriate, and shall have the power and authority to:
(i) act for some or all of the Pre-Reverse Split Company Stockholders and the Company Stockholders with regard to all matters pertaining to this Agreement or any other agreements ancillary hereto;
(ii) act for the Pre-Reverse Split Company Stockholders and the Company Stockholders to transact matters of litigation;
(iii) execute and deliver all amendments, waivers, ancillary agreements, certificates and documents that the Company Stockholder Representative deems necessary or appropriate in connection with the consummation of the transactions contemplated by this Agreement, including delivering any update to or correction, amendment or modification of the Closing Date Allocation Schedule permitted by this Agreement;
| - 27 - |
(iv) do or refrain from doing, on behalf of the Pre-Reverse Split Company Stockholders and the Company Stockholders, any further act or deed that the Company Stockholder Representative deems necessary or appropriate in the Company Stockholder Representative’s discretion relating to the subject matter of this Agreement, in each case as fully and completely as the Pre-Reverse Split Company Stockholders and the Company Stockholders could do if personally present;
(v) give and receive all notices required to be given or received by the Pre-Reverse Split Company Stockholders and the Company Stockholders under this Agreement;
(vi) agree to, negotiate, enter into settlements and compromises and/or comply with arbitration awards and court orders with respect to claims for indemnification made by Buyer under Article XI; and
(vii) receive service of process in connection with any claims under this Agreement or any ancillary agreement contemplated hereby.
(b) All decisions and actions of the Company Stockholder Representative on behalf of the Pre-Reverse Split Company Stockholders and the Company Stockholders shall be deemed to be facts ascertainable outside of this Agreement and shall be binding upon all Pre-Reverse Split Company Stockholders and Company Stockholders, and no Pre-Reverse Split Company Stockholder or Company Stockholder shall have the right to object, dissent, protest or otherwise contest the same.
(c) At the First Effective Time, Buyer shall pay an amount in cash equal to $25,000 (the “Company Stockholder Representative Expense Amount”) to the Company Stockholder Representative, which Company Stockholder Representative Expense Amount shall be held by the Company Stockholder Representative in a segregated account (the “Company Stockholder Representative Expense Fund”). The Company Stockholder Representative Expense Fund will be used solely for the purposes of paying directly, or reimbursing the Company Stockholder Representative for, any third party expenses pursuant to this Agreement and the agreements ancillary hereto. The Company Stockholders will not receive any interest or earnings on the Company Stockholder Representative Expense Fund and irrevocably transfer and assign to the Company Stockholder Representative any ownership right that they may otherwise have had in any such interest or earnings. The Company Stockholder Representative will not be liable for any loss of principal of the Company Stockholder Representative Expense Fund other than as a result of its gross negligence or willful misconduct. The Company Stockholder Representative will hold these funds separate from its corporate funds, will not use these funds for its operating expenses or any other corporate purposes and will not voluntarily make these funds available to its creditors in the event of bankruptcy. For tax purposes, the Company Stockholder Representative Expense Fund will be treated as having been received and voluntarily set aside by the Company Stockholders at the time of Closing. In no event shall Buyer or the Final Surviving Corporation (or any of their respective Affiliates) be obligated to reimburse the Company Stockholder Representative for any expenses payable from the Company Stockholder Representative Expense Fund. Upon the determination of the Company Stockholder Representative that retaining any portion of the Company Stockholder Representative Expense Fund is no longer necessary, or as directed by the advisory committee to the Company Stockholder Representative as set forth in the engagement letter between the Company and the Company Stockholder Representative, the Company Stockholder Representative shall deliver any then remaining portion of the Company Stockholder Representative Expense Fund to Buyer, after which Buyer shall, subject to Section 3.14, promptly issue to each Company Stockholder a number of Buyer Ordinary Shares with a value, based on the Buyer Share Value, equal to such Company Stockholder’s Pro Rata Share of such remaining portion of the Company Stockholder Representative Expense Fund.
| - 28 - |
(d) The Company Stockholder Representative shall act for the Pre-Reverse Split Company Stockholders and the Company Stockholders on all of the matters set forth in this Agreement and any other agreements ancillary hereto in the manner the Company Stockholder Representative believes to be in the best interest of the Pre-Reverse Split Company Stockholders and the Company Stockholders. The Company Stockholder Representative is authorized to act on behalf of the Pre-Reverse Split Company Stockholders and the Company Stockholders notwithstanding any dispute or disagreement among the Pre-Reverse Split Company Stockholders or the Company Stockholders. In taking any action as Company Stockholder Representative, the Company Stockholder Representative may rely conclusively, without any further inquiry or investigation, upon any certification or confirmation, oral or written, given by any Person whom the Company Stockholder Representative reasonably believes to be authorized thereunto. The Company Stockholder Representative may, in all questions arising hereunder, rely on the advice of counsel, and the Company Stockholder Representative shall not be liable to any Pre-Reverse Split Company Stockholder or Company Stockholder for anything done, omitted or suffered in good faith by the Company Stockholder Representative based on such advice. The Company Stockholder Representative undertakes to perform such duties and only such duties as are specifically set forth in this Agreement and no implied covenants or obligations shall be read into this Agreement against the Company Stockholder Representative. The Company Stockholder Representative shall not have any liability to any of the Company Stockholders for any act done or omitted hereunder as Company Stockholder Representative while acting in good faith and pursuant to the engagement letter between the Company and the Company Stockholder Representative. The Company Stockholder Representative shall be indemnified by the Pre-Reverse Split Company Stockholders and the Company Stockholders from and against any loss, liability or expense incurred in good faith on the part of the Company Stockholder Representative and arising out of or in connection with the acceptance or administration of the Company Stockholder Representative’s duties hereunder, in each case as such loss, liability or expense is suffered or incurred. Any such claim for indemnification shall be satisfied first from any then available portion of the remaining Company Stockholder Representative Expense Fund and, if such amount is insufficient to satisfy any such loss, liability or expense, from the first proceeds from any payments to be made by Buyer pursuant to this Agreement or the CVR Agreement otherwise available for distribution to the Pre-Reverse Split Company Stockholders and/or the Company Stockholders or by a claim against the Company Stockholders (with each Company Stockholder liable for the Pro Rata Share of any such claim that is represented by such Company Stockholder’s Company Capital Stock). Notwithstanding anything in this Agreement to the contrary, nothing herein shall relieve the Company Stockholders from their obligation to promptly pay such losses, liabilities and expenses as they are suffered or incurred, nor does it prevent the Company Stockholder Representative from seeking any remedies available to it at law or otherwise. In no event will the Company Stockholder Representative be required to advance its own funds on behalf of the Company Stockholders or otherwise. Notwithstanding anything in this Agreement to the contrary, any restrictions or limitations on liability or indemnification obligations of, or provisions limiting the recourse against non-parties otherwise applicable to, the Company Stockholders set forth elsewhere in this Agreement are not intended to be applicable to the indemnities provided to the Company Stockholder Representative under this section. The foregoing indemnities will survive the Closing, the resignation or removal of the Company Stockholder Representative or the termination of this Agreement.
| - 29 - |
(e) In the event the Company Stockholder Representative becomes unable to perform the Company Stockholder Representative’s responsibilities hereunder or resigns from such position, the Company Stockholder Representative shall select another representative to fill the vacancy of the Company Stockholder Representative, and upon such substituted representative’s written agreement to assume the rights and responsibilities of the Company Stockholder Representative hereunder, such substituted representative shall be deemed to be the Company Stockholder Representative for all purposes of this Agreement. Except as contemplated by the previous sentence, the Company Stockholder Representative may be removed only upon delivery of written notice to Buyer signed by Company Stockholders who, as of immediately prior to the First Effective Time, held a majority (by voting power) of the then outstanding shares of Company Capital Stock; provided that no such removal shall be effective until such time as a successor Company Stockholder Representative shall have been validly appointed hereunder. The Company Stockholder Representative shall provide Buyer prompt written notice of any replacement of the Company Stockholder Representative, including the identity and address of the new Company Stockholder Representative. Upon any replacement of the Company Stockholder Representative, the Company Stockholder Representative being replaced shall transfer to the new Company Stockholder Representative the balance of any unexpended Company Stockholder Representative Expense Fund.
(f) The Company Stockholder Representative agrees not to, directly or indirectly, disclose the existence or terms of this Agreement or any other agreement contemplated hereby or any other information regarding this Agreement, the Merger or any of the other matters contemplated hereby, including information provided to the Company Stockholder Representative pursuant to the terms of this Agreement, except, in each case (i) to the extent such information is or becomes generally known to the public (other than as a result of a disclosure by the Company Stockholder Representative without a breach of its obligations under this Section 3.9(f)), (ii) as required by applicable Law, (iii) to employees, advisors, agents or consultants of the Company Stockholder Representative (if applicable) and to the Company Stockholders, in each case who have a need to know such information, and further provided that such persons are subject to confidentiality obligations with respect thereto, or (iv) is in connection with, and only to the extent required for, enforcement of rights or defense of claims (including, in each case, on behalf of the Company Stockholders) under this Agreement and the transactions contemplated hereby and thereby.
(g) For all purposes of this Agreement:
(i) Buyer shall be entitled to rely conclusively on the instructions and decisions of the Company Stockholder Representative as to the settlement of any disputes or claims under this Agreement, or any ancillary agreement contemplated hereby, or any other actions required or permitted to be taken by the Company Stockholder Representative hereunder, and no party hereunder or any Company Stockholder shall have any cause of action against Buyer for any action taken by Buyer in reliance upon the instructions or decisions of the Company Stockholder Representative;
| - 30 - |
(ii) the provisions of this Section 3.9 are independent and severable, are irrevocable (subject only to Section 3.9(e)) and coupled with an interest and shall be enforceable notwithstanding any rights or remedies that any Company Stockholder may have in connection with the transactions contemplated by this Agreement;
(iii) except as specifically set forth herein, no Company Stockholder will have any right to bring any claim, cause of action, objection or complaint except through the Company Stockholder Representative, and the Company Stockholder Representative shall have the sole authority to act for, and enforce the rights of, all Company Stockholders in connection with this Agreement and the transactions contemplated hereby; and
(iv) the provisions of this Section 3.9 shall be binding upon the executors, heirs, legal representatives, personal representatives, successor trustees and successors of each Company Stockholder, and any references in this Agreement to a Company Stockholder shall mean and include the successors to the rights of each applicable Company Stockholder hereunder, whether pursuant to testamentary disposition, the Laws of descent and distribution or otherwise.
3.10 Dissenting Shares. Notwithstanding the foregoing provisions of this Article III, the Dissenting Shares shall not be converted into a right to receive any portion of the Merger Consideration and the holders thereof shall be entitled to such rights as are granted by Section 262 of the DGCL. Each holder of Dissenting Shares who becomes entitled to payment for such shares pursuant to Section 262 of the DGCL shall receive payment therefor from the Final Surviving Corporation in accordance with the DGCL; provided, however, that (i) if any such holder of Dissenting Shares shall have failed to establish such holder’s entitlement to appraisal rights as provided in Section 262 of the DGCL, or (ii) if any such holder of Dissenting Shares shall have effectively withdrawn such holder’s demand for appraisal of such shares or lost such holder’s right to appraisal and payment for such holder’s shares under Section 262 of the DGCL, such holder shall forfeit the right to appraisal of such shares and each such share shall not constitute a Dissenting Share and shall be treated as if it had been a Share, as applicable, immediately prior to the First Effective Time and converted, as of the First Effective Time, into a right to receive from the Final Surviving Corporation the portion of the Merger Consideration deliverable in respect thereof as determined in accordance with this Article III, without any interest thereon (and such holder shall be treated as a Company Stockholder). The Company will give Buyer reasonable notice of all written notices received by the Company pursuant to Section 262 of the DGCL. Without the prior written consent of Buyer (which shall not be unreasonably withheld, conditioned or delayed), the Company shall not voluntarily make any payment with respect to, or settle or offer to settle, any such demand for payment. From and after the First Effective Time, no stockholder who has properly exercised and perfected appraisal rights pursuant to Section 262 of the DGCL shall be entitled to vote his or her Shares for any purpose or receive payment of dividends or other distributions with respect to his or her Shares (except dividends and distributions payable to stockholders of record at a date which is prior to the First Effective Time). Notwithstanding anything herein to the contrary, any payments required to be made to holders of Dissenting Shares pursuant to this Section 3.10 shall be made by the Final Surviving Corporation out of its own funds. No funds will be supplied for that purpose, directly or indirectly, by Buyer (or any of its Affiliates except for the Final Surviving Corporation), nor will Buyer (or any of its Affiliates except for the Final Surviving Corporation) directly or indirectly reimburse the Final Surviving Corporation for any payments to holders of Dissenting Shares.
| - 31 - |
3.11 Withholding. Buyer, the Company, the Final Surviving Corporation, the Company Stockholder Representative and the Exchange Agent shall be entitled to deduct and withhold from the consideration (including any payments or other distributions pursuant to the CVR Agreement) otherwise payable or deliverable in connection with the transactions contemplated by this Agreement or the CVR Agreement such amounts that Buyer, the Company, the Final Surviving Corporation, the Company Stockholder Representative and the Exchange Agent are required to deduct and withhold with respect to any such deliveries and payments under the Code or any provision of state, local, provincial or foreign Law. To the extent that amounts are withheld, and duly and timely remitted to the appropriate Governmental Authority, by Buyer, the Company, the Final Surviving Corporation, the Company Stockholder Representative or the Exchange Agent, such withheld amounts shall be treated for all purposes of this Agreement and/or the CVR Agreement as having been paid to the person in respect of which such deduction and withholding was made.
3.12 Transfer Restrictions on Share Consideration.
(a) The Company and the Company Stockholder Representative (on behalf of the Company Stockholders) acknowledge that the Buyer Ordinary Shares issued pursuant to this Agreement to any Company Stockholder (i) will not have been registered under the Securities Act or qualified under any applicable state securities Laws, (ii) will be subject to such additional restrictions as are set forth in any Lock-up Agreement entered into by such Company Stockholder and (iii) will be subject to escrow and held on the issuer sponsored subregister and subject to a holding lock for any required holding period under Rule 144 of the Securities Act and, if applicable, for the relevant period set out in any Lock-up Agreement entered into by such Company Stockholder.
(b) Any certificates or book-entry records evidencing the Buyer Ordinary Shares shall bear the following or any similar legend:
“THE SHARES REPRESENTED HEREBY HAVE NOT BEEN REGISTERED WITH THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED, AND, ACCORDINGLY, MAY NOT BE TRANSFERRED UNLESS (I) SUCH SECURITIES HAVE BEEN REGISTERED FOR SALE PURSUANT TO THE SECURITIES ACT OF 1933, AS AMENDED, (II) SUCH SECURITIES ARE SOLD IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR (III) THE ISSUER HAS RECEIVED AN OPINION OF COUNSEL REASONABLY SATISFACTORY TO IT THAT SUCH TRANSFER MAY LAWFULLY BE MADE WITHOUT REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED.”
3.13 No Fractional Shares. No fractional Buyer Ordinary Shares will be issued to the Company Stockholders under this Agreement, and any fraction of a Buyer Ordinary Share shall be rounded to the nearest whole number.
3.14 Non-Accredited Investors. Notwithstanding anything herein to the contrary, with the approval of the Company Stockholder Representative (not to be unreasonably withheld, conditioned or delayed), Buyer may elect to pay to any Company Stockholder that Buyer determines is not, or may not be, an Accredited Investor, in lieu of the Share Consideration that such Company Stockholder would have otherwise been entitled to receive, cash in an amount, determined based on the Buyer Share Price, equal to the dollar value of such Share Consideration.
Article
IV.
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except (i) as disclosed in the Company SEC Reports filed or furnished on or after January 1, 2021 and prior to the date of this Agreement (other than any forward looking disclosures set forth in any “Risk Factors” section of any Company SEC Report, any forward-looking disclosures in any “Forward Looking Information” section of any Company SEC Report and any other disclosures included in any Company SEC Report to the extent they are predictive or forward-looking in nature) (provided, however, that nothing set forth in such Company SEC Reports shall be deemed to modify or qualify any representation or warranty set forth in any Fundamental Representations of the Company) or (ii) as set forth in the Company Disclosure Schedule, the Company represents and warrants to Buyer and Merger Subs that the statements contained in this Article IV are true and correct as of the date of this Agreement and will be true and correct as of the Closing with the same effect as though made at and as of such time (provided, however, that representations and warranties that are made as of a particular date or period will be true and correct only as of such date or period).
| - 32 - |
4.1 Corporate Organization of the Company. The Company has been duly incorporated and is validly existing as a corporation in good standing under the Laws of the State of Delaware and has the corporate power and authority to own or lease its properties and to conduct its business as it is now being conducted. The copies of the Company Charter and the Company Bylaws previously made available by the Company to Buyer or its representatives are true and complete. The Company is duly licensed or qualified to do business and (where applicable) is in good standing as a foreign corporation in each jurisdiction in which the ownership of its property or the character of its activities is such as to require it to be so licensed or qualified or in good standing, as applicable, except where the failure to be so licensed or qualified or in good standing would not reasonably be expected to result in the loss of a material benefit of, or the incurrence of a material Liability by, the Company or any of its Subsidiaries.
4.2 Subsidiaries.
(a) Section 4.2 of the Company Disclosure Schedule sets forth: (i) the name of each Subsidiary of the Company; (ii) the number and type of outstanding equity securities of each Subsidiary and a list of the holders thereof; (iii) the jurisdiction of organization of each Company Subsidiary; (iv) the names of the officers and directors of each Subsidiary; and (v) the jurisdictions in which each Company Subsidiary is qualified or holds licenses to do business as a foreign corporation or other entity.
(b) Each Company Subsidiary is a corporation duly organized, validly existing and in corporate and Tax good standing under the Laws of the jurisdiction of its incorporation. Each Company Subsidiary is duly qualified to conduct business and is in corporate and Tax good standing under the Laws of each jurisdiction in which the nature of its businesses or the ownership or leasing of its properties requires such qualification. Each Company Subsidiary has all requisite power and authority to carry on the businesses in which it is engaged and to own and use the properties owned and used by it. The Company has made available to Buyer complete and accurate copies of the charter, by-laws or other organizational documents of each Company Subsidiary. No Company Subsidiary is in default under or in violation of any provision of its charter, by-laws or other organizational documents. All of the issued and outstanding shares of capital stock of each Company Subsidiary are duly authorized, validly issued, fully paid, nonassessable and free of preemptive rights. All shares of each Company Subsidiary that are held of record or owned beneficially by either the Company or any Company Subsidiary are held or owned free and clear of any restrictions on transfer (other than restrictions under the Securities Act and state securities Laws), claims, Liens, options, warrants, rights, contracts, calls, commitments, equities and demands. There are no outstanding or authorized options, warrants, rights, agreements or commitments to which the Company or any Company Subsidiary is a party or which are binding on any of them providing for the issuance, disposition or acquisition of any capital stock of any Company Subsidiary. There are no forms of equity or equity-based compensation or similar rights with respect to any Company Subsidiary. There are no voting trusts, proxies or other agreements or understandings with respect to the voting of any capital stock of any Company Subsidiary.
(c) The Company does not own or control directly or indirectly or have any direct or indirect equity participation or similar interest in, or any obligation to providing funding to, any corporation, partnership, limited liability company, joint venture, trust or other business association or entity that is not a Company Subsidiary.
4.3 Due Authorization. The Company has all requisite corporate power and authority to execute and deliver this Agreement and (subject to the consents, approvals, authorizations and other requirements described in Section 4.5) to consummate the transactions contemplated hereby. The execution and delivery of this Agreement by the Company and the consummation by the Company of the transactions contemplated hereby have been duly and validly authorized and approved by the Board of Directors of the Company, and no other corporate proceeding on the part of the Company is necessary to authorize this Agreement (other than the Merger Consent). Without limiting the generality of the foregoing, the Board of Directors of the Company, at a meeting duly called and held, by the unanimous vote of all directors (a) determined that the First Merger is advisable, fair and in the best interests of the Company and its stockholders, (b) approved this Agreement in accordance with the provisions of the DGCL, and (c) directed that this Agreement and the First Merger be submitted to the stockholders of the Company for their adoption and approval and resolved to recommend that the stockholders of the Company vote in favor of the adoption of this Agreement and the approval of the First Merger. This Agreement has been duly and validly executed and delivered by the Company and (assuming this Agreement constitutes a legal, valid and binding obligation of Buyer, Merger Subs and the Company Stockholder Representative) constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, subject to applicable bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium and similar Laws affecting creditors’ rights generally and subject, as to enforceability, to general principles of equity (collectively, the “Remedies Exception”).
| - 33 - |
4.4 No Conflict. Subject to the receipt of the consents, approvals, authorizations and other requirements set forth in Section 4.5 or on Section 4.5 of the Company Disclosure Schedule, the execution and delivery of this Agreement by the Company and the consummation by the Company and its Subsidiaries of the transactions contemplated hereby do not and will not, as of the Closing, (a) violate any provision of, or result in the breach of, any applicable Law to which the Company or any of its Subsidiaries is subject or by which any property or asset of the Company or any of its Subsidiaries is bound, (b) conflict with or violate any provision of the Company Charter, Company Bylaws or other organizational documents of the Company or any of its Subsidiaries, (c) violate any provision of or result in a breach of, or require a consent or constitute (with or without due notice or lapse of time or both) a default under, any Contract listed on Section 4.12 of the Company Disclosure Schedule, or terminate or result in the termination of any such Contract, or result in the creation of any Lien under any such Contract upon any of the properties or assets of the Company or any of its Subsidiaries, or constitute an event which, after notice or lapse of time or both, would result in any such violation, breach, termination or creation of a Lien or create in any party the right to accelerate or modify such Contract, or (d) result in a violation or revocation of any required Permit from any Governmental Authority, except to the extent that the occurrence of any of the foregoing items set forth in clauses (a), (c) or (d) would not reasonably be expected to result the loss of a material right or benefit of, or the incurrence of a material Liability by, the Company or any of its Subsidiaries.
4.5 Governmental Consents. Assuming the truth and completeness of the representations and warranties of Buyer contained in this Agreement, no consent, approval or authorization of, or designation, declaration or filing with, any Governmental Authority is required on the part of the Company or any of its Subsidiaries with respect to the Company’s execution or delivery of this Agreement or the consummation by the Company of the transactions contemplated hereby, except (a) for any material consents, approvals, authorizations, designations, declarations or filings set forth in Section 4.5 of the Company Disclosure Schedules, (b) compliance with any applicable securities Laws, (c) as otherwise disclosed on Section 4.5 of the Company Disclosure Schedule and (d) for the filing of the First Certificate of Merger in accordance with the DGCL.
4.6 Capitalization of the Company; Preliminary Allocation Schedule.
(a) The authorized capital stock of the Company consists of:
(i) 300,000,000 shares of Company Common Stock, 4,387,282 of which are issued and outstanding as of the date of this Agreement, inclusive of 1,509 shares of Company Series B Preferred Stock (out of 2,500 authorized) which were issued and outstanding as of immediately prior to the execution of this Agreement and were automatically converted into 658,968 shares of Company Common Stock concurrently with the execution of this Agreement; and
| - 34 - |
(ii) 1,500 shares of Company Series A Preferred Stock, 65 of which are issued and outstanding as of the date of this Agreement and which are convertible into 93,000 shares of Company Common Stock as of the date of this Agreement, inclusive of all accrued unpaid dividends. All issued and outstanding shares of Company Preferred Stock will have been converted into shares of Company Common Stock in accordance with the Company Charter prior to the Reverse Split.
(b) All of the issued and outstanding shares of Company Capital Stock have been duly authorized and validly issued and are fully paid and nonassessable and have not been issued in violation of any preemptive or similar rights. All of the issued and outstanding shares of capital stock of the Company have been offered, issued and sold by the Company in material compliance with all applicable federal and state securities Laws.
(c) The Company has made available to Buyer a complete and accurate list (set forth in Section 4.6(c) of the Company Disclosure Schedule) of all of the Company Equity Plans, indicating for each Company Equity Plan, as of the date hereof, (i) the number of shares of Company Common Stock issued under such Company Equity Plan, (ii) the number of shares of Company Common Stock subject to outstanding Company Options under such Company Equity Plan, (iii) the number of shares of Company Common Stock reserved for future issuance under such Company Equity Plan, and (iv) the exercise price of each of the outstanding Company Options under such Company Equity Plan. The Company has made available to Buyer complete and accurate copies of all (A) Company Equity Plans, (B) forms of stock option agreements evidencing Company Options, (C) the forms of agreements evidencing any other equity or equity-linked award or compensation arrangement and (D) any equity or equity-based award agreements that deviate in any material respect from the forms of agreement described in (B) and (C). All of the shares of capital stock of the Company subject to Company Options will be, upon issuance pursuant to the exercise of such instruments, duly authorized, validly issued, fully paid, nonassessable and free of all preemptive rights.
(d) With respect to each Company Option (whether outstanding or previously exercised), (i) each such Company Option intended to qualify as an “incentive stock option” under Section 422 of the Code so qualifies, (ii) each grant of a Company Option was duly authorized no later than the date on which the grant of such Company Option was by its terms to be effective (the “Grant Date”) by all necessary corporate action, including, as applicable, approval by the Company’s Board of Directors (or a duly constituted and authorized committee thereof), or a duly authorized delegate thereof, and any required stockholder approval by the necessary number of votes or written consents, and the award agreement governing such grant (if any) was duly executed and delivered by each party thereto no later than the Grant Date and (iii) each such grant was made in accordance with, in all material respects, the terms of the applicable Company Equity Plan, the Exchange Act, to the extent applicable, and all other applicable Laws. No Company Option granted by the Company has an exercise price that has been or may be less than the fair market value of the underlying stock as of the date such Company Option was granted or has any feature for the deferral of compensation other than the deferral of recognition of income until the later of exercise or disposition of such Company Option.
| - 35 - |
(e) Except as set forth in Section 4.6(a) and Section 4.6(c) of the Company Disclosure Schedule, (i) there are no equity interests of any class of the Company, or any security exchangeable into or exercisable for such equity interests, issued, reserved for issuance or outstanding, (ii) there are no options, warrants, equity securities, calls, rights, commitments or Contracts to which the Company is a party or by which the Company is bound obligating the Company to issue, exchange, transfer, deliver or sell, or cause to be issued, exchanged, transferred, delivered or sold, additional shares of capital stock or other equity interests of the Company or any security or rights convertible into or exchangeable or exercisable for any such shares or other equity interests, or obligating the Company to grant, extend, otherwise modify or amend or enter into any such option, warrant, equity interest, call, right, commitment or Contract, (iii) the Company has no obligation (contingent or otherwise) to issue any subscription, warrant, option, convertible security or other such right, or to issue or distribute to holders of any equity interests of the Company any evidences of Indebtedness or assets of the Company, and (iv) the Company has no obligation (contingent or otherwise) to purchase, redeem or otherwise acquire any equity interests or to pay any dividend or to make any other distribution in respect thereof.
(f) There is no Contract, written or oral, between the Company and any holder of its securities, or, to the Company’s Knowledge, among any holders of its securities, relating to the sale or transfer (including Contracts relating to rights of first refusal, co-sale rights or “drag along” rights), registration under the Securities Act or the securities Laws of any other jurisdiction, or voting, of the capital stock of the Company.
(g) The Preliminary Allocation Schedule sets forth a true, correct and complete summary of the allocation (estimated as of the date hereof) of the amounts payable to the Company Stockholders pursuant to this Agreement.
4.7 SEC Filings; Financial Statements.
(a) The Company has filed or furnished, as applicable, all registration statements, forms, reports and other documents (including exhibits and other information incorporated therein) required to be filed or furnished by the Company with the SEC since January 1, 2021. All such registration statements, forms, reports and other documents (including exhibits and all other information incorporated therein and those registration statements, forms, reports and other documents that the Company may file or furnish after the date hereof until the Closing) are referred to herein as the “Company SEC Reports.” The Company SEC Reports (i) were or will be filed or furnished on a timely basis, (ii) at the time filed or furnished, complied, or will comply when filed or furnished, as to form in all material respects with the requirements of the Securities Act, the Exchange Act, the Sarbanes-Oxley Act and the Dodd-Frank Act of 2010, as amended, as the case may be, and applicable to such Company SEC Reports and (iii) except to the extent that information contained in a Company SEC Report has been revised, amended, modified or superseded by a later filed or furnished Company SEC Report, did not or will not at the time they were or are filed or furnished contain any untrue statement of a material fact or omit to state a material fact required to be stated in such Company SEC Reports or necessary in order to make the statements in such Company SEC Reports, in the light of the circumstances under which they were made, not misleading in any material respect.
| - 36 - |
(b) Each of the consolidated financial statements (including, in each case, any related notes and schedules) contained or to be contained (including by incorporation by reference) in the Company SEC Reports (the “Financial Statements”) at the time filed (i) complied or will comply as to form in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto, (ii) were or will be prepared in accordance with GAAP applied on a consistent basis throughout the periods involved (except as may be indicated in the notes to such financial statements or, in the case of unaudited interim financial statements, as permitted by the SEC on Form 10-Q under the Exchange Act), and (iii) fairly presented or will fairly present in all material respects the consolidated financial position of the Company and its Subsidiaries as of the dates indicated and the consolidated results of its operations and cash flows for the periods indicated, all in accordance with GAAP, except that the unaudited interim financial statements were or are subject to normal and recurring year-end adjustments (none of which are reasonably expected to be material).
(c) The Company is in compliance in all material respects with the applicable provisions of the Sarbanes-Oxley Act. Each required form, report and document containing financial statements that has been filed with or submitted to the SEC was accompanied by any certifications required to be filed or submitted by the Company’s principal executive officer and principal financial officer pursuant to the Sarbanes-Oxley Act and, at the time of filing or submission of each such certification, any such certification complied in all material respects with the applicable provisions of the Sarbanes-Oxley Act.
(d) The Company maintains disclosure controls and procedures required by Rule 13a-15 or 15d-15 under the Exchange Act. Such disclosure controls and procedures are designed to provide reasonable assurance that all information concerning the Company that could have a material effect on the financial statements is made known on a timely basis to the individuals responsible for the preparation of the Company’s filings with the SEC and other public disclosure documents.
4.8 Undisclosed Liabilities.
(a) There is no Liability of the Company or any of its Subsidiaries, except for Liabilities (i) reflected or reserved for on the Company Balance Sheet, (ii) that have arisen since the date of the Company Balance Sheet in the ordinary course of the operation of business of the Company, (iii) disclosed in the Company Disclosure Schedule or (iv) contractual and other liabilities incurred in the ordinary course of business that are not required by GAAP to be reflected on a balance sheet and that are not in the aggregate material (in each case, none of which results from, arises out of, relates to, is in the nature of, or was caused by any breach of contract, breach of warranty, tort, infringement or violation of Law).
(b) Section 4.8(b) of the Company Disclosure Schedule contains a complete and accurate list, including the applicable amounts, of each item constituting Indebtedness of the Company or any of its Subsidiaries as of the date hereof.
| - 37 - |
4.9 Litigation and Proceedings. There are no pending or, to the Knowledge of the Company, threatened, lawsuits, actions, suits, claims or other proceedings at law or in equity or, to the Knowledge of the Company, investigations, in each case, before or by any Governmental Authority against or involving the Company, any of its Subsidiaries, or any current or former officer, director, employee, consultant, agent or stockholder of the Company or any of its Subsidiaries in its, his or her capacity as such or with respect to the Company or such Subsidiary that, in each case, if resolved adversely to the Company or such Subsidiary, would not reasonably be expected to result in the loss of a material benefit of, or the incurrence of a material Liability by, the Company or any of its Subsidiaries. There are no judgments, orders, injunctions, decrees, stipulations or awards (whether rendered by a court, administrative agency or other Governmental Authority, by arbitration or otherwise) against or involving the Company or any of its Subsidiaries. There is no Action by the Company or any of its Subsidiaries pending, or which the Company or any of its Subsidiaries, as applicable, has commenced preparations to initiate, against any other Person.
4.10 Compliance with Laws.
(a) The Company and each of its Subsidiaries are in compliance, and have, in the past three (3) years, conducted its business in compliance, in all material respects with all applicable Laws, including all applicable Anti-Corruption Laws, Anti-Money Laundering Laws, Human Rights Laws and Modern Slavery Laws. Neither the Company nor any of its Subsidiaries has received any written notice from any Governmental Authority (including the FDA) of a material violation of any applicable Law at any time during the past three (3) years.
(b) Without limiting Section 4.10(a), neither the Company nor any of its Subsidiaries or, to the Company’s Knowledge, any agent acting on their behalf, has during the past three (3) years committed a violation of the U.S. Foreign Corrupt Practices Act of 1977, as amended, or any other anti-bribery or anticorruption Law, including the UK Anti-Bribery Act 2010 and the Australian Criminal Code Act 1995 (Cth) and similar Australian state laws of any jurisdiction, to the extent applicable to the Company or such Subsidiary (collectively, “Anti-Bribery Laws”), or received any written or to the Company’s Knowledge, oral communication from any Governmental Authority that alleges that the Company or any of its Subsidiaries is or may be in violation of, or has or may have any Liability under, any Anti-Bribery Law. There is no pending or, to the Company’s Knowledge, threatened investigation, claim, or any other proceeding, in each case by or from a Governmental Authority, regarding any actual or possible violation of the Anti-Bribery Laws by the Company or any of its Subsidiaries. To the Company’s Knowledge, none of the Company, any of its Subsidiaries or any of their respective representatives to the extent acting on behalf of their behalf has, during the past three (3) years, directly or indirectly, offered, given, reimbursed, paid or promised to pay, or authorized the payment of, any material money or other thing of material value (including any fee, gift, sample, travel expense or entertainment) or any commission payment payable to (a) any Person who is an official, officer, agent, employee or representative of any Governmental Authority or of any existing or prospective customer (whether or not owned by a Governmental Authority), (b) any political party or official thereof, or (c) any candidate for political or political party office, in each case while knowing or having reason to believe that all or any portion of such money or thing of value would be offered, given, reimbursed, paid or promised, directly or indirectly, in violation of the Anti-Bribery Laws of any jurisdiction applicable to the Company or any of its Subsidiaries. During the past three (3) years, neither the Company nor any of its Subsidiaries has made any disclosures to any Governmental Authority concerning potential violations of any Anti-Bribery Laws.
| - 38 - |
(c) Without limiting Section 4.10(a), neither the Company nor any of its Subsidiaries or, to the Company’s Knowledge, any agent acting on their behalf, has during the past three (3) years committed a violation of any Law applicable to the Company or any of its Subsidiaries relating to modern slavery and anti-human trafficking, including the U.S. Trafficking Victims Protection Act (TVPA) of 2000, the California Transparency in Supply Chains Act 2015 and the Australian Modern Slavery Act 2018 (Cth).
4.11 FDA Matters.
(a) As to each of the product candidates of each of the Company and its Subsidiaries, including compounds currently under research and/or development by the Company and subject to the jurisdiction of the FDA or any equivalent Governmental Authority in any legal jurisdiction other than the U.S. (each such product, a “Company Regulated Product”), such Company Regulated Product is being researched, investigated, developed, manufactured, packaged, labeled, stored, distributed, imported and exported, and tested in compliance in all material respects with all applicable Laws. To the extent that any Company Regulated Product involves the use of a radioisotope, the Company and its Subsidiaries are in compliance in all material respects with respect to the applicable Laws governing such isotopes.
(b) The Company and its Subsidiaries hold, directly or by virtue of its agreements with its vendors, all required Permits to research, investigate, develop, manufacture, package, label, store, distribute, and test each Company Regulated Product and any radioisotope thereof and no such Permit has been revoked, withdrawn, suspended, cancelled or terminated or modified in any adverse manner. To the Knowledge of the Company, there is no basis for believing that any such Permit will not be renewable upon expiration. The Company and its Subsidiaries are, to the extent applicable, in compliance in all material respects with such Permits and have not received any written notice or other written communication, or to the Knowledge of the Company, any other communication from any Governmental Authority regarding (i) any material violation of or failure to comply materially with any term or requirement of any Permit or (ii) any revocation, withdrawal, suspension, cancellation, termination or material modification of any Permit. No Action is pending or, to the Knowledge of the Company, threatened, which seeks to revoke, limit, suspend, or materially modify any such Permit.
(c) There are no Actions pending or, to the Knowledge of the Company, threatened against the Company or any of its Subsidiaries with respect to an alleged material violation of the FDCA or any similar Law administered or promulgated by any FDA-equivalent Governmental Authority in any legal jurisdiction other than the U.S. None of the Company, its Subsidiaries or their respective officers or employees has been or is subject to any enforcement Actions by the FDA or other Governmental Authority and, to the Knowledge of the Company, no such Actions have been threatened. There is not any Form FDA-483 observation, civil, criminal or administrative Action, demand letter, warning letter or untitled letter pending or in effect against the Company or any of its Subsidiaries or any of their respective officers or employees, and the Company and its Subsidiaries have no liability for failure to comply with the FDCA or other similar Laws. There is no act, omission, event, or circumstance of which the Company has Knowledge that would reasonably be expected to give rise to or form the basis for any civil, criminal or administrative Action, demand letter, warning letter, untitled letter or request for information or any Liability for failure to comply with the FDCA or other similar Laws. Neither the Company nor any of its Subsidiaries has received any written notice that the FDA or any other Governmental Authority has commenced, or, to the Company’s Knowledge, threatened in writing to initiate, any action to enjoin the manufacture and production of the Company Regulated Products or any component thereof at any of its or its suppliers’ facilities.
| - 39 - |
(d) All preclinical studies and clinical trials, and other studies and tests of any Company Regulated Product conducted by or on behalf of the Company or any of its Subsidiaries have been, and if still pending are being, conducted in material compliance, to the extent applicable with the applicable protocol for such study or trial, good laboratory practices, good clinical practices and all applicable Laws, including the FDCA and its implementing regulations governing good laboratory practices and good clinical practices (e.g., 21 C.F.R. Parts 50, 54, 56, and 312 of the U.S. Code of Federal Regulations) and the respective counterparts thereof outside the United States. No clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has been terminated or placed on full or partial clinical hold by the FDA or by the applicable Institutional Review Board (“IRB”) for safety reasons or otherwise prior to scheduled completion, and neither the FDA, an IRB nor any other applicable Governmental Authority, clinical investigator that has participated or is participating in, or institutional review board that has or has had jurisdiction over, a clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has initiated, or, to the Company’s Knowledge, threatened to initiate, any action to place a full or partial clinical hold order on, or otherwise terminate or suspend, any proposed or ongoing clinical investigation of the Company Regulated Products conducted or proposed to be conducted by or on behalf of the Company or any of its Subsidiaries.
(e) All manufacturing operations conducted by or for the benefit of the Company and its Subsidiaries have been and are being conducted in material compliance with applicable Laws, including provisions of the FDA’s current good manufacturing practice regulations and comparable regulatory requirements of foreign Governmental Authorities. The Company and its Subsidiaries have established and maintain a quality agreement with each of the third party vendors that manufacture, process, package, or supply ingredients and packaging materials for or distribute the Company Regulated Products. The Company and its Subsidiaries, and to the Company’s Knowledge their respective third party vendors, have filed all required notices, registration applications, order forms, reports, supplemental applications and annual or other reports or documents, including adverse experience reports, that are material to the continued development, handling, manufacture, sale, and distribution of the Company Regulated Products. No supplier or manufacturing site for any Company Regulated Product (whether owned by the Company and its Subsidiaries or that of a contract manufacturer) has been subject to a Governmental Authority (including FDA) shutdown or import or export prohibition, nor received and not closed out any FDA Form 483 or any other Governmental Authority notice of inspectional observations, “warning letters,” “untitled letters” or similar correspondence or notice from the FDA or other Governmental Authority.
(f) Neither the Company nor any of its Subsidiaries has made any untrue statement of a material fact or fraudulent statement to the FDA or any Governmental Authority or otherwise failed to disclose a material fact required to be disclosed to the FDA or any Governmental Authority. The Company and its Subsidiaries are not the subject of any pending or, to the Knowledge of the Company, threatened investigation in respect of any Company Regulated Product pursuant to the FDA’s “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy or FDA’s Application Integrity Policy. All documents and information filed by the Company or any of its Subsidiaries with the FDA or any other Governmental Authority with respect to the Company Regulated Products, or the manufacturing, handling, storage or shipment of the Company Regulated Products were, at the time of filing, true, complete and accurate in all material respects.
| - 40 - |
(g) None of the Company, its Subsidiaries, or any of their respective officers, directors, or employees has been, is, or is in anticipation of being (based on a conviction by the courts or a finding of fault by a regulatory authority): (i) debarred pursuant to the Generic Drug Enforcement Act of 1992 (21 U.S.C. § 335a), as amended from time to time; (ii) disqualified from participating in clinical trials pursuant to 21 C.F.R. §312.70, as amended from time to time; (iii) disqualified as a testing facility under 21 C.F.R. Part 58, Subpart K, as amended from time to time; (iv) excluded, debarred or suspended from or otherwise ineligible to participate in a “Federal Health Care Program” as that term is defined in 42 U.S.C. 1320a-7b(f), including under 42 U.S.C. § 1320a-7 or relevant regulations in 42 C.F.R. Part 1001; (v) assessed or threatened with assessment of civil money penalties pursuant to 42 C.F.R. Part 1003; or (vi) included on the HHS/OIG List of Excluded Individuals/Entities, the General Services Administration’s System for Award Management, or the FDA Debarment List or the FDA Disqualified/Restricted List. None of the Company, its Subsidiaries or any of their respective officers, directors or employees has engaged in any activities that are prohibited, or are cause for civil penalties, or grounds for mandatory or permissive exclusion, debarment, or suspension pursuant to any of these authorities. The Company and its Subsidiaries are not using, nor have they ever used, in any capacity any person that has ever been, or to the Knowledge of the Company, is the subject of an Action that could lead to the persons becoming debarred, excluded, disqualified, restricted or suspended pursuant to any of these authorities.
(h) Each of the Company and its Subsidiaries have materially complied with all applicable Laws relating to patient, medical or individual health information, including the Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”), and the standards for the privacy of Individually Identifiable Health Information at 45 C.F.R. Parts 160 and 164, Subparts A and E, the standards for the protection of Electronic Protected Health Information set forth at 45 C.F.R. Part 160 and 45 C.F.R. Part 164, Subpart A and Subpart C, the standards for transactions and code sets used in electronic transactions at 45 C.F.R. Part 160, Subpart A and Part 162, and the standards for Breach Notification for Unsecured Protected Health Information at 45 C.F.R. Part 164, Subpart D, all as amended from time to time. Each of the Company and its Subsidiaries have entered into, where required, and are in compliance in all material respects with the terms of all Business Associate Agreements (as defined in HIPAA) to which Company or any Subsidiary is a party or otherwise bound. Company and its Subsidiaries where required, have (i) created and maintained written policies and procedures to protect the privacy of Protected Health Information (as defined in HIPAA) in its possession or control, (ii) provided training to all employees and agents, and (iii) implemented security procedures, including physical, technical and administrative safeguards, to protect all Protected Health Information stored or transmitted in electronic form. Neither the Company nor any of its Subsidiaries has received written notice from the Office for Civil Rights for the U.S. Department of Health and Human Services or any other Governmental Authority alleging a failure to comply with HIPAA or any other federal or state law or regulation applicable to the protection of individually identifiable health information or personally identifiable information. To the Knowledge of the Company, there has been no Breach (as defined in HIPAA) of Unsecured Protected Health Information (as defined in HIPAA), unpermitted disclosure of Personal Health Information (as defined in HIPAA), or breach of personally identifiable information with respect to information maintained or transmitted to the Company or any of its Subsidiaries that would require notice to a Governmental Authority.
| - 41 - |
All capitalized terms in Section 4.11(h) not otherwise defined in this Agreement shall have the meanings set forth under HIPAA.
4.12 Contracts; No Defaults.
(a) Section 4.12 of the Company Disclosure Schedule contains a listing of all of the following Contracts to which the Company or any of its Subsidiaries is a party or otherwise has any remaining rights or obligations (other than Company Benefit Plans covering more than one individual):
(i) each Contract that the Company reasonably anticipates will involve annual payments or consideration furnished by or to the Company or any of its Subsidiaries of more than $50,000;
(ii) each Contract relating to Indebtedness, including the borrowing of money, or mortgaging, pledging or otherwise placing a Lien on any assets of the Company or any of its Subsidiaries;
(iii) each Contract for the acquisition of any Person or any business division thereof or the disposition of any material assets of the Company or any of its Subsidiaries;
(iv) each lease, rental or occupancy agreement, real property license, installment and conditional sale agreement or other Contract that, in each case, provides for the ownership of, leasing of, title to, use of, or any leasehold or other interest in any real or personal property;
(v) each Contract providing for any royalty, milestone or similar payments by, or owed to, the Company or any of its Subsidiaries on or after the date hereof;
(vi) each joint venture Contract, partnership agreement or limited liability company agreement with a third party;
(vii) each Contract requiring capital expenditures after the date of this Agreement in an annual amount in excess of $20,000;
(viii) each Contract in which the Company or any of its Subsidiaries is subject to noncompetition or non-solicitation (other than confidentiality agreements with customers of the Company or any of its Subsidiaries entered into in the ordinary course of business and set forth in the Company’s standard terms and conditions of sale or standard form of employment agreement, forms of which have previously been made available to Buyer) that restricts the Company or any of its Subsidiaries in any material respect;
(ix) each (A) employment Contract (excluding offer letters for at-will employment that do not provide for severance or for advance notice of termination or for any change of control, transaction, retention or other special remuneration) ;
| - 42 - |
(x) each Contract, plan, policy or program providing for severance, termination compensation, retention or stay pay, change in control payments or transaction-based bonuses;
(xi) each settlement Contract settling claims against the Company or any of its Subsidiaries or any of their respective current or former directors, officers, employees or consultants (including any Contract in connection with which any employment-related claim is settled);
(xii) each Contract which contains any provisions with ongoing obligations requiring the Company or any of its Subsidiaries to indemnify any other party (excluding indemnities contained in Contracts for the purchase, sale or license of products or services entered into in the ordinary course of business);
(xiii) each Contract containing covenants materially limiting (A) the types of business in which the Company or any of its Subsidiaries (or, after giving effect to the First Merger, Buyer or any of its Affiliates) may engage, (B) the geographic locations in which the Company or any of its Subsidiaries (or, after giving effect to the First Merger, Buyer or its Affiliates) may so engage in any business or (C) the products that the Company or any of its Subsidiaries (or, after giving effect to the First Merger, Buyer or any of its Affiliates) may research, develop, manufacture or commercialize;
(xiv) each Contract entered into by the Company or any of its Subsidiaries with any Affiliate of the Company or with any current or former officer, director or stockholder of the Company or any of its Subsidiaries or any Affiliate thereof;
(xv) each Contract relating to grants, funding or other forms of assistance received by the Company or any of its Subsidiaries from any Governmental Authority;
(xvi) each Contract relating the research, development, clinical trial, manufacturing, distribution, supply, marketing or co-promotion of any products, product candidates or devices in development by or which has been or which is being researched, developed, marketed, distributed, supported, sold or licensed out, in each case by or on behalf of the Company or any of its Subsidiaries; and
(xvii) each Contract pursuant to which the Company or any of its Subsidiaries (A) licenses from, or has otherwise been assigned, transferred or granted any covenant not to assert by, a third party, any Intellectual Property used in connection with the Exploitation of any Company Regulated Product that is material to the Company’s business (other than (1) (x) click-wrap, shrink-wrap and off-the-shelf software licenses, and (y) any other software licenses that are available on standard terms to the public generally, in each case of (x) and (y) with license, maintenance, support and other fees less than $10,000 per year) and (2) standard employee and consultant assignment agreements in the form made available to Buyer, (B) has licensed, assigned, sold or transferred to a third party, or otherwise granted to a third party, any right or covenant not to assert under any Company Intellectual Property, or (C) has agreed to indemnify a third party against any claim of infringement, violation or misappropriation of any Intellectual Property.
| - 43 - |
(b) True and complete copies of the Contracts listed (or required to be listed) on Section 4.12 of the Company Disclosure Schedule have been delivered to or made available to Buyer or its representatives. All of the Contracts set forth (or required to be set forth) on Section 4.12 of the Company Disclosure Schedule are (i) in full force and effect, subject to the Remedies Exception, and (ii) represent the valid and binding obligations of the Company or its Subsidiary or Subsidiaries party thereto and, to the Knowledge of the Company, represent the valid and binding obligations of the other parties thereto. Neither the Company nor, to the Knowledge of the Company, any other party thereto is in breach of or default under any such Contract. Neither Company nor any of its Subsidiaries has received any claim or notice of breach of or default under any such Contract. To the Knowledge of the Company, no event has occurred which, individually or together with other events, would reasonably be expected to result in a breach of or a default under any such Contract (in each case, with or without notice or lapse of time or both).
4.13 Company Benefit Plans.
(a) Section 4.13 of the Company Disclosure Schedule lists each “employee benefit plan” (as defined in Section 3(3) of ERISA) and each other compensation plan, program, agreement or arrangement that is maintained, sponsored or contributed to by the Company or any of its Subsidiaries for the benefit of its current or former employees or with respect to which the Company or any of its Subsidiaries has any Liability (collectively, without regard to materiality, the “Company Benefit Plans”). The Company has made available to Buyer true and complete copies of (i) each Company Benefit Plan and any summary plan descriptions thereof, (ii) Forms 5500 in each of the most recent three (3) plan years, including all schedules thereto, (iii) with respect to any Company Benefit Plan that purports to meet the requirements of Section 401(a) of the Code, the most recent determination, advisory, or opinion letter issued by the IRS, (iv) all material notices that were given by any Governmental Authority to the Company any Company Benefit Plan during the past five (5) years, and (v) any trust documents, funding vehicles and any material third-party Contracts with respect to such Company Benefit Plan. The Company does not utilize a “professional employer organization” (PEO), employee leasing company or other similar organization to provide benefits to its workforce.
(b) Neither the Company nor any of its Subsidiaries maintains or has ever maintained any compensatory arrangement that would, if maintained, be within the definition of “Company Benefit Plan” nor has it failed to maintain to a Company Benefit Plan when required to do so.
(c) Each Company Benefit Plan that is intended to be qualified under Section 401(a) of the Code has received a favorable determination, advisory, or opinion letter from the IRS, or has pending or has time remaining in which to file an application for such a determination from the IRS. Any operational failures under such plan have been corrected in accordance with applicable guidance. There has been no prohibited transaction (within the meaning of Section 406 of ERISA or Section 4975 of the Code, other than a transaction that is exempt under a statutory or administrative exemption) with respect to any Company Benefit Plan that could result in the loss of a material benefit of, or the incurrence of a material Liability by the Company or its Subsidiaries.
| - 44 - |
(d) None of the Company, any of its Subsidiaries or any ERISA Affiliate has ever maintained, contributed to, or had any Liability with respect to, any (i) “defined benefit plan” (as defined in Section 3(35) of ERISA) or any other plan that is or was subject to the funding requirements of Section 412 or 430 of the Code or Section 302 or Title IV of ERISA, (ii) “multiemployer plan” (as defined in Section 3(37) of ERISA), (iii) multiple employer plan (as described in Section 413(c) of Code or Section 210 of ERISA), (iv) “multiple employer welfare arrangement” (as defined in Section 3(40) of ERISA), or (v) funded welfare benefit plan within the meaning of Section 419 of the Code, nor has the Company or any of its Subsidiaries maintained or participated in any Company Benefit Plan that has covered employees outside of the United States or that has been subject to the Laws of any jurisdiction other than the United States.
(e) Section 4.13(e) of the Company Disclosure Schedule discloses each: (i) agreement with any stockholder, director, executive officer or other employee of the Company or its Subsidiaries (A) the benefits of which are contingent, or the terms of which are altered, upon the occurrence of a transaction involving the Company of the nature of any of the transactions contemplated by this Agreement, (B) providing any term of employment or compensation guarantee or (C) providing severance benefits or other benefits after the termination of employment of such stockholder, director, executive officer or employee; and (ii) agreement or plan binding the Company or its Subsidiaries, including any stock option plan, stock appreciation right plan, restricted stock plan, stock purchase plan, severance benefit plan or Company Benefit Plan, any of the benefits of which will be increased, or the vesting of the benefits of which will be accelerated, by the occurrence of any of the transactions contemplated by this Agreement or the value of any of the benefits of which will be calculated on the basis of any of the transactions contemplated by this Agreement.
(f) Except as required by Law, no Company Benefit Plan provides any post-employment medical or life insurance benefits.
(g) No act or omission has occurred and no condition exists with respect to any Company Benefit Plan that would subject Buyer, the Company, any of its Subsidiaries, or any plan participant to (i) any fine, penalty, Tax or Liability of any kind imposed under ERISA, the Code or any other applicable Law (other than Liabilities associated with the routine operation of the Company Benefit Plan) or (ii) any contractual indemnification or contribution obligation protecting any fiduciary, insurer or service provider with respect to any Company Benefit Plan, nor will the transactions contemplated by this Agreement give rise to any such Liability.
(h) There are no loans or extensions of credit from the Company or any of its Subsidiaries to any Company Employee or any service provider to the Company or any of its Subsidiaries (other than advances of business expenses in the ordinary course of business). There is no corporate-owned life insurance (COLI), split-dollar life insurance policy or any other life insurance policy on the life of any Company Employee or on any Company Stockholder.
(i) Each Company Benefit Plan that is a “nonqualified deferred compensation plan” (as defined in Code Section 409A(d)(1)) has been operated in all material respects in compliance with Code Section 409A. No service provider to the Company or its Subsidiaries has incurred liability for tax imposed under Section 409A(a)(1)(B) in connection with participation in any Company Benefit Plan or otherwise as a result of the service provider’s arrangements with the Company. No stock option or equity unit granted by the Company has an exercise price that has been or may be less than the fair market value of the underlying stock or equity units (as the case may be) as of the date such option or unit was granted or has any feature for the deferral of compensation other than the deferral of recognition of income until the later of exercise or disposition of such option.
| - 45 - |
4.14 Employment and Labor Relations.
(a) Neither the Company nor any Subsidiary has breached or violated in any material respect any (i) applicable Law regarding employment or employment practices, terms and conditions of employment and wages and hours, including any such Law or Contract respecting employment discrimination, employee classification (for overtime purposes or as employee versus independent contractor), overtime (including the proper determination of regular pay and the treatment of bonuses), meal and rest periods, equal pay or pay equity, workers’ compensation, family and medical or other employee leave, the Immigration Reform and Control Act, labor relations, disability rights or benefits, privacy, unlawful harassment, retaliation, whistleblowing, wrongful discharge or violation of the personal rights of Company Employees or prospective employees, equal opportunity/affirmative action, plant closure or mass layoff issues, unemployment insurance, and occupational safety and health requirements, (ii) order, ruling, decree, judgment or arbitration award of any arbitrator or any court or other Governmental Authority with respect to any Company Employee or any other current or former service provider, or (iii) employment agreement, other individual service providing agreement or other agreement entered into with any Company Employee or other current or former service provider. Neither the Company nor any of its Subsidiaries is a party to a conciliation agreement, consent decree or other Contract or order with any Governmental Authority with respect to employment practices. No claims, controversies, investigations, audits or other legal proceedings are pending or, to the Knowledge of the Company, threatened, with respect to such Laws or employment agreements, either by private persons or by Governmental Authorities.
(b) Neither the Company nor any of its Subsidiaries has ever been a party to or bound by any collective bargaining agreement. Neither the Company nor any of its Subsidiaries has ever experienced any actual or, to the Company’s Knowledge, threatened strikes, grievances, claims of unfair labor practices, other collective bargaining disputes, organizational efforts, or filings of petition for certification nor is the Company or any of its Subsidiaries, to the Company’s Knowledge, the subject of threatened organizational efforts.
(c) Section 4.14(c) of the Company Disclosure Schedule contains a list of all current Company Employees (by employee identification number), along with the employer, position, date of hire, annual rate of compensation (or, where applicable, the hourly or per diem rate of compensation, or, if by commissions, a description of or cross-reference to the applicable terms), estimated or target annual incentive compensation of each such person, employment status of each such person (including whether the person is on leave of absence and the dates of such leave), part-time or full-time status, weekly working hours where not full-time, status as exempt or non-exempt from overtime, assigned work location, and remote work location. Section 4.14(c) of the Company Disclosure Schedule sets forth all bonuses earned by any Company Employee through the Closing Date that are expected to be accrued but unpaid as of the Closing Date and the amounts of accrued vacation or paid time off, accrued sick time, and the amount of such liabilities as of one (1) Business Day prior to the date of this Agreement. Each such Company Employee is retained at-will or is a party to an employment Contract with the Company or any of its Subsidiaries that has been made available to Buyer. Each Company Employee has entered into the Company’s or such Subsidiary’s standard form of confidentiality, non-competition (where permitted by applicable Law), non-solicitation and assignment of inventions agreement, a copy of which has previously been made available to Buyer. All of the agreements referenced in the preceding sentence will continue to be legal, valid, binding and enforceable and in full force and effect immediately following the Closing in accordance with the terms thereof as in effect immediately prior to the Closing. To the Knowledge of the Company, no key Company Employee or group of Company Employees has any plans to terminate employment with the Company or any of its Subsidiaries.
| - 46 - |
(d) All Company Employees employed in the United States are citizens or permanent residents. Neither the Company nor any of its Subsidiaries employs or engages or has ever employed or engaged any individual outside the United States.
(e) Section 4.14(e) of the Company Disclosure Schedule contains a list of all individual consultants and individual independent contractors currently engaged by either the Company or any of its Subsidiaries (including any engaged through an entity in which the consultant or contractor is a substantial owner), along with the position, date of retention, expected end date, category of services provided, whether engaged directly or through a third party, and rate of remuneration for each such Person. All Persons treated as independent contractors rather than as employees have been properly so treated, and any compensation paid to them has been reported on IRS Form 1099 or other applicable Tax form. Except as disclosed in Section 4.14(e) of the Company Disclosure Schedule, each such consultant or independent contractor is a party to a written agreement or Contract directly with the Company or its applicable Subsidiary or is engaged through written agreements between the Company or such Subsidiary and staffing agencies that treat such consultant or independent contractor as employees of the agency. Each such consultant and independent contractor has entered into the Company’s or such Subsidiary’s standard form of confidentiality, non-solicitation and assignment of inventions agreement with the Company or such Subsidiary, a copy of which has previously been made available to Buyer, or is bound by similar confidentiality, non-solicitation and assignment of inventions covenants pursuant to the master agreements signed with such consultant or independent contractor or such consultant’s employer. Neither the Company nor any of its Subsidiaries has or has had any temporary or leased employees.
(f) No charges or complaints are open and pending (or in the past three (3) years have been settled or otherwise closed) against the Company or any of its Subsidiaries with the Equal Employment Opportunity Commission, the Office of Federal Contract Compliance Programs, or other Governmental Authority regulating the employment or compensation of individuals (or, with respect to discrimination, retaliation, or similar wrongdoing, pursuant to internal complaint procedures), and no Company Employee has made, in the past three (3) years, a written complaint of discrimination, harassment, retaliation, or other similar wrongdoing or, to the Knowledge of the Company, in the past year, an oral complaint. In the past three (3) years, neither the Company nor any of its Subsidiaries has received any requests for, or conducted, an internal investigation of any officer or supervisor of the Company or any of its Subsidiaries with respect to any such claims.
| - 47 - |
(g) Neither the Company nor any of its Subsidiaries has any Liability with respect to (i) any misclassification of any person as an independent contractor rather than as an employee, as an employee rather than as an independent contractor, or as a non-employee when in fact employed, (ii) any employee or contractor leased from or staffed by another employer, or (iii) any person currently or formerly classified as exempt from, or otherwise not paid where required, overtime and minimum or other wages
(h) To the Company’s Knowledge, no current Company Employee is in violation of any term of any patent disclosure agreement, non-competition agreement, or any restrictive covenant to a former employer relating to the right of any such employee to be employed by the Company or its Subsidiaries because of the nature of the business conducted or presently proposed to be conducted by the Company or its Subsidiaries or to the use of trade secrets or proprietary information of others, nor, to the Company’s Knowledge, will any current Company Employee be in violation under any such agreement or covenant upon employment by or performance of services for the group of companies including Buyer.
(i) Section 4.14(i) of the Company Disclosure Schedule (i) contains a complete and accurate list of all of the Company’s and its Subsidiaries’ written employee handbooks, employment manuals, and employment policies, and (ii) sets forth the policy of the Company and its Subsidiaries with respect to accrued vacation, paid time off, accrued sick time and earned time off.
4.15 Taxes.
(a) Each of the Company and its Subsidiaries has properly filed all Tax Returns that it was required to file, and all such Tax Returns are true, correct and complete in all material respects. Each of the Company and its Subsidiaries has paid all Taxes, whether or not shown on any Tax Return, that were due and payable. The unpaid Taxes of the Company and each of its Subsidiaries (i) for taxable periods (or portions thereof) through the date of the Company Balance Sheet do not exceed the accruals and reserves for Taxes (excluding accruals and reserves for deferred Taxes established to reflect timing differences between book and Tax income) set forth on the Company Balance Sheet and (ii) for taxable periods (or portions thereof) though the Closing Date, will not exceed the reserve as adjusted for the passage of time through the Closing Date in accordance with GAAP. All unpaid Taxes of the Company and each of its Subsidiaries for all taxable periods (or portions thereof) commencing after the date of the Company Balance Sheet arose in the ordinary course of business.
(b) All Taxes that the Company or any of its Subsidiaries is or was required by Law to withhold or collect have been duly withheld or collected and, to the extent required, have been properly paid to the appropriate Governmental Authority, and each of the Company and its Subsidiaries has complied in all material respects with all information reporting and backup withholding requirements, including the maintenance of required records with respect thereto, in connection with amounts paid to any employee, independent contractor, creditor, or other third party.
(c) Neither the Company nor any of its Subsidiaries is or has ever been a member of an affiliated group with which it has filed (or been required to file) consolidated, combined, unitary or similar Tax Returns, other than a group of which the common parent is the Company. Neither the Company nor any of its Subsidiaries (i) has any liability under Treasury Regulation Section 1.1502-6 (or any comparable or similar provision of federal, state, local or foreign Law), as a transferee or successor, pursuant to any contractual obligation, or otherwise for any Taxes of any Person other than the Company or any of its Subsidiaries, or (ii) is a party to or bound by any Tax indemnity, Tax sharing, Tax allocation or similar agreement.
| - 48 - |
(d) The Company has delivered or made available to Buyer (i) complete and correct copies of all Tax Returns of the Company and its Subsidiaries relating to Taxes for all taxable periods for which the applicable statute of limitations has not yet expired, (ii) complete and correct copies of all private letter rulings, revenue agent reports, information document requests, notices of proposed deficiencies, deficiency notices, protests, petitions, closing agreements, settlement agreements, pending ruling requests and any similar documents submitted by, received by, or agreed to by or on behalf of the Company or any of its Subsidiaries relating to Taxes for all taxable periods for which the statute of limitations has not yet expired, and (iii) complete and correct copies of all material agreements, rulings, settlements or other Tax documents with or from any Governmental Authority relating to Tax incentives of the Company or any of its Subsidiaries.
(e) No examination or audit or other action of or relating to any Tax Return of the Company or any of its Subsidiaries by any Governmental Authority is currently in progress or, to the Knowledge of the Company, threatened. No deficiencies for Taxes of the Company or any of its Subsidiaries have been claimed, proposed or assessed by any Governmental Authority in writing. Neither the Company nor any of its Subsidiaries has been informed in writing by any jurisdiction in which the Company or any Subsidiary does not file a Tax Return that the jurisdiction believes that the Company or Subsidiary was required to file any Tax Return that was not filed or is subject to Tax in such jurisdiction. Neither the Company nor any of its Subsidiaries has (i) waived any statute of limitations with respect to Taxes or agreed to extend the period for assessment or collection of any Taxes, which waiver or extension is still in effect, (ii) requested any extension of time within which to file any Tax Return, which Tax Return has not yet been filed, or (iii) executed or filed any power of attorney with any Tax Authority, which is still in effect.
(f) Except as provided Section 4.15(f) of the Company Disclosure Schedule, neither the Company nor any of its Subsidiaries has made any payment, is obligated to make any payment, or is a party to any agreement, contract, arrangement or plan that could obligate it to make any payment that may be treated as an “excess parachute payment” under Section 280G of the Code (without regard to Sections 280G(b)(4) and 280G(b)(5) of the Code).
(g) Neither the Company nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of (i) any adjustments under Section 481 of the Code (or any similar adjustments under any provision of the Code or the corresponding foreign, state or local Tax Law), (ii) deferred intercompany gain or any excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding provision of state, local or foreign Tax Law), (iii) a closing agreement as described in Section 7121 of the Code (or any corresponding or similar provision of state, local or foreign Tax Law) executed on or prior to the Closing Date, (iv) an installment sale or open transaction disposition made on or prior to the Closing Date, or (v) a prepaid amount or deferred revenue received on or prior to the Closing Date.
| - 49 - |
(h) Neither the Company nor any of its Subsidiaries has been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(l)(A)(ii) of the Code.
(i) Neither the Company nor any of its Subsidiaries has distributed to its shareholders or security holders stock or securities of a controlled corporation, nor has stock or securities of the Company or any of its Subsidiaries been distributed, in a transaction to which Section 355 of the Code applies (i) in the two years prior to the date of this Agreement or (ii) in a distribution that could otherwise constitute part of a “plan” or “series of related transactions” (within the meaning of Section 355(e) of the Code) that includes the transactions contemplated by this Agreement.
(j) There are no liens or other encumbrances with respect to Taxes upon any of the assets of the Company or any of its Subsidiaries, other than with respect to Taxes not yet due and payable.
(k) Neither the Company nor any of its Subsidiaries (i) is a party to any joint venture, partnership, or other arrangement that is treated as a partnership for federal income Tax purposes, (ii) has made an entity classification (“check-the-box”) election under Section 7701 of the Code, (iii) is a stockholder of a “controlled foreign corporation” as defined in Section 957 of the Code (or any similar provision of state, local or foreign Law), (iv) is a stockholder in a “passive foreign investment company” within the meaning of Section 1297 of the Code, or (v) has made an election under Section 965(h) with respect to any deferred foreign income corporation in which it was a United States shareholder within the meaning of Section 951(b).
(l) Neither the Company nor any of its Subsidiaries is subject to tax in any country other than its country of incorporation, organization or formation by virtue of having employees, a permanent establishment or other place of business in that country.
(m) All related party transactions involving the Company or any of its Subsidiaries have been conducted at arm’s length in compliance with Section 482 of the Code and the Treasury Regulations promulgated thereunder and any comparable provisions of any other Tax Law. Each of the Company and its Subsidiaries has maintained documentation (including any applicable transfer pricing studies) in connection with such related party transactions in accordance with Sections 482 and 6662 of the Code and the Treasury Regulations promulgated thereunder and any comparable provisions of any other Tax Law.
(n) Neither the Company nor any of its Subsidiaries has engaged in a “reportable transaction” as set forth in Treasury Regulation Section 1.6011-4(b) or a “listed transaction” as set forth in Treasury Regulation Section 301.6111-2(b)(2) or any analogous provision of state or local Law. Each of the Company and its Subsidiaries has disclosed on its federal income Tax Returns all positions taken therein that could give rise to a substantial understatement of federal income Tax within the meaning of Section 6662 of the Code.
| - 50 - |
(o) The Company has no Knowledge of any facts, and has not taken or agreed to take any action, that would reasonably be expected to prevent or impede the Merger from qualifying as a “reorganization” within the meaning of Section 368(a) of the Code.
(p) The Company is not an investment company as defined in Section 68(a)(2)(F)(iii) of the Code.
4.16 Brokers’ Fees. No broker, finder, investment banker or other Person is entitled to any brokerage fee, finders’ fee or other similar commission, for which Buyer, the Company or any Company Subsidiary would be liable in connection with the transactions contemplated by this Agreement based upon arrangements made by the Company or any of its Affiliates.
4.17 Insurance. Section 4.17 of the Company Disclosure Schedule contains a list of all material policies of property, fire and casualty, product liability, workers’ compensation, and other forms of insurance held by, or for the benefit of, the Company and its Subsidiaries as of the date of this Agreement. True and complete copies of such insurance policies have been made available to Buyer or its representatives. As of the date hereof, the Company has not received any written notice from any insurer under any such insurance policies, canceling or materially adversely amending any such policy or denying renewal of coverage thereunder, and all premiums on such insurance policies due and payable as of the date hereof have been paid.
4.18 Licenses, Permits and Authorizations. The Company and each of its Subsidiaries hold, and, to the Company’s Knowledge, is in compliance in all material respects with, all of the Permits issued by Governmental Authorities, including the FDA, that are required by applicable Laws to permit the Company and any of its Subsidiaries to own, operate, use, investigate, and maintain its assets in the manner in which they are now operated, used and maintained and to conduct the business of the Company or any of its Subsidiaries (collectively, the “Company Permits”). There are no pending or, to the Knowledge of the Company, threatened claims, actions, suits or other proceedings or, to the Knowledge of the Company, investigations before or by any Governmental Authority that would reasonably be expected to result in the revocation or termination of any such material Company Permit.
4.19 Real Property. The Company maintains no Leased Real Property, except for any shared office space that is cancellable on a month-to-month basis or which may subject the Company or any Subsidiary to payments in the aggregate of no greater than $5,000. Neither the Company nor any of its Subsidiaries owns real property.
4.20 Intellectual Property.
(a) Section 4.20(a) of the Company Disclosure Schedule lists (i) each Patent Right (A) that is in the Company Owned Intellectual Property or (B) that is in the Company Licensed Intellectual Property with respect to which the Company or any of its Subsidiaries has a right to participate in the prosecution or maintenance, and (ii) each trademark, service mark, domain name and copyright owned by the Company or any of its Subsidiaries, in each case for which applications have been filed or registrations or issued patents have been obtained, whether in the United States or in any country internationally (all of the items to be listed on Section 4.20(a) of the Company Disclosure Schedule, the “Company Registered IP”), in each case enumerating specifically the applicable filing or registration number, title, jurisdiction in which filing was made or from which registration issued, date of filing and issuance, names of all current applicant(s) and registered owners(s), as applicable. The Company and each of its Subsidiaries have made all filings and payments required to be made to maintain each item of such Company Registered IP in full force and effect by the applicable deadline and otherwise in accordance with all applicable Laws. All assignments to the Company or any of its Subsidiaries of Company Registered IP that is Company Owned Intellectual Property have been properly executed and recorded.
| - 51 - |
(b) Section 4.20(b) of the Company Disclosure Schedule sets forth a true and complete list of the third party Contracts pursuant to which Company or any of its Subsidiaries receives a license or other rights under any Company Intellectual Property (the “Existing In-License Agreements”). The Existing In-License Agreements are in full force and effect in accordance with their terms. Neither the Company nor its applicable Subsidiary, or, to the Company’s Knowledge, any of the other parties thereto, is in breach of any Existing In-License Agreement. Neither the Company nor any of its Subsidiaries has sent, provided, or received any notice of breach or intent to terminate any Existing In-License Agreement. The Company has made available to Buyer true, accurate and complete copies of the Existing In-License Agreements, including all amendments thereto.
(c) No inventorship challenge, opposition, nullity proceeding, inter partes review, post grant review proceeding or interference has been filed, or to the Knowledge of the Company, threatened, with respect to any Patent Rights included in the Company Registered IP. The Knowledge of the Company, the Company and each of its Subsidiaries have complied with its duty of candor and disclosure to the United States Patent and Trademark Office and any relevant foreign patent office with respect to all patent and trademark applications in the Company Registered IP filed by or on behalf of the Company or its Subsidiaries and have made no material misrepresentation in such applications. The Company has clear title to the Company Registered IP that is Company Owned Intellectual Property.
(d) The Company or any of its Subsidiaries, as applicable, owns (free and clear of all Liens), or has the right to use pursuant to license, sublicense, agreement or permission as set forth in a Contract set forth in Section 4.12 of the Company Disclosure Schedule, (i) all Company Registered IP and (ii) all other Intellectual Property used in, or necessary for the development, manufacture and commercialization of each Company Regulated Product and otherwise for the operation of the business of the Company and its Subsidiaries as presently conducted or as conducted at any time within the last three (3) years or as currently contemplated to be conducted, including, in each of cases (i) and (ii), any tangible embodiments thereof (all of the foregoing, collectively, the “Necessary Company IP”). The Company Intellectual Property includes all Necessary Company IP.
(e) Neither the Company nor any of its Subsidiaries has received from any Person in the past three (3) years any written notice, charge, complaint, claim or other written assertion of any direct or indirect infringement, violation or misappropriation of any Intellectual Property of any Person by the Company or any of its Subsidiaries. Neither the Exploitation of any of the Company Regulated Products, nor any other activity by the Company of any of its Subsidiaries, has infringed or violated, or constituted a misappropriation of, any Intellectual Property rights of any third party.
| - 52 - |
(f) To the Knowledge of the Company, no third party (including any current or former employee, consultant or contractor of the Company and its Subsidiaries) is infringing upon, misappropriating or otherwise violating any Company Intellectual Property, except for any such infringement that, individually or in the aggregate, would not reasonably be expected to result in material Liability to the Company, any of its Subsidiaries or Buyer.
(g) Neither the execution, delivery, or performance of this Agreement nor the consummation of any of the transactions or agreements contemplated by this Agreement will result in (i) any loss, termination or impairment of, or any change in the Company’s rights in, any Company Intellectual Property, (ii) a breach of or default under any Contract governing any Company Intellectual Property, (iii) the grant or transfer to any third party of any new license or other right or interest under, the abandonment, assignment to any third party, the modification or loss of any right with respect to, or the creation of any Lien on, any Company Intellectual Property, or (iv) the Company, any of its Subsidiaries, Buyer or any of Buyer’s Affiliates (including the Final Surviving Corporation) being obligated to pay any penalty or new or increased royalty or fee to any Person under any Contract governing any Company Intellectual Property.
(h) The Company and its Subsidiaries have used commercially reasonable efforts to maintain in confidence the trade secrets and other confidential information in the Company Intellectual Property. The Company and its Subsidiaries have complied in all material respects with all applicable Contracts and Laws pertaining to information privacy, data protection or security, including the Health Insurance Portability and Accountability Act of 1996, the EU Data Protection Directive and any Laws in any country relating thereto, and the General Data Protection Regulation and any Laws in any country relating thereto. No complaint relating to an improper use or disclosure of, or a breach in the security of, any trade secrets, confidential information or protected information has been made or, to the Knowledge of the Company, threatened against the Company of any of its Subsidiaries. To the Knowledge of the Company, there has been no (i) unauthorized disclosure of any third party proprietary or confidential information in the possession, custody or control of the Company or any of its Subsidiaries, or (ii) breach of the Company’s of any of its Subsidiaries’ security procedures wherein confidential information has been disclosed to a third party.
(i) Each individual who is or was an employee of the Company or any of its Subsidiaries has executed a valid, binding and enforceable written agreement expressly and presently assigning to the Company all right, title and interest in any inventions, works of authorship and data invented, conceived, reduced to practice, authored, created or otherwise developed, during the term of such individual’s employment work for the Company and its Subsidiaries, and all Intellectual Property rights therein, and has waived all moral rights therein to the extent legally permissible. With respect to any Necessary Company IP invented, conceived, reduced to practice, authored, created or otherwise developed by any third party, each such third party has executed a valid, binding and enforceable written agreement expressly and presently assigning or licensing to the Company all right, title and interest in and to such Necessary Company IP.
(j) The Company has no Company Intellectual Property that is subject to the Bayh-Dole Act or a comparable Law outside the United States or any other Law granting any Governmental Authority any license, retained right or march-in right with respect to such Intellectual Property or any right as a result of funding by a Governmental Authority. Neither the Company, or any assignor or licensor to the Company or any of its Subsidiaries, has received any support, funding, resources or assistance from any Governmental Authority or quasi-governmental agency or funding source in connection with the Exploitation of any Company Regulated Product, any facilities or equipment used in connection therewith or any Company Intellectual Property. No university or Governmental Authority has sponsored any research or development conducted by or on behalf of the Company or any of its Subsidiaries, or has any claim of right or ownership of or Lien on any Company Intellectual Property.
| - 53 - |
4.21 Environmental Matters. Except as would not reasonably be expected to result in the loss of a material benefit of, or the incurrence of a material Liability by, the Company or any of its Subsidiaries, (a) the Company and each of its Subsidiaries is (and has been for the last three (3) years) in compliance with all Environmental Laws, (b) the Company and each of its Subsidiaries holds, and is (and has been for the last three (3) years) in material compliance with, all Company Permits required under applicable Environmental Laws to permit the Company and its Subsidiaries to operate their respective assets in a manner in which they are now operated and maintained and to conduct the business of the Company and each of its Subsidiaries as currently conducted, and (c) there are no written claims or notices of violation pending or, to the Knowledge of the Company, threatened against the Company or any of its Subsidiaries alleging violations of or Liability under any Environmental Law.
4.22 Data Privacy. In connection with its collection, storage, transfer (including any transfer across national borders), disclosure and/or use of any personally identifiable information from any individuals (collectively “Personal Information”), the Company and each of its Subsidiaries is and for the past three (3) years has been in compliance with all applicable Law related to the collection, storage, use, disclosure or processing of any Personal Information, including, to the extent applicable to the Company or such Subsidiary, HIPAA, the federal Privacy Act of 1974, the California Online Privacy Protection Act, the EU General Data Protection Regulation ((EU) 2016/679) and the Australian Privacy Act 2018 (Cth), as well as any privacy and security policies of the Company and its Subsidiaries and the requirements of any Contract to which the Company or any of its Subsidiaries are bound. The Company and its Subsidiaries implement, monitor and maintain reasonable physical, technical, organizational and administrative security measures and policies in place to protect the operation of all their information systems and the confidentiality of all Personal Information and confidential information collected by them from and against unauthorized access, use and/or disclosure. To the Company’s Knowledge, there has not been any material actual or reasonably suspected compromise or unlawful, accidental, or unauthorized loss of, use, acquisition, encryption, theft, disclosure of, access to, or other processing of Personal Information (“Security Incident”) or other material security breach impacting the security, confidentiality, operation or integrity of the information systems of the Company and its Subsidiaries.
4.23 Absence of Changes.
(a) From December 31, 2021 to the date of this Agreement, there has not been any Material Adverse Effect on the Company or any of its Subsidiaries.
| - 54 - |
(b) From the date of the most recent balance sheet included in the Financial Statements through the date of this Agreement, the Company and each of its Subsidiaries have in all material respects, conducted its business and operated its properties in the ordinary course of business.
(c) From the date of the most recent balance sheet included in the Financial Statements through the date of this Agreement, neither the Company nor any of its Subsidiaries has taken any of the actions set forth in Section 6.1.
4.24 Affiliate Matters. The Company is not party to any Contract with any (i) present or former officer or director of the Company or (ii) Affiliate of the Company. No Affiliate of the Company, directly or indirectly, (a) owns any property or right, tangible or intangible, which is used in the business of the Company or any of its Subsidiaries, (b) to the Knowledge of the Company, has any claim or cause of action against the Company or any of its Subsidiaries, or (c) other than employment-related arrangements and the payment of compensation and benefits in the ordinary course of business and travel advances and employee loans in the ordinary course of business, owes any money to, or is owed any money by, the Company or any of its Subsidiaries.
4.25 Accredited Investors. Section 4.25 of the Company Disclosure Schedules sets forth a list of each holder of Company Capital Stock, and whether such person is an Accredited Investor. Prior to the date hereof, the Company has used reasonable best efforts to obtain completed and signed accredited investor questionnaires, in the form attached hereto as Annex H (each such completed and signed accredited investor questionnaire, an “Investor Questionnaire”), from each holder of Company Capital Stock as of the date hereof and has made available to Buyer each such completed and signed Investor Questionnaire. The statements regarding any such holder’s status(es) as set forth in Section 4.25 of the Company Disclosure Schedule or as set forth in the Investor Questionnaires delivered by the holders of Company Capital Stock are, to the Knowledge of the Company, true, accurate and complete in all respects.
4.26 No Additional Representations or Warranties. The Company hereby acknowledges and agrees that, except for the representations and warranties set forth in Article V, (a) neither Buyer nor any its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, has made or is making any express or implied representation or warranty with respect to Buyer or any of its Subsidiaries or Affiliates or their respective business or operations, including with respect to any information provided or made available to the Company or any of its Affiliates, stockholders or representatives, or any other Person, or, except as otherwise expressly set forth in this Agreement, had or has any duty or obligation to provide any information to the Company or any of its Affiliates, stockholders or representatives, or any other Person, in connection with this Agreement, the transactions contemplated hereby or otherwise, and (b) to the fullest extent permitted by Law, neither Buyer nor its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, will have or be subject to any Liability or other obligation of any kind or nature to the Company or any of its Affiliates, stockholders or representatives, or any other Person, resulting from the delivery, dissemination or any other distribution to the Company or any of its Affiliates, stockholders or representatives, or any other Person, or the use by the Company or any of its Affiliates, stockholders or representatives, or any other Person, of any such information provided or made available to any of them by Buyer or any of its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, including any information, documents, estimates, projections, forecasts or other forward-looking information, business plans or other material provided or made available to the Company or any of its Affiliates, stockholders, or representatives, or any other Person in anticipation or contemplation of the Merger, the issuance of the Merger Consideration or any other transaction contemplated by this Agreement, and (subject to the express representations and warranties of Buyer set forth in Article V or in the case of fraud) neither the Company nor any of its Affiliates, stockholders or representatives, or any other Person, has relied on any such information (including the accuracy or completeness thereof).
| - 55 - |
Article V.
REPRESENTATIONS AND WARRANTIES OF BUYER AND MERGER SUBS
Except as disclosed in the Buyer Financial Reports, Buyer, Merger Sub I and Merger Sub II hereby jointly and severally represent and warrant to the Company that the statements contained in this Article V are true and correct as of the date of this Agreement and will be true and correct as of the Closing with the same effect as though made at and as of such time (provided, however, that representations and warranties that are made as of a particular date or period will be true and correct only as of such date or period).
5.1 Corporate Organization. Buyer has been duly incorporated and is validly existing as a public limited company under the Laws of the Commonwealth of Australia. Merger Sub I has been duly incorporated and is validly existing as a corporation in good standing under the Laws of the State of Delaware. Merger Sub II has been duly incorporated and is validly existing as a corporation in good standing under the Laws of the State of Delaware. Merger Sub I is a corporation newly formed for the sole purpose of effecting the First Merger, and has not engaged in any activity other than as contemplated in this Agreement. Merger Sub II is a corporation newly formed for the sole purpose of effecting the Second Merger, and has not engaged in any activity other than as contemplated in this Agreement. Each of Buyer and each of the Merger Subs has the requisite power and authority to own or lease its properties and to conduct its business as it is now being conducted. Each of Buyer and each of the Merger Subs are duly licensed or qualified and (where applicable) in good standing in each jurisdiction in which the ownership of its property or the character of its activities is such as to require it to be so licensed or qualified or in good standing, as applicable, except where failure to be so licensed or qualified or in good standing would not reasonably be expected to have a Material Adverse Effect on Buyer or any of its Subsidiaries. Buyer owns, beneficially and of record, all of the outstanding shares of capital stock of each of the Merger Subs, free and clear of all Liens.
5.2 Due Authorization. Each of Buyer, Merger Sub I and Merger Sub II has all requisite power and authority to execute and deliver this Agreement and (subject to the consents, approvals, authorizations and other requirements described in Section 5.4) to perform all obligations to be performed by it hereunder and under the CVR Agreement. The execution and delivery of this Agreement and the CVR Agreement by Buyer, Merger Sub I and Merger Sub II and the consummation by them of the transactions contemplated hereby and thereby have been duly and validly authorized and approved by the boards of directors of each of Buyer, Merger Sub I and Merger Sub II, and no other corporate proceeding on the part of Buyer, Merger Sub I or Merger Sub II is necessary to authorize this Agreement or the CVR Agreement (other than the adoption of this Agreement by Buyer in its capacity as the sole stockholder of Merger Sub I and Merger Sub II, which adoption will occur immediately following the execution of this Agreement by Merger Subs). This Agreement has been, and upon execution the CVR Agreement will have been, duly and validly executed and delivered by each of Buyer and/or Merger Subs, as applicable, and (assuming this Agreement and the CVR Agreement constitute a legal, valid and binding obligation of the other parties thereto) each constitutes a legal, valid and binding obligation of each of Buyer and/or Merger Subs, as applicable, enforceable against Buyer and/or Merger Subs, as applicable, in accordance with their respective terms, subject to the Remedies Exception.
| - 56 - |
5.3 No Conflict. Subject to the receipt of the consents, approvals, authorizations and other requirements set forth in Section 5.4 or on Schedule 5.4 the execution and delivery of this Agreement by Buyer and Merger Subs and the consummation by them of the transactions contemplated hereby do not and will not, as of the Closing, (a) violate any provision of, or result in the breach of any applicable Law to which Buyer or either Merger Sub is subject or by which any property or asset of Buyer or either Merger Sub is bound, (b) conflict with or violate any provision of the certificate of incorporation, constitution, bylaws or other organizational documents of Buyer or any Subsidiary of Buyer (including either Merger Sub), (c) violate any provision of or result in a breach of, or require a consent or constitute (with or without due notice or lapse of time or both) under, any agreement, indenture or other instrument to which Buyer or any Subsidiary of Buyer (including either Merger Sub) is a party or by which Buyer or any Subsidiary of Buyer (including either Merger Sub) may be bound, or terminate or result in the termination of any such agreement, indenture or instrument, or result in the creation of any Lien under any such agreement, indenture or instrument upon any of the properties or assets of Buyer or any Subsidiary of Buyer (including either Merger Sub), or constitute an event which, after notice or lapse of time or both, would result in any such violation, breach, termination or creation of a Lien or create in any party the right to accelerate or modify such Contract, or (d) result in a violation or revocation of any required Permit from any Governmental Authority, except to the extent that the occurrence of the foregoing items set forth in clauses (a), (c) or (d) would not reasonably be expected to have a Material Adverse Effect on Buyer or any of its Subsidiaries.
5.4 Governmental Consents. Assuming the truth and completeness of the representations and warranties of the Company contained in this Agreement, no consent, approval or authorization of, or designation, declaration or filing with, any Governmental Authority is required on the part of Buyer or Merger Subs with respect to Buyer’s or either Merger Subs’ execution or delivery of this Agreement or the consummation by Buyer or Merger Subs of the transactions contemplated hereby, except for (a) any consents, approvals, authorizations, designations, declarations or filings, the absence of which would not reasonably be expected to have a Material Adverse Effect on Buyer or any of its Subsidiaries, (b) compliance with any applicable securities Laws, (c) in the case of Merger Sub I, the filing of the First Certificate of Merger in accordance with the DGCL and (d) in the case of Merger Sub II, the filing of the Second Certificate of Merger in accordance with the DGCL.
5.5 Litigation and Proceedings. As of the date of this Agreement, there are no pending or, to the knowledge of Buyer, threatened, lawsuits, actions, suits, claims or other proceedings at law or in equity or, to the knowledge of Buyer, investigations, in each case, before or by any Governmental Authority against Buyer or any of its Subsidiaries that, in each case, if resolved adversely to Buyer, would reasonably be expected to have a Material Adverse Effect on Buyer or any of its material Subsidiaries.
| - 57 - |
5.6 Issuance of Buyer Ordinary Shares. The issuance and delivery of Buyer Ordinary Shares in accordance with this Agreement, if, when and as issued, has been or will be, as of the applicable time of issuance, duly authorized by all necessary corporate action on the part of Buyer and, when issued as contemplated hereby, such Buyer Ordinary Shares shall be duly and validly issued, fully paid and nonassessable. Buyer has or will, as of the applicable time of issuance, the power to issue the Buyer Ordinary Shares in accordance with this Agreement in accordance with its constitution and the ASX Listing Rules without the approval of its shareholders (or any other Person) being required, has the existing capacity under ASX Listing Rule 7.1 to issue such Buyer Ordinary Shares and is not issuing such Buyer Ordinary Shares for the purpose described in section 707(3)(b)(i) of the Corporations Act. The Buyer Ordinary Shares, when so issued and delivered in accordance with the provisions of this Agreement, shall be free and clear of all Liens, other than those contemplated by this Agreement and any restrictions on transfer created by applicable securities Laws and will not have been issued in violation of applicable Laws or stock market rules or regulations, or any preemptive rights or rights of first refusal or similar rights.
5.7 No Additional Representations or Warranties. Buyer and Merger Subs hereby acknowledge and agree that, except for the representations and warranties set forth in Article IV or in the case of fraud, (a) neither the Company nor any its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, has made or is making any express or implied representation or warranty with respect to the Company or any of its Subsidiaries or Affiliates or their respective business or operations, including with respect to any information provided or made available to Buyer or any of its Affiliates, stockholders or representatives, or any other Person, or, except as otherwise expressly set forth in this Agreement, had or has any duty or obligation to provide any information to Buyer or any of its Affiliates, stockholders or representatives, or any other Person, in connection with this Agreement, the transactions contemplated hereby or otherwise, and (b) to the fullest extent permitted by Law, neither the Company nor its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, will have or be subject to any Liability or other obligation of any kind or nature to Buyer or any of its Affiliates, stockholders or representatives, or any other Person, resulting from the delivery, dissemination or any other distribution to Buyer or any of its Affiliates, stockholders or representatives, or any other Person, or the use by Buyer or any of its Affiliates, stockholders or representatives, or any other Person, of any such information provided or made available to any of them by the Company or any of its Subsidiaries, Affiliates, stockholders or representatives, or any other Person, including any information, documents, estimates, projections, forecasts or other forward-looking information, business plans or other material provided or made available to Buyer or any of its Affiliates, stockholders, or representatives, or any other Person in anticipation or contemplation of the Merger or any other transaction contemplated by this Agreement, and (subject to the express representations and warranties of the Company set forth in Article IV or in the case of fraud) neither Buyer nor any of its Affiliates, stockholders or representatives, or any other Person, has relied on any such information (including the accuracy or completeness thereof).
| - 58 - |
Article VI.
COVENANTS OF THE COMPANY
6.1 Conduct of Business.
(a) From the date of this Agreement through the Closing, the Company and its Subsidiaries shall, except (A) as would constitute a violation of applicable Law, (B) as set forth on Section 6.1 of the Company Disclosure Schedule, (C) as contemplated by this Agreement or (D) as consented to by Buyer in writing, operate its business in the ordinary course of business and maintain in all material respects satisfactory relationships with its material business relationships. Without limiting the generality of the foregoing, except (1) as would constitute a violation of applicable Law, (2) as set forth on Section 6.1 of the Company Disclosure Schedule or (3) as consented to by Buyer in writing (which consent, in the case of clause (v) below, shall not be unreasonably conditioned, withheld, delayed or denied), the Company shall not, except as otherwise contemplated by this Agreement:
(i) except as required to effect the Reverse Split, (A) change or amend the Company Charter, Company Bylaws or other organizational documents of the Company or any of its Subsidiaries, except as otherwise required by Law; or (B) authorize for issuance, issue, grant, sell, deliver, dispose of, pledge or otherwise encumber any equity securities of the Company, or any of its Subsidiaries except for shares of Company Common Stock upon the exercise of outstanding Company Options or upon the conversion of Company Preferred Stock into Company Common Stock;
(ii) except for the Reverse Split, split, combine or reclassify any shares of its capital stock; declare, set aside or pay any dividend or other distribution (whether in cash, stock or property or any combination thereof) in respect of its capital stock; or enter into any agreement with respect to voting of any shares of Company Capital Stock;
(iii) make or declare any dividend or distribution to the stockholders of the Company;
(iv) adopt a plan of complete or partial liquidation or dissolution, recapitalization or other reorganization;
(v) hire any new officers or, except in the ordinary course of business, any new employees or consultants or terminate, other than for cause, any officer or employee;
(vi) create, incur or assume any Indebtedness; assume, guarantee, endorse or otherwise become liable or responsible (whether directly, contingently or otherwise) for the obligations of any other Person; or make any loans, advances or capital contributions to, or investments in, any other Person;
(vii) subject any of its material property or assets to any Lien, other than any Permitted Lien;
(viii) incur any capital expenditure or commitment for capital expenditures;
(ix) (A) modify or terminate (excluding any expiration in accordance with its terms) any Contract of a type required to be listed on Section 4.12 of the Company Disclosure Schedule or providing for aggregate payments of more than $50,000, or any material insurance policy required to be listed on Section 4.17 of the Company Disclosure Schedule, outside of the ordinary course of business; (B) enter into any Contract outside of the ordinary course of business or (C) take or omit to take any action that would constitute a material violation of or material default under, or waive any material rights under, any Contract of a type required to be listed on Section 4.12 of the Company Disclosure Schedule;
| - 59 - |
(x) change the nature or scope of its business being carried on as of the date of this Agreement or commence any new business not being ancillary or incidental to such business or take any action to alter its organizational or management structure;
(xi) materially change its accounting methods, principles or practices, except insofar as may be required by a generally applicable change in GAAP;
(xii) institute or settle any Action;
(xiii) sell, assign, transfer, convey, lease, license, sublicense or otherwise dispose of (A) any Company Intellectual Property, or (B) outside of the ordinary course of business, any assets or properties of Company with a value, individually or in the aggregate, of $50,000;
(xiv) (A) adopt, enter into, terminate or amend any employment, severance, retention or change in control plan or Contract, any Company Benefit Plan or any collective bargaining agreement, (B) increase the compensation or fringe benefits of, or pay any bonus to, any director, officer, employee or consultant, (C) pay any benefit to any employee or consultant of the Company or any of its Subsidiaries except as required as of the date of this Agreement under any Company Benefit Plan, (D) grant any awards to any employee or consultant of the Company or any of its Subsidiaries under any bonus, incentive, performance or other compensation plan or arrangement or benefit plan, including the grant of equity or equity-based compensation, or the removal of existing restrictions in any benefit plans or agreements or awards made thereunder, or (E) take any action to fund or in any other way secure the payment of compensation or benefits to any employee or consultant of the Company or any of its Subsidiaries under any employee plan, agreement, Contract or arrangement or Company Benefit Plan, other than payment of premiums due or contributions owed in the ordinary course of business;
(xv) acquire by merger or consolidation, or merge or consolidate, with any corporation, partnership, association, joint venture or other business organization or division thereof, or acquire any business, assets or property, or make any investment in, any Person;
(xvi) make any material loans or material advances of money to any Person (other than the Company), except for advances to employees or officers of the Company for expenses incurred in the ordinary course of business;
(xvii) (A) make or change any material Tax election, change an annual accounting period, file any material amended Tax Return, enter into any closing agreement, settle or compromise any claim, notice, audit report or assessment in respect of Taxes or consent to any extension or waiver of the statute of limitations period applicable to any Tax claim or assessment, or take any other similar action relating to the filing of any Tax Return or the payment of any Tax or (B) except as required or permitted by GAAP, make any material change to any accounting principles, methods or practices;
| - 60 - |
(xviii) other than in the ordinary course of business, abandon, or fail to prosecute or maintain, any Company Owned Intellectual Property, or any Company Licensed Intellectual Property that the Company has the right to prosecute or maintain; or
(xix) enter into any agreement, or otherwise become obligated, to do any action prohibited under this Section 6.1(a).
(b) Prior to the Closing, each of the Company and Buyer shall exercise, consistent with and subject to the terms and conditions of this Agreement, control and supervision over their respective businesses.
(c) From the date of this Agreement through the Closing, the Company shall not, without the prior written consent of Buyer, discharge or cause to be discharged or forgiven, any of the Company’s Indebtedness, except for the payment of interest and principal as such amounts become due under the terms of such Indebtedness in the ordinary course of business (and without regard to the transactions contemplated by this Agreement), and shall not amend or terminate or cause to be amended or terminated any Contracts in respect to such Indebtedness, or seek any forgiveness of any such Indebtedness.
(d) The Company shall give prompt notice to Buyer upon becoming aware of the occurrence, or failure to occur, of any event, which occurrence or failure to occur would be reasonably likely to cause (a) (i) any representation or warranty of such party contained in this Agreement that is qualified as to materiality to be untrue or inaccurate in any respect or (ii) any other representation or warranty of such party contained in this Agreement to be untrue or inaccurate in any material respect, in each case, at any time from and after the date of this Agreement until the First Effective Time, or (b) any material failure of the Company or any of its Subsidiaries, as the case may be, or of any officer, director, employee or agent thereof, to comply with or satisfy any covenant, condition or agreement to be complied with or satisfied by it under this Agreement.
6.2 Inspection. Subject to applicable Law, confidentiality obligations and similar restrictions that may be applicable to information furnished to the Company by third parties that may be in the Company’s possession from time to time, and except for any information that is subject to attorney-client privilege or other privilege from disclosure, prior to the Closing, the Company shall afford to Buyer and its accountants, counsel and other representatives, upon reasonable notice, reasonable access, during normal business hours, in such manner as to not interfere with the normal operation of the Company to its properties, books, Contracts, commitments, Tax Returns, records and appropriate officers and employees of the Company, and shall furnish such representatives with such financial and operating data and other information concerning the affairs of the Company, in each case, as such representatives may reasonably request; provided, that, in each case, Buyer and the Company shall reasonably cooperate in seeking to find a way to allow disclosure of such documents (or portions thereof) or information without resulting in violating applicable Law or confidentiality obligations, or waiving such privileges, or, to the extent legally permissible, reasonably necessary and practicable, make appropriate substitute arrangements under circumstances in which the foregoing restrictions apply. All information obtained by Buyer, Merger Subs and their respective representatives shall be subject to the Confidentiality Agreement.
| - 61 - |
6.3 Information Statement.
(a) Promptly following the public filing of Buyer’s audited consolidated balance sheet and the related consolidated statements of operations and comprehensive income (loss) and stockholder’s equity (deficit) as of the end of and for the fiscal year ended December 31, 2023, the Company shall (i) deliver to each Company Stockholder that did not execute and deliver a Written Consent the notices and information required by the DGCL (including a copy of Section 262 of the DGCL), together with any other information, documents and notices required by the DGCL or any other applicable Laws or by the Company Charter, Company Bylaws or other organizational documents of the Company, and (ii) file, in accordance with the rules and regulations of the Exchange Act, including Regulation 14C and Schedule 14C thereunder, a preliminary information statement (the “Preliminary Information Statement,” and together with all notices and information described in the immediately preceding clause (i), the “Preliminary Stockholder Materials”).
(b) Promptly following, but in no event later than three (3) Business Days following the expiration of the 10 calendar day period as provided in Rule 14c-5 under the Exchange Act, the Company shall file, in accordance with the rules and regulations of the Exchange Act, including Regulation 14C and Schedule 14C thereunder, a definitive information statement (the “Information Statement,” and together with the Preliminary Stockholder Materials, the “Stockholder Materials”).
(c) The Company shall afford Buyer the opportunity to review and comment upon the Stockholder Materials and shall not file or deliver any Stockholder Materials until Buyer has provided its prior written consent as to the form and substance thereof. Buyer and its representatives shall provide any comments on such Stockholder Materials as promptly as reasonably practicable. The Company covenants and agrees to ensure that the Stockholder Materials comply in all material respects with the DGCL, the Securities Act, the Exchange Act, the rules and regulations promulgated by the SEC and other applicable Laws and do not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading.
(d) Each of Buyer and the Company shall furnish all information concerning it as may reasonably be requested by the other party in connection with such actions and the preparation of the Preliminary Information Statement and the Information Statement. Each of Buyer and the Company shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld or delayed) any response to comments of the SEC or its staff with respect to the Preliminary Information Statement, the Information Statement and any amendment filed in response thereto. If either Buyer or the Company becomes aware that any information contained in the Preliminary Information Statement or the Information Statement shall have become false or misleading in any material respect or that the Preliminary Information Statement or the Information Statement is required to be amended in order to comply with applicable Law, then (i) such party shall promptly inform the other and (ii) Buyer and the Company shall cooperate and mutually agree upon (such agreement not to be unreasonably withheld or delayed) an amendment or supplement to the Preliminary Information Statement or the Information Statement, as applicable. The Company shall use reasonable best efforts to cause the Preliminary Information Statement and the Information Statement, as so amended or supplemented, to be filed with the SEC and to be delivered to the Company Stockholders, pursuant to applicable Law. The Company shall provide Buyer with copies of any written comments, and shall inform Buyer of any oral comments, that the Company receives from the SEC or its staff with respect to the Preliminary Information Statement promptly after the receipt of such comments and shall give Buyer a reasonable opportunity to review and comment on any proposed written or oral responses to such comments prior to the Company responding to the SEC or its staff.
| - 62 - |
6.4 Director & Officer Tail Policy. The Company shall obtain prior to the Closing a prepaid, non-cancelable six-year “tail” policy containing terms not less favorable than the terms of the Company’s current directors’ and officers’ liability insurance coverage with respect to matters existing or occurring at or prior to the First Effective Time (the cost of which, to the extent not paid by the Company prior to the Closing, shall be an Excess Transaction Expense) (the “D&O Tail Policy”).
6.5 Exclusivity.
(a) The Company agrees that between the date of this Agreement and the earlier of the Closing and the termination of this Agreement, the Company shall not, and shall ensure that none of its Subsidiaries or any of their respective directors, managers, officers, employees, representatives, equityholders or Affiliates shall, (i) solicit, initiate, encourage or accept any proposal or offer that constitutes an Acquisition Proposal or (ii) participate in any discussions or negotiations regarding, furnish to any other Person any information with respect to, or otherwise facilitate, any proposal that constitutes an Acquisition Proposal. For purposes of this Agreement, “Acquisition Proposal” means any offer or proposal by a third party other than Buyer or any of its Affiliates for any of the following: (A) any direct or indirect acquisition or purchase of the capital stock or other equity or ownership interest of the Company or any of its Subsidiaries or all or any material portion of the assets of the Companies or any of its Subsidiaries, (B) any merger, joint venture, consolidation or other business combination relating to the Company or any of its Subsidiaries or (C) any recapitalization, liquidation, dissolution, share exchange or reorganization involving the Company or any of its Subsidiaries.
(b) The Company shall immediately notify any Person with which discussions or negotiations of the nature described in Section 6.5(a) were pending that the Company is terminating such discussions or negotiations. If the Company or any of its Subsidiaries receives any inquiry, proposal or offer of the nature described in Section 6.5(a), the Company shall, within one (1) Business Day after such receipt, notify Buyer of such inquiry, proposal or offer, including the identity of the other party and the material terms of such inquiry, proposal or offer.
6.6 Reverse Split. At or prior to the First Effective Time, the Company shall file an amendment to the Company Charter, in form and substance reasonably acceptable to Buyer, to effect the Reverse Split.
6.7 Security Holder Litigation. The Company shall promptly (and in any event within one (1) day) notify Buyer in writing after becoming aware of any Action commenced against the Company and/or its officers or directors relating to the Merger, the Reverse Split or the other transactions contemplated by this Agreement, and shall keep Buyer reasonably informed with respect to the status thereof. The Company shall give Buyer the right to (a) review and comment on all filings or responses to be made by the Company in connection with such Action, (b) participate in the defense (including discussions or negotiations regarding settlement or mooting of any such Action) of any such Action, and (c) consult on the settlement with respect to such Action with counsel of Buyer’s choice, and the Company shall accept any reasonable comments of Buyer. Notwithstanding anything else contained herein, the Company shall not settle or enter into any negotiations or settlement of any such Action or without the prior written consent of Buyer, including that, for the avoidance of doubt, the Company shall not enter into any settlement which does not include full release of Buyer and its Affiliates or which imposes an injunction or other equitable relief upon Buyer or any of its Affiliates (including, after the First Effective Time, the First Surviving Corporation or Final Surviving Corporation).
| - 63 - |
Article VII.
COVENANTS OF BUYER
7.1 Appendix 3B. Following execution of this Agreement by the parties hereto, Buyer will lodge an Appendix 3B with ASX to announce the proposed issue of Buyer Ordinary Shares pursuant to this Agreement.
7.2 Director & Officer Indemnification and Insurance.
(a) Buyer shall cause the Company for a period of not less than six (6) years from the First Effective Time (i) to maintain provisions in its certificate of incorporation, bylaws or other organizational documents concerning the indemnification and exoneration (including provisions relating to expense advancement) of the Company’s former and current officers, directors and employees that are no less favorable to those Persons than the provisions of the certificate of incorporation, bylaws or other organizational documents of the Company, in each case, as of the date of this Agreement, and (ii) not to amend, repeal or otherwise modify such provisions in any respect that would adversely affect the rights of those Persons thereunder, in each case, except as required by Law.
(b) The rights of indemnification and to receive advancement of expenses as provided by this Agreement shall not be deemed exclusive of any other rights to which any Person entitled to indemnification under this Section 7.1 (an “Indemnified Person”) may at any time be entitled. No right or remedy herein conferred by this Agreement is intended to be exclusive of any other right or remedy, and every other right and remedy shall be cumulative and in addition to every other right and remedy given hereunder or now or hereafter existing at Law or in equity or otherwise. The assertion of any right or remedy hereunder, or otherwise, shall not prevent the concurrent or subsequent assertion of any other right or remedy.
(c) Notwithstanding anything contained in this Agreement to the contrary, this Section 7.1 shall survive the consummation of the First Merger for a period of six (6) years after the First Effective Time and shall be binding, jointly and severally, on all successors and assigns of Buyer and the Final Surviving Corporation. In the event that Buyer or the Final Surviving Corporation or any of their respective successors or assigns consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or transfers or conveys all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that the successors and assigns of Buyer or the Final Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 7.1.
| - 64 - |
Article VIII.
JOINT COVENANTS
8.1 Support of Transaction. Without limiting any covenant contained in Article VI or Article VII, Buyer and the Company shall each, and Buyer shall cause its Subsidiaries to use commercially reasonable efforts to: (a) assemble, prepare and file any information (and, as needed, to supplement such information) as may be reasonably necessary to obtain as promptly as practicable all governmental and regulatory consents required to be obtained in connection with the transactions contemplated hereby, (b) obtain all material consents and approvals of third parties that any of Buyer, the Company or their respective Affiliates are required to obtain in order to consummate the First Merger, and (c) take such other action as may reasonably be necessary or as another party may reasonably request to satisfy the conditions of Article IX or otherwise to comply with this Agreement and to consummate the transactions contemplated hereby as soon as practicable (but in any event prior to the Outside Date).
8.2 Stockholder Approval. Within one (1) Business Day after the execution and delivery of this Agreement, the Company shall, in accordance with the DGCL, the Company Charter and the Company Bylaws, obtain and deliver to Buyer a true, correct and complete copy of an irrevocable written consent of holders of at least a majority of the issued and outstanding shares of Company Capital Stock to adopt this Agreement and approve the First Merger and the other transactions contemplated hereby, and (b) the amendment of the Company Charter to effect Reverse Split (the “Merger Consent”). Immediately following the execution and delivery of this Agreement, Buyer, as sole stockholder of Merger Subs, shall adopt this Agreement and approve each Merger and the related transactions contemplated hereby in accordance with the DGCL and each Merger Sub’s certificate of incorporation and bylaws.
8.3 Further Assurances. Each party hereto agrees that, from time to time after the Closing Date, it will execute and deliver, or cause its Affiliates to execute and deliver, such further instruments, and take (or cause its Affiliates to take) such other action, as may be reasonably necessary to carry out the purposes and intents of this Agreement.
8.4 Tax Matters.
(a) Buyer shall prepare and file or shall cause to be prepared and filed any Tax Returns of the Company and its Subsidiaries for any Pre-Closing Tax Period or any Straddle Period required to be filed after the Closing Date, and if Taxes are due with respect to such a Tax Return for which an indemnification claim may be made under this Agreement, Buyer shall provide the Company Stockholder Representative with a draft of such Tax Return (and such additional information regarding such Tax Return as may reasonably be requested by the Company Stockholder Representative) for review and comment at least 45 days prior to the filing of such Tax Return in the case of income Tax Returns, and in such period of time prior to filing as Buyer shall reasonably determine to be practicable in the case of other Tax Returns. Buyer shall consider in good faith any comments reasonably requested by the Company Stockholder Representative in writing and received by Buyer prior to the filing of such Tax Return.
| - 65 - |
(b) Buyer and the Company Stockholders agree that if the Company or any of its Subsidiaries is permitted but not required under applicable foreign, state or local Tax Laws to treat the Closing Date as the last day of a taxable period, Buyer and the Company Stockholders shall treat such day as the last day of a taxable period. In the case of any Straddle Period, the portion of any Tax that is allocable to the taxable period that is deemed to end on the Closing Date will be: (i) in the case of Property Taxes, deemed to be the amount of such Taxes for the entire Straddle Period multiplied by a fraction, the numerator of which is the number of calendar days of such Straddle Period in the Pre-Closing Tax Period and the denominator of which is the number of calendar days in the entire Straddle Period, and (ii) in the case of all other Taxes, determined as though the taxable year of the Company terminated on the Closing Date.
(c) Buyer and the Company Stockholders and their respective Affiliates shall cooperate in the preparation of all Tax Returns and the conduct of all Tax audits or other administrative or judicial proceedings relating to the determination of any Tax for any Tax periods for which one party could reasonably require the assistance of the other party in obtaining any necessary information.
(d) Without the Company Stockholder Representative’s prior written consent (which shall not be unreasonably withheld, conditioned or delayed), Buyer and its respective Affiliates will not, and will not cause the Company to, take any of the following actions with respect to Taxes or Tax Returns of the Company or its Subsidiaries, if such action could reasonably be expected to give rise to an indemnification claim under this Agreement: (i) amend or otherwise modify any Tax Return relating to a Pre-Closing Tax Period or (ii) (A) initiate discussions or examinations or contact with a Governmental Authority or (B) make any voluntary disclosures with respect to or relating to Taxes.
(e) Buyer, on one hand, and Company Stockholders, on the other, shall each be responsible for the payment of 50% of any Transfer Taxes. Buyer and Company Stockholder Representative will cooperate in the filing of all necessary Tax Returns and other documentation with respect to all such Transfer Taxes.
(f) The Merger is intended to qualify as a “reorganization” within the meaning of Section 368(a) of the Code and to not be subject to Section 367(a)(1) of the Code (the “Intended Tax Treatment”). The parties adopt this Agreement as a “plan of reorganization” for purposes of Sections 354 and 361 of the Code and within the meaning of Section 1.368-2(g) of the Treasury Regulations. None of the parties shall (and each of the parties shall cause their respective Subsidiaries not to) knowingly take (or fail to take) any action that could reasonably be expected to cause the Merger to fail to qualify for the Intended Tax Treatment. The parties shall treat the Merger for all Tax purposes in a manner consistent with the Intended Tax Treatment and shall not file any U.S. federal, state or local Tax Return in a manner that is inconsistent with the Intended Tax Treatment unless otherwise required by a determination within the meaning of Section 1313(a) of the Code. If the Company requests a Tax opinion from its tax advisors regarding the Intended Tax Treatment, then Buyer will cooperate to provide customary Tax representation letters reasonably requested by such tax advisors, it being understood and agreed that this section does not require that a Tax opinion reach any particular legal conclusion regarding the Tax treatment of the Merger and such an opinion is not a condition to Closing.
| - 66 - |
8.5 Private Placement. Each of the Company and Buyer shall take all reasonably necessary action on its part such that the issuance of Buyer Ordinary Shares pursuant to this Agreement constitutes a transaction exempt from registration under the Securities Act under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder.
8.6 CVR Agreement. Each of the Company and Buyer shall take all reasonably necessary action on its part to ensure that a duly qualified Rights Agent executes and delivers the CVR Agreement at or prior to the Closing.
Article IX.
CONDITIONS TO OBLIGATIONS
9.1 Conditions to the Obligations of Buyer and Merger Subs. The obligations of Buyer and Merger Subs to consummate, or cause to be consummated, the First Merger are subject to the satisfaction of the following additional conditions, any one or more of which may be waived in writing by Buyer and Merger Subs:
(a) (i) The representations and warranties of the Company in this Agreement (other than the Fundamental Representations of the Company) shall be true and correct (without giving regard to any qualifications or limitations as to “materiality” or “Material Adverse Effect”, and words of similar import set forth therein, other than with respect to Section 4.23(a)) in all respects as of the date of this Agreement and at and as of the Closing with the same effect as though made at and as of such time, except where the failure to be true and correct would not reasonably be expected to have a Material Adverse Effect and (b) the Fundamental Representations of the Company will be true and correct in all respects (other than Fundamental Representations of the Company set forth in Section 4.6 or Section 4.24, which will be true and correct in all but de minimis respects) as of the date of this Agreement and at and as of the Closing with the same effect as though made at and as of such time; provided, however, that representations and warranties that are made as of a particular date or period will be true and correct (in the manner set forth above) only as of such date or period.
(b) Each of the covenants of the Company to be performed at or prior to the Closing shall have been performed in all material respects.
(c) The Company shall have delivered to Buyer a certificate signed by an officer of the Company, dated as of the Closing Date, certifying that the conditions specified in Section 9.1(a), Section 9.1(b) and Section 9.1(d) have been fulfilled (the “Closing Certificate”).
(d) Since the date of this Agreement, there shall have not have occurred a Material Adverse Effect on the Company.
(e) No Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, executive order, decree, injunction or other order (whether temporary, preliminary or permanent) which is in effect and which prohibits, restrains, enjoins or makes illegal the consummation of the Merger, and there shall not be any threatened, instituted or pending action by a Governmental Authority seeking to prohibit, restrain or enjoin the consummation of the Merger or other transactions under this Agreement.
| - 67 - |
(f) The Merger Consent shall have been validly obtained.
(g) The Reverse Split shall have been effected.
(h) The Company shall have used reasonable best efforts to obtain completed and signed Investor Questionnaires from each Company Stockholder and shall have delivered all such Investor Questionnaires to Buyer; Buyer shall have no reason to believe that the statements set forth in the Investor Questionnaires are not true; and Buyer shall be reasonably satisfied that the issuance of the Buyer Ordinary Shares pursuant to this Agreement is exempt from the registration requirements of the Securities Act.
(i) There shall be no more than 27 Company Stockholders that are not Accredited Investors.
(j) The aggregate number of Dissenting Shares, together with the shares of Company Capital Stock eligible to become Dissenting Shares, shall not exceed two percent (2%) of the number of outstanding shares of Company Capital Stock as of the First Effective Time (calculated after giving effect to the conversion into shares of Company Common Stock of all outstanding shares of Company Preferred Stock).
(k) Buyer shall have received copies of Written Consents evidencing the receipt of the Merger Consent.
(l) Buyer shall have received the items contemplated to be delivered by the Company in accordance with Section 3.4.
(m) The aggregate amount of the Closing Indebtedness Amount, together with all Excess Transaction Expenses, to be paid by Buyer pursuant to Section 3.5 shall not exceed $500,000.
(n) The Company shall have delivered to Buyer a signed certification that the shares of Company Capital Stock are not United States real property interests as defined in Section 897(c) of the Code, together with a notice to the IRS (which shall be filed by Buyer with the IRS following the Closing), in accordance with the Treasury Regulations under Sections 897 and 1445 of the Code.
(o) Buyer shall have received duly executed counterparts to the CVR Agreement from the other parties thereto.
(p) Buyer shall have received duly executed copies of each Option Acknowledgment Agreement executed by all holders of outstanding Company Options.
(q) Buyer shall have received such other certificates and instruments (including certificates of good standing of the Company and its Subsidiaries in their respective jurisdictions of organization) as it shall reasonably request in connection with the Closing.
| - 68 - |
9.2 Conditions to the Obligations of the Company. The obligations of the Company to consummate, or cause to be consummated, the First Merger are subject to the satisfaction of the following additional conditions, any one or more of which may be waived in writing by the Company:
(a) (i) The representations and warranties of Buyer and Merger Subs in this Agreement (other than the Fundamental Representations of Buyer) shall be true and correct (without giving regard to any qualifications or limitations as to “materiality” or “Material Adverse Effect”, and words of similar import set forth therein) in all respects as of the date of this Agreement and at and as of the Closing with the same effect as though made at and as of such time, except where the failure to be true and correct would not reasonably be expected to have a Material Adverse Effect on Buyer and (ii) the Fundamental Representations of Buyer will be true and correct in all respects as of the date of this Agreement and at and as of the Closing with the same effect as though made at and as of such time; provided, however, that representations and warranties that are made as of a particular date or period will be true and correct (in the manner set forth above) only as of such date or period.
(b) Each of the covenants of Buyer and Merger Subs to be performed at or prior to the Closing shall have been performed in all material respects.
(c) Buyer shall have delivered to the Company a certificate signed by an officer of Buyer, dated as of the Closing Date, certifying that the conditions specified in Section 9.2(a) and Section 9.2(b) have been fulfilled (the “Buyer Closing Certificate”).
(d) Buyer shall have delivered a duly executed counterpart to the CVR Agreement to the other parties thereto.
(e) No Governmental Authority of competent jurisdiction shall have enacted, issued, promulgated, enforced or entered any statute, rule, regulation, executive order, decree, injunction or other order (whether temporary, preliminary or permanent) which is in effect and which prohibits, restrains, enjoins or makes illegal the consummation of the Merger, and there shall not be any threatened, instituted or pending action by a Governmental Authority seeking to prohibit, restrain or enjoin the consummation of the Merger or other transactions under this Agreement.
9.3 Waiver of Conditions; Frustration of Conditions. All conditions to the Closing shall be deemed to have been satisfied or waived following the First Effective Time. None of the Company, Buyer or Merger Subs may rely on the failure of any condition set forth in this Article IX to be satisfied if such failure was caused by the failure of the Company, on the one hand, or Buyer or Merger Subs, on the other hand, respectively, to (a) use reasonable best efforts to consummate the First Merger and the other transactions contemplated hereby and (b) otherwise comply with its obligations under this Agreement.
Article X.
TERMINATION/EFFECTIVENESS
10.1 Termination. This Agreement may be terminated and the transactions contemplated hereby abandoned at any time prior to the Closing:
(a) by duly authorized mutual written consent of Buyer and the Company;
| - 69 - |
(b) by written notice to the Company from Buyer if:
(i) there is any breach of any representation, warranty, covenant or agreement on the part of the Company set forth in this Agreement, such that the conditions specified in Section 9.1(a) or Section 9.1(b) would not be satisfied at the Closing, except that, if such breach is curable by the Company through the exercise of commercially reasonable efforts, then, for a period of up to thirty (30) days after receipt by the Company of notice from Buyer of such breach, but only as long as the Company continues to use commercially reasonable efforts to cure such breach (the “Company Cure Period”), such termination shall not be effective and the Outside Date shall be automatically extended until the end of the Company Cure Period, and such termination shall become effective only if such breach is not cured within the Company Cure Period; or
(ii) the Closing has not occurred on or before August 7, 2024 (subject to extension as set forth in this Article X, the “Outside Date”), unless Buyer’s or Merger Subs’ breach is the primary reason for the Closing not occurring on or before such date; or
(iii) the consummation of any of the transactions contemplated hereby is permanently enjoined, prohibited or otherwise restrained by the terms of a final, non-appealable order or judgment of a court of competent jurisdiction; or
(iv) if the Merger Consent shall not have been obtained prior to 5:00 p.m., New York time, on the first (1st) Business Day immediately following the date of this Agreement.
(c) by written notice to Buyer from the Company if:
(i) there is any breach of any representation, warranty, covenant or agreement on the part of Buyer or Merger Subs set forth in this Agreement, such that the conditions specified in Section 9.2(a) or Section 9.2(b) would not be satisfied at the Closing, except that, if any such breach is curable by Buyer through the exercise of commercially reasonable efforts, then, for a period of up to thirty (30) days after receipt by Buyer of notice from the Company of such breach, but only as long as Buyer continues to exercise such commercially reasonable efforts to cure such breach (the “Buyer Cure Period”), such termination shall not be effective and the Outside Date shall automatically be extended until the end of the Buyer Cure Period, and such termination shall become effective only if such breach is not cured within the Buyer Cure Period;
(ii) the Closing has not occurred on or before the Outside Date, unless the Company’s breach is the primary reason for the Closing not occurring on or before such date; or
(iii) the consummation of any of the transactions contemplated hereby is permanently enjoined, prohibited or otherwise restrained by the terms of a final, non-appealable order or judgment of a court of competent jurisdiction.
10.2 Effect of Termination. Except as otherwise set forth in this Section 10.2, in the event of the termination of this Agreement pursuant to Section 10.1, this Agreement shall forthwith become void and have no effect, without any liability on the part of any party hereto or its respective Affiliates, officers, directors, employees or stockholders, other than liability of the Company, Buyer or Merger Subs, as the case may be, for any intentional and willful breach of this Agreement occurring prior to such termination; provided, however, that any such termination shall not relieve any party from liability for damages for any willful breach on the part of Buyer or the Company, as the case may be, including such party’s obligation to close if it was otherwise obligated to do so under the terms of this Agreement. The provisions of this Section 10.2, Article XI and Article XII, and the Confidentiality Agreement shall survive any termination of this Agreement. In the event of any termination of this Agreement, the $2,000,000 Pre-Closing Collaboration and Option Fee (as defined in the Term Sheet) paid by Telix Pharmaceuticals (US) Inc. to the Company shall be automatically deemed an equity investment in the Company made by Telix Pharmaceuticals (US) Inc., and the Company shall promptly (and any event within five Business Days) issue to Telix Pharmaceuticals (US) Inc. 298,507 duly authorized, validly issued, fully paid, nonassessable shares of Company Common Stock, free of preemptive rights, in respect thereof.
| - 70 - |
Article XI.
INDEMNIFICATION
11.1 Survival of Representations, Warranties and Covenants. Each representation warranty, covenant and obligation contained herein and any certificate related to any such representation, warranty, covenant or obligation will survive the Closing and continue in full force and effect for twelve (12) months after the Closing Date (the “Survival Expiration Date”); provided, however, that (a) any covenant contained in this Agreement that, by its terms, provides for performance following the Closing shall survive for the period provided in such covenant, if any, or until such covenant is performed and (b) each Fundamental Representation and the representations and warranties of the Company in Section 4.20 shall survive for the later of a period of six (6) years after the Closing Date or the expiration of the applicable statute of limitations. If any Buyer Indemnified Party delivers to the Company Stockholder Representative, before expiration of a representation, warranty, covenant or agreement, a written notice asserting a claim for indemnification in accordance with this Article XI based upon a breach of such representation, warranty, covenant or agreement, then the applicable representation, warranty, covenant or agreement shall survive until, but only for purposes of, the resolution of the matter covered by such notice.
11.2 Indemnification.
(a) Subject to Section 11.4, from and after the Closing, the Pre-Reverse Split Company Stockholders, severally (and not jointly), shall defend, indemnify and hold harmless Buyer and its Affiliates (including, after the Closing, the Company, the Final Surviving Corporation and the Subsidiaries) and its and their respective officers, directors, employees, shareholders, agents and representatives (collectively, the “Buyer Indemnified Parties”) and will compensate and reimburse the Buyer Indemnified Parties for, any and all Losses incurred or suffered by any Buyer Indemnified Party (regardless of whether such Losses relate to any Third-Party Claim) resulting from, relating to or constituting:
(i) any breach of any representation or warranty the Company has made in Article IV of this Agreement or in the Closing Certificate;
| - 71 - |
(ii) any breach by the Company of any covenant or agreement of the Company in this Agreement that, by its terms, provides for performance by the Company prior to the Closing;
(iii) any Closing Indebtedness and any Closing Transaction Expenses, in each case to the extent not included in the calculation of the Adjustment Amount;
(iv) any portion of the True-up Amount in excess of the Holdback Amount;
(v) any inaccuracy in the Closing Date Allocation Schedule;
(vi) any Pre-Closing Taxes;
(vii) any claim by a stockholder or former stockholder of the Company of any of its Subsidiaries (including any stockholder whose ceases to own shares of the Company as a result of the Reverse Split) or holder of Company Options or any Person who purports to be a current or former stockholder of the Company or any of its Subsidiaries, holder of Company Options and/or other current of former equityholder of the Company or any of its Subsidiaries, seeking to assert, or based upon: (A) the ownership or rights to ownership of any shares of stock or other equity of the Company or any of its Subsidiaries; (B) any rights of a stockholder or holder of other equity of the Company or any of its Subsidiaries (other than the right to receive the consideration pursuant to Article III), including any option, preemptive rights or rights to notice or to vote; (C) any rights of such Person under the Company Charter, Company Bylaws or other organizational Documents of the Company or any of its Subsidiaries; (D) any claim that his, her or its shares were wrongfully repurchased by the Company or any its Subsidiaries or otherwise related to the Reverse Split; (E) any claim for appraisal or dissenters rights, including any payment in respect of Dissenting Shares in excess of the amount of payments otherwise payable to the stockholder seeking such rights under this Agreement, or (F) any breach of fiduciary duty by any officer or director of the Company at or prior to the Closing;
(viii) any fraud on the part of the Company in connection with the transactions contemplated by this Agreement; and
(ix) any claim for indemnification, exculpation and/or the advancement or reimbursement of expenses by any Person who was an officer or director of the Company at any time prior to the Closing (solely to the extent the Losses arising therefrom exceed amounts actually recovered (net of the costs and expenses of collection) under the D&O Tail Policy.
(b) The amount of indemnification to which a Buyer Indemnified Party shall be entitled under this Article XI shall be determined: (i) by the written agreement between the Buyer Indemnified Party and the Indemnitor; (ii) by a final judgment or decree of any court of competent jurisdiction; or (iii) by any other means to which the Buyer Indemnified Party and the Indemnitor shall agree. The judgment or decree of a court shall be deemed final when the time for appeal, if any, shall have expired and no appeal shall have been taken or when all appeals taken shall have been finally determined.
| - 72 - |
11.3 Indemnification Claim Procedures.
(a) If any Action is commenced or threatened by a third party that may give rise to a claim for indemnification (a “Third-Party Claim”) by any Buyer Indemnified Party, then such Buyer Indemnified Party shall promptly (i) notify the Indemnitor and (ii) deliver to the Indemnitor a written notice (A) describing in reasonable detail the nature of the Action, (B) including a copy of all papers served with respect to such Action, (C) including the Buyer Indemnified Party’s good faith estimate of the amount of Losses that may arise from such Action, and (D) describing in reasonable detail the basis for the Buyer Indemnified Party’s request for indemnification under this Agreement. Failure to notify the Indemnitor in accordance with this Section 11.3(a) will not relieve the Indemnitor of any liability that it may have to the Buyer Indemnified Party, except to the extent the defense of such Action is prejudiced by the Buyer Indemnified Party’s failure to give such notice.
(b) An Indemnitor may elect at any time to assume and thereafter conduct the defense of any Action subject to any such Third-Party Claim with counsel of the Indemnitor’s choice and each Buyer Indemnified Party shall cooperate in all respects with the conduct of such defense by the Indemnitor (including the making of any related claims, counterclaim or cross complaint against any Person in connection with the Action) and the settlement of such Action by the Indemnitor; provided, that (i) the Company Stockholder Representative may only assume control of such defense if (A) the maximum amount of Losses related to such Third-Party Claim, taken together with the estimated costs of defense thereof and the claimed amount of indemnification with respect to any unresolved claims for indemnification then pending, is less than or equal to $3,310,000, and (B) it acknowledges in writing to Buyer on behalf of all of the Pre-Reverse Split Company Stockholders that any damages, fines, costs or other liabilities that may be assessed against the Buyer Indemnified Party in connection with such Third-Party Claim constitute Losses for which the Buyer Indemnified Party shall be indemnified pursuant to this Article XI, and (ii) the Company Stockholder Representative may not assume control of (but may participate in, at its sole cost and expense) the defense of any Third-Party Claim involving Taxes, any Governmental Authority or criminal liability or in which equitable relief is sought against the Buyer Indemnified Party or its Affiliates; provided, further that the Indemnitor will not approve of the entry of any judgment or enter into any settlement or compromise with respect to such Action without the Buyer Indemnified Party’s prior written approval (which must not be unreasonably withheld or delayed), unless the terms of such settlement provide for a complete release of the claims that are the subject of such Action in favor of the Buyer Indemnified Party. If the Buyer Indemnified Party gives an Indemnitor notice of a Third-Party Claim and either (A) the Indemnitor does not, within sixty (60) days after such notice is given, (1) give notice to the Buyer Indemnified Party of its election to assume the defense of the Action or Actions subject to such Third-Party Claim and (2) thereafter promptly assume such defense or (B) the Indemnitor does not otherwise have the right to assume defense of such Third-Party Claim under the terms of this Article XI, then the Buyer Indemnified Party may conduct the defense of such Action; provided, however, that the Buyer Indemnified Party will not agree to the entry of any judgment or enter into any settlement or compromise with respect to such Action or Actions without the prior written consent of the Indemnitor (which consent shall not be unreasonably withheld).
(c) In circumstances where the Indemnitor assumes the defense of a Third-Party Claim in accordance with Section 11.3(b), the Buyer Indemnified Party shall be entitled to participate in the defense of such Third-Party Claim and to employ separate counsel of its choice for such purpose, in which case the fees and expenses of such separate counsel shall be borne by such Buyer Indemnified Party.
| - 73 - |
(d) If any Buyer Indemnified Party becomes aware of any circumstances that may give rise to claim for indemnification for any matter not involving a Third-Party Claim, then such Buyer Indemnified Party shall promptly (i) notify the Indemnitor and (ii) deliver to the Indemnitor a written notice (A) describing in reasonable detail the nature of the circumstances giving rise to such claim, (B) including the Buyer Indemnified Party’s good faith estimate of the amount of Losses that may arise from such circumstances, and (C) describing in reasonable detail the basis for the Buyer Indemnified Party’s request for indemnification under this Agreement. Failure to notify the Indemnitor in accordance with this Section 11.3(d) will not relieve the Indemnitor of any liability that it may have to the Buyer Indemnified Party, except to the extent the defense of such claim is prejudiced by the Buyer Indemnified Party’s failure to give such notice. If the Indemnitor disputes its indemnity obligations for any Losses with respect to any such claim, the parties shall proceed in good faith to negotiate a resolution of such dispute and, if not resolved through negotiations, such dispute shall be resolved by litigation in an appropriate court of jurisdiction determined pursuant to Section 12.13.
(e) At the reasonable request of the Indemnitor, each Buyer Indemnified Party shall grant the Indemnitor and its representatives all reasonable access to the books, records, employees and properties of such Buyer Indemnified Party to the extent reasonably related to the matters to which the applicable claim for indemnification relates. All such access shall be granted during normal business hours and shall be granted under the conditions which shall not unreasonably interfere with the business and operations of such Buyer Indemnified Party.
11.4 Limitations on Indemnification Liability. Notwithstanding any provision of this Agreement to the contrary, any claims a Buyer Indemnified Party makes under this Article XI will be limited as follows:
(a) Indemnification Cap. With respect to claims for indemnification under Section 11.2(a), except in cases of fraud, such claims shall be satisfied solely pursuant to Section 11.5.
(b) Claims Basket. The Buyer Indemnified Parties shall not be entitled to indemnification pursuant to Section 11.2(a)(i) (except for claims based on fraud, intentional or knowing misrepresentation or willful breach, and except for claims for breaches of Fundamental Representations) unless and until the aggregate amount of all Losses incurred by the Buyer Indemnified Parties for which the Buyer Indemnified Parties are entitled to indemnification pursuant to this Article XI exceeds a dollar amount equal to the product of (i) three quarters of one percent (0.75%) multiplied by (ii) the Base Purchase Price (the “Basket Amount”), and the Buyer Indemnified Parties shall only be entitled to indemnification for such Losses to the extent such Losses exceed the Basket Amount.
(c) Losses Net of Insurance Proceeds and Other Third-Party Recoveries. All Losses for which any Buyer Indemnified Party would otherwise be entitled to indemnification under this Article XI shall be reduced by the amount of insurance proceeds any Buyer Indemnified Party actually received in respect of any Losses incurred by such Buyer Indemnified Party (net of all costs of collection and increases in insurance premiums). In the event that any insurance or other recovery is made by any Buyer Indemnified Party with respect to any Loss for which such Buyer Indemnified Party has been indemnified hereunder, then a refund equal to the aggregate amount of the insurance or other recovery shall be made promptly by such Buyer Indemnified Party to the Rights Agent for distribution to the Pre-Reverse Split Company Stockholders to the extent necessary to prevent duplication of recovery by the Buyer Indemnified Parties.
| - 74 - |
(d) Assignment of Claims. If any Buyer Indemnified Party receives any indemnification payment pursuant to this Article XI, at the election of the Indemnitor, such Buyer Indemnified Party shall assign to the Indemnitor all of its claims for recovery against third Persons as to such Losses, whether by insurance coverage, contribution claims, subrogation or otherwise.
(e) Certain Other Damages. Notwithstanding anything to the contrary contained herein, with respect to indemnification pursuant to Section 11.2 (other than claims based on fraud, intentional or knowing misrepresentation or willful breach), no Losses shall be recoverable under this Article XI that constitute punitive, exemplary or special damages, unless such Losses are required to be paid to a third party pursuant to a Third-Party Claim for which the Buyer Indemnified Parties were entitled to indemnification pursuant to this Article XI and such claim for indemnification was actually made.
(f) No Duplicate Claims. In the event a Buyer Indemnified Party recovers Losses in respect of a claim for indemnification, no other Buyer Indemnified Party may recover the same Losses in respect of a claim for indemnification under this Agreement.
(g) Materiality Qualifications. Notwithstanding anything to the contrary in this Agreement, for purposes of determining (i) whether there has been a breach of any representation or warranty set forth in Article IV or the Closing Certificate and (ii) the amount of Losses for which any Buyer Indemnified Party may be entitled to indemnification under this Article XI, each such representation or warranty shall be deemed to have been made without any qualifications or limitations as to materiality (including any qualifications or limitations made by reference to a Material Adverse Effect).
11.5 Offset. Any amounts owed or claimed in good faith to be owed by any Pre-Reverse Split Company Stockholder to any Buyer Indemnified Party pursuant to this Article XI shall be automatically offset or set off against any amount that is or may become payable to the Pre-Reverse Split Company Stockholders pursuant to the CVR Agreement. For the avoidance of doubt, any claims pursuant to this Article XI (including claims pursuant to which Buyer claims the right of offset or set off pursuant to this Section 11.5), shall be finally resolved in accordance with the terms of this Article XI.
11.6 Indemnification Sole and Exclusive Remedy. Except with respect to claims based on fraud or claims for specific performance of covenants, following the Closing, indemnification pursuant to this Article XI shall be the sole and exclusive remedy of the parties and any parties claiming by or through any party (including the Buyer Indemnified Parties) related to or arising from any breach of any representation, warranty, covenant or agreement contained in, or otherwise pursuant to, this Agreement and none of Buyer, Merger Subs, the Company, the Company Stockholder Representative or any Pre-Reverse Split Company Stockholder or Company Stockholder shall have any other rights or remedies in connection with any breach of this Agreement or any other liability arising out of the negotiation, entry into or consummation of the transactions contemplated by this Agreement, whether based on contract, tort, strict liability, other Laws or otherwise; provided that no provision of this sentence shall operate as a release of any Pre-Reverse Split Company Stockholder or Company Stockholder from any claim against or liability of such Pre-Reverse Split Company Stockholder or Company Stockholder under any Contract delivered by such Pre-Reverse Split Company Stockholder or Company Stockholder to Buyer or any Merger Sub in connection with this Agreement or the transactions contemplated hereby.
| - 75 - |
11.7 Tax Treatment. All amounts paid with respect to claims for indemnification under Article XI of this Agreement shall be treated by the parties hereto for all Tax purposes as adjustments to the Merger Consideration to the greatest extent permitted by applicable Law, and shall be reported as such by the parties hereto on their Tax Returns, as applicable.
Article XII.
MISCELLANEOUS
12.1 Waiver. Any party to this Agreement may, at any time prior to the Closing, by action taken by its Board of Directors, or officers thereunto duly authorized, waive any of the terms or conditions of this Agreement or (without limiting Section 12.10) agree to an amendment or modification to this Agreement by an agreement in writing executed in the same manner (but not necessarily by the same Persons) as this Agreement. No waiver by any of the parties hereto of any default, misrepresentation or breach of representation, warranty, covenant or other agreement hereunder, whether intentional or not, shall be deemed to extend to any prior or subsequent default, misrepresentation or breach or affect in any way any rights arising by virtue of any prior or subsequent such occurrence. No waiver by any of the parties of any of the provisions hereof shall be effective unless explicitly set forth in writing and executed by the party sought to be charged with such waiver.
12.2 Notices. All notices and other communications among the parties shall be in writing and shall be deemed to have been duly given (i) when delivered in person, (ii) when delivered after posting in the United States mail having been sent registered or certified mail return receipt requested, postage prepaid, (iii) when delivered by FedEx or other nationally recognized overnight delivery service, or (iv) when delivered by email, with affirmative confirmation of delivery (i.e., an electronic record of the sender that the email was sent to the intended recipient thereof without an “error” or similar message that such email was not received by such intended recipient), addressed as follows:
| (a) | If to Buyer, Merger Subs or the Final Surviving Corporation, to: |
Telix Pharmaceuticals Limited
55 Flemington Road
North Melbourne, Victoria, 3051, Australia
| Attention: | Lena Moran-Adams | |
| Email: | [●] |
| - 76 - |
with copies (which shall not constitute notice) to:
Wilmer
Cutler Pickering Hale and Dorr LLP
7 World Trade Center
250 Greenwich Street
New York, New York 10007
| Attention: | Christopher D. Barnstable-Brown | |
| Jason L. Kropp | ||
| Craig Hilts | ||
| Email: | Chris.Barnstable-Brown@wilmerhale.com | |
| Jason.Kropp@wilmerhale.com | ||
| Craig.Hilts@wilmerhale.com |
| (b) | If to the Company, prior to the Closing, to: |
QSAM Biosciences, Inc.
Attn: Christopher Nelson, General Counsel
9442 Capital of Texas Hwy N, Plaza 1, Suite 500
Austin, TX 78759
Email: [●]
with copies (which shall not constitute notice) to:
Dickinson Wright PLLC
350 East Las Olas Blvd
Suite 1750
Ft. Lauderdale FL 33301
Attention: Joel Mayersohn
Email: jmayersohn@dickinson-wright.com
| (c) | If to the Company Stockholder Representative, to: |
David H. Clarke
[●]
Email: [●]
with a copy (which shall not constitute notice) to:
Christopher Nelson
[●]
Email: [●]
or to such other address or addresses as the parties may from time to time designate in writing.
12.3 Assignment. No party hereto shall assign this Agreement or any part hereof without the prior written consent of the other parties, except that Buyer or the Merger Subs may transfer or assign their respective rights and obligations under this Agreement, in whole or from time to time in part, to one (1) or more of their Affiliates or any acquiror of all or substantially all of Buyer’s business or assets; provided that, in the case of an assignment by Buyer to any of its Affiliates, such assignment shall not relieve Buyer of any of its obligations hereunder. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns.
| - 77 - |
12.4 Rights of Third Parties. Nothing expressed or implied in this Agreement is intended or shall be construed to confer upon or give any Person, other than the parties hereto, any right or remedies under or by reason of this Agreement; provided, however, that in the event the Closing occurs, the Indemnified Persons (and their successors, heirs and representatives) are intended third-party beneficiaries of, and may enforce, Section 7.1.
12.5 Expenses. Except as otherwise specified herein, each party hereto, other than the Company Stockholder Representative (whose expenses shall be paid out of the Company Stockholder Representative Expense Fund pursuant to Section 3.9(c)), shall bear its own expenses incurred in connection with this Agreement and the transactions contemplated hereby whether or not such transactions shall be consummated, including all fees of its legal counsel, financial advisers and accountants; provided, however, that the amounts set forth on Section 1.1(a) of the Company Disclosure Schedule which are expressly contemplated by this Agreement and Section 1.1(a) of the Company Disclosure Schedule to be paid or assumed by Buyer will be borne by Buyer.
12.6 Governing Law. This Agreement, and all claims or causes of action based upon, arising out of, or related to this Agreement or the transactions contemplated hereby, shall be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to principles or rules of conflict of laws to the extent such principles or rules would require or permit the application of Laws of another jurisdiction; provided, that the Laws of Victoria and the Commonwealth of Australia shall govern the issuance of Buyer Ordinary Shares pursuant to this Agreement.
12.7 Captions; Counterparts. The captions in this Agreement are for convenience only and shall not be considered a part of or affect the construction or interpretation of any provision of this Agreement. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts and any other document required to be executed and delivered hereunder may be delivered via facsimile, electronic mail (including .pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000 (e.g., www.docusign.com)) or other transmission method and any counterpart or such document so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
12.8 Schedules and Annexes. The Schedules and Annexes referenced herein, including the Company Disclosure Schedule are a part of this Agreement as if fully set forth herein. All references herein to the Schedules and Annexes, including the Company Disclosure Schedule, shall be deemed references to such parts of this Agreement unless the context shall otherwise require. The Company Disclosure Schedule shall be arranged in sections and paragraphs corresponding to the numbered and lettered sections and paragraphs contained in Article IV. Any disclosure made by a party in the Company Disclosure Schedules with reference to any section or schedule of this Agreement shall be deemed to be a disclosure with respect to all other sections or schedules to which the relevance of such disclosure is reasonably apparent based on a reading of the disclosure.
| - 78 - |
12.9 Entire Agreement. This Agreement (together with the Company Disclosure Schedules and Annexes to this Agreement) and that certain Confidential Disclosure Agreement, dated as of June 8, 2023, between Telix International Pty Ltd. and the Company (the “Confidentiality Agreement”), constitute the entire agreement among the parties relating to the transactions contemplated hereby and supersede any other agreements, whether written or oral, that may have been made or entered into by or among any of the parties hereto or any of their respective Subsidiaries relating to the transactions contemplated hereby, including the Term Sheet. No representations, warranties, covenants, understandings or agreements, oral or otherwise, relating to the transactions contemplated by this Agreement exist between the parties, except as expressly set forth in this Agreement and the Confidentiality Agreement.
12.10 Amendments. This Agreement may be amended or modified in whole or in part, only by a duly authorized agreement in writing executed in the same manner as this Agreement and which makes reference to this Agreement. The approval of this Agreement by the stockholders of the Company shall not restrict the ability of the Board of Directors of the Company to terminate this Agreement in accordance with Section 10.1 or to cause the Company to enter into an amendment to this Agreement pursuant to this Section 12.10 to the extent permitted under Section 251(d) of the DGCL.
12.11 Publicity. The Company and Buyer agree that, from the date hereof through the Closing Date, the Company shall not make any public release or announcement concerning the transactions contemplated hereby shall be issued or made by or on behalf of any party without the prior consent of the other parties, except that the Company may make any disclosures or announcements necessary to comply with applicable Law or securities exchange regulations, including, if applicable, filing a copy of this Agreement with the SEC or similar Governmental Authority. The Company and Buyer and Merger Subs agree to keep the terms of this Agreement confidential, except to the extent and to the Persons to whom disclosure is required by applicable Law or securities exchange regulation or for purposes of compliance with financial reporting obligations; provided, that the parties may disclose such terms to their respective employees, accountants, advisors and other representatives as necessary in connection with the ordinary conduct of their respective businesses (so long as such Persons agree to, or are bound by contract or professional or fiduciary obligations to, keep the terms of this Agreement confidential and so long as the parties shall be responsible to the other parties hereto for breach of this Section 12.11 or such confidentiality obligations by the recipients of its disclosure).
12.12 Severability. If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this Agreement shall remain in full force and effect. The parties further agree that if any provision contained herein is, to any extent, held invalid or unenforceable in any respect under the Laws governing this Agreement, they shall take any actions necessary to render the remaining provisions of this Agreement valid and enforceable to the fullest extent permitted by Law and, to the extent necessary, shall amend or otherwise modify this Agreement to replace any provision contained herein that is held invalid or unenforceable with a valid and enforceable provision giving effect to the intent of the parties.
| - 79 - |
12.13 Jurisdiction; Waiver of Jury Trial.
(a) Any Action based upon, arising out of or related to this Agreement or the transactions contemplated hereby may be brought in the Delaware Chancery Court (or, if the Delaware Chancery Court shall be unavailable, any other court of the State of Delaware or, in the case of claims to which the federal courts have exclusive subject matter jurisdiction, any federal court of the United States of America sitting in the State of Delaware), and, in each case, appellate courts therefrom, and each of the parties irrevocably submits to the exclusive jurisdiction of each such court in any such Action, waives any objection it may now or hereafter have to personal jurisdiction, venue or to convenience of forum, agrees that all claims in respect of such Action shall be heard and determined only in any such court, and agrees not to bring any Action arising out of or relating to this Agreement or the transactions contemplated hereby in any other court. Nothing herein contained shall be deemed to affect the right of any party to serve process in any manner permitted by Law or to commence Actions or otherwise proceed against any other party in any other jurisdiction, in each case, to enforce judgments obtained in any Action brought pursuant to this Section 12.13(a).
(b) Each party hereto hereby waives, to the fullest extent permitted by applicable Law, any right it may have to a trial by jury in respect of any Action arising out of this Agreement or the transactions contemplated hereby. Each party hereto (i) certifies that no representative, agent or attorney of any other party has represented, expressly or otherwise, that such party would not, in the event of any Action, seek to enforce the foregoing waiver and (ii) acknowledges that it and the other parties hereto have been induced to enter into this Agreement by, among other things, the mutual waiver and certifications in this Section 12.13(b).
12.14 Enforcement. The parties hereto agree that irreparable damage would occur, and that the parties would not have any adequate remedy at law, in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that the parties shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to specifically enforce the terms and provisions of this Agreement, without proof of actual damages or otherwise, in addition to any other remedy to which any party is entitled at law or in equity. Each party agrees to waive any requirement for the securing or posting of any bond in connection with such remedy. The parties further agree not to assert that a remedy of specific enforcement is unenforceable, invalid, contrary to Law or inequitable for any reason, nor to assert that a remedy of monetary damages would provide an adequate remedy. To the extent any party hereto brings an Action to enforce specifically the performance of the terms and provisions of this Agreement (other than an Action to enforce specifically any provision that by its terms requires performance after the Closing or expressly survives termination of this Agreement), the Outside Date shall automatically be extended to (a) the twentieth (20th) Business Day following the resolution of such Action or (b) such other time period established by the court presiding over such Action.
12.15 Tax Advice. Each party hereto acknowledges and agrees that it has not received and is not relying upon Tax advice from any other party hereto, and that it has and will continue to consult its own advisors with respect to Taxes.
[Remainder of page intentionally left blank]
| - 80 - |
IN WITNESS WHEREOF the parties have hereunto caused this Agreement to be duly executed as of the date first above written.
| TELIX PHARMACEUTICALS LIMITED | ||
| By: | /s/ Christian Behrenbruch | |
| Name: | Dr. Christian Behrenbruch | |
| Title: | Managing Director and Group Chief Executive Officer | |
| CYCLONE Merger Sub I, Inc. | ||
| By: | /s/ Darren Smith | |
| Name: | Darren Smith | |
| Title: | Treasurer | |
| CYCLONE Merger Sub II, Inc. | ||
| By: | /s/ Darren Smith | |
| Name: | Darren Smith | |
| Title: | Treasurer | |
| QSAM BIOSCIENCES INC. | ||
| By: | /s/ C. Richard Piazza | |
| Name: | C. Richard Piazza | |
| Title: | Executive Chairman | |
| DAVID H. CLARKE, as the Company Stockholder Representative | ||
| By: | /s/ David Clarke | |
| Name: | David Clarke | |
Form of CVR Agreement
CONTINGENT VALUE RIGHTS AGREEMENT
THIS CONTINGENT VALUE RIGHTS AGREEMENT, dated as of [_____], 2024, (this “Agreement”), is entered into by and between Telix Pharmaceuticals Limited ACN 616 620 369, a public limited company registered under the Laws of the Commonwealth of Australia (“Parent”), QSAM Biosciences, Inc., a Delaware corporation (the “Company”), David H. Clarke (“Holder Representative”), and [______], a [______], as Rights Agent.
PREAMBLE
WHEREAS, Parent, Cyclone Merger Sub I, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Parent (“Merger Sub I”), Cyclone Merger Sub II, Inc., a Delaware corporation and a direct, wholly owned subsidiary of Parent (“Merger Sub II”, and together with Merger Sub I, “Merger Subs”), the Company, and Holder Representative, solely in his capacity as the Company Stockholder Representative, have entered into an Agreement and Plan of Merger, dated as of [_____] (as it may be amended or supplemented from time to time, the “Merger Agreement”), pursuant to which (a) Merger Sub I will merge with and into the Company, Merger Sub I will cease to exist, and the Company will survive as a direct, wholly owned subsidiary of Parent (the “First Merger”), and as part of the same overall transaction, the Company will merge with and into Merger Sub II, the Company will cease to exist, and Merger Sub II will survive as a direct, wholly owned subsidiary of Parent (the “Second Merger” and, collectively or ad seriatim with the First Merger, as appropriate, the “Merger”);
WHEREAS, pursuant to the Merger Agreement, and in accordance with the terms and conditions thereof, at or prior to the Closing Date (as defined below), Parent has agreed to provide Holders (as defined below) the right to receive one or more contingent cash or stock payments upon the achievement of certain milestones as hereinafter described in accordance with the terms hereof and of the Merger Agreement;
WHEREAS, prior to the time at which the First Merger become effective pursuant to the terms of the Merger Agreement (the “First Effective Time”) the Company shall effect the Reverse Split (in accordance with the Merger Agreement), pursuant to which Pre-Reverse Split Company Stockholders holding fractional shares of Company Common Stock (after giving effect to the Reverse Split) shall receive, among other things, one (1) CVR for each share of Company Common Stock that was converted into a fractional share (and not aggregated into a whole number of shares held by the applicable holder) pursuant to such Reverse Split (each such CVR, a “Reverse Split CVR”); and
NOW, THEREFORE, in consideration of the premises and the consummation of the transactions referred to above, and for other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, it is mutually covenanted and agreed, for the proportionate benefit of all Holders (as defined below), as follows:
Article 1
DEFINITIONS
Section 1.01. Definitions.
Capitalized terms used but not otherwise defined herein shall have the meanings ascribed thereto in the Merger Agreement. The following terms shall have the meanings ascribed to them as follows:
“Acquired Products” means any Company Regulated Product (as defined in the Merger Agreement), including the CycloSam® product candidate developed by the Company prior to the Closing.
“Affiliate” means, with respect to any specified Person, any Person that, directly or indirectly, controls, is controlled by, or is under common control with, such specified Person, through one or more intermediaries or otherwise. For the avoidance of doubt, following the Closing, (a) the Company shall constitute an Affiliate of Parent and (b) neither Parent nor any of its Subsidiaries (including the Company) shall constitute an Affiliate of any Holder.
“ASX” means ASX Limited and, where applicable, the securities exchange operated by it.
“ASX Listing Rules” means the official listing rules of the ASX.
“Business Day” means any day that is not a Saturday, a Sunday or other day on which the commercial banking institutions in New York, New York or Melbourne, Australia are authorized to close for business.
“Cash-Out CVR Holder” means any Holder that, pursuant to Section 3.14 of the Merger Agreement, received cash in lieu of the Share Consideration that such Holder would have otherwise been entitled to receive (in such Holder’s capacity as a Company Stockholder) pursuant to the Merger Agreement.
“Closing” means the closing of the First Merger.
“Closing Date” means the date on which the Closing actually occurs.
“Commercially Reasonable Efforts” means, with respect to Parent’s obligations to develop or commercialize the Acquired Products, the level of efforts consistent with the efforts normally used by similarly situated biotechnology or biopharmaceutical company relating to the development and commercialization of a product with similar market potential as the Acquired Product at a similar stage of development or commercialization and taking into all relevant factors, including: (a) efficacy and safety clinical data; (b) patent and regulatory exclusivity; (c) target product profile; (d) market competition (including generic or biosimilar competition); (e) anticipated or approved labelling; (f) present and future market potential; (g) the likelihood of and scope obtained for regulatory approval; (h) the likelihood of and scope obtained for pricing and reimbursement; (i) the profitability and commercial potential of the product; and (j) all necessary medical, sales, marketing and other costs required for successful commercialization. For the avoidance of doubt, Commercially Reasonable Efforts shall be determined on a market-by-market and product-by-product basis, and it is anticipated that the level of effort will be different for different markets, and will change over time, reflecting changes in the status of the product and the market(s) involved.
| - 2 - |
“Company Common Stock” means the common stock, par value $0.0001 per share, of the Company.
“CVRs” means the rights of Holders to receive contingent payments pursuant to this Agreement.
“EMA” means the European Union Medicines Agency.
“FDA” means the United States Food & Drug Administration.
“First Commercial Sale” means, with respect to an Acquired Product, the first sale for monetary value for use or consumption by the end user (which for avoidance of doubt does not include any licensee or distributor) of such Acquired Product in a Major Market Country after receipt of all required Regulatory Approvals in the applicable country. First Commercial Sale of an Acquired Product expressly excludes any distribution or other sale at or below cost solely for ‘treatment IND’ sales, named patient use, compassionate use, or test marketing programs or non-registrational studies.
“Governmental Authority” means any U.S. or foreign federal, state, local or municipal government or any agency, instrumentality, commission, office, legislative body, court, arbitrational tribunal, mediator, securities exchange, administrative agency, government authority or other governmental or quasi-governmental regulatory authority or body.
“Holder” means, at the relevant time, a Person in whose name a CVR is registered in the CVR Register.
“Indication” means, with respect to an Acquired Product, a therapeutic use for a specified disease or medical condition, including but not limited to, for the treatment of symptoms of disease such as pain or for conditioning prior to a medical procedure such as bone marrow transplantation. Notwithstanding the foregoing, the following shall not constitute a new or additional Indication: (a) moving from one line of therapy to another within an Indication (e.g. the use of the Acquired Product for the same disease or medical condition in a second line therapy after approval for a first line of therapy); (b) use of the Acquired Product for the same disease or medical condition for different populations or population sub-types in the same line of therapy; (c) the use of the Acquired Product for the same disease or medical condition in different combinations or co-administration of therapies; and (d) treatment of the same disease or medical condition with the Acquired Product in an expanded, modified or additional patient population in the same line of therapy.
“Law” means any United States federal, state, municipal, or local or foreign law, common law, constitution, treaty, statute, standard, ordinance, code, rule, regulation, resolution, guidance or promulgation, or any decree, order, injunction, rule, judgment, consent of or by any Governmental Authority, or any Permit or similar right granted under any of the foregoing, or any similar provision having the force or effect of law.
| - 3 - |
“Major Market Country” means any of the following: the United States, France, Germany, Italy, Spain, Japan, United Kingdom, Australia, Canada, Brazil or China.
“Milestone 1” means the first achievement of Successful Completion with respect to any Acquired Product for one or more Indication(s).
“Milestone 1 Amount” means USD $10.0 million.
“Milestone 2” means the First Commercial Sale of an approved Acquired Product in any Major Market Country for any Indication.
“Milestone 2 Amount” means USD $20.0 million.
“Milestone 3” means the First Commercial Sale of an Acquired Product in any Major Market Country after receipt of Regulatory Approval for an Indication other than the Indication which resulted in the achievement of Milestone 2.
“Milestone 3 Amount” means USD $10.0 million.
“Milestones” means, collectively, Milestone 1, Milestone 2, Milestone 3 and the Net Sales Milestone.
“Milestone Payment(s)” means, as applicable, any of the Milestone 1 Amount, Milestone 2 Amount, Milestone 3 Amount or Net Sales Milestone Amount, in each case payable (a) subject to Section 2.04(e), if due and payable in accordance with the terms of this Agreement prior to the fifth anniversary of the date of this Agreement, by a number of Parent Ordinary Shares equal to (i) the applicable milestone amount, divided by (ii) the Milestone Share Price or (b) if due and payable in accordance with the terms of this Agreement on or after the fifth anniversary of the date of this Agreement, in cash in the applicable amount.
“Milestone Period” means the date beginning on the Closing Date and ending on the ten (10) year anniversary of such date.
“Milestone Share Price” means the volume weighted average price at which Parent Ordinary Shares traded on the ASX (excluding special crossings and overnight sales) over the twenty (20) trading-day period ending on the Business Day immediately prior to the date on which Parent delivers a Milestone Notice, as converted from AUD to USD at the exchange rate published in the Wall Street Journal as of the day that is one (1) Business Day prior to the applicable date of determination.
| - 4 - |
“Net Sales” means the total amount received in USD or USD equivalent for all sales, transfers or other supply of Acquired Products by or on behalf of Parent, its sublicensees or its Affiliates in an arms’ length bona fide commercial transaction, excluding: (a) taxes and duties; (b) customs tariffs, duties and charges; (c) product returns; (d) currency fluctuations and currency hedging; (e) regulatory and market access license approval and maintenance costs; (f) third party license fees (including royalty, milestone and patent costs payments or reimbursement); (g) other usual arms’ length trade discounts, rebates and costs of supplying and commercializing the Acquired Products such as freight, transportation, warehousing, packaging and shipping, dose preparation or compounding fees, product bad debt, product related-insurance charges. For avoidance of doubt: (i) Net Sales shall be deemed to not include transfers free of charge as part of the development of product, samples, product for clinical trials, compassionate use or demo/evaluation purposes, or intercompany transfers between Parent and its Affiliates, provided that Net Sales shall apply to Affiliates’ sales, transfers or other supply of Acquired Products to third parties; and (ii) the amount of Net Sales must be determined from Parent and its Affiliates books and records, as further described herein.
“Net Sales Milestone” means cumulative worldwide Net Sales of any or all Acquired Product(s) of USD$500.0 million.
“Net Sales Milestone Amount” means USD $50.0 million.
“Officer’s Certificate” means a certificate (i) signed by an authorized officer of Parent, in his or her capacity as such, and (ii) delivered to the Rights Agent.
“Parent Ordinary Shares” means the ordinary shares of Parent.
“Permitted Deductions” means (i) amounts Parent is permitted to offset or set off pursuant to Section 11.5 of the Merger Agreement and (ii) amounts for which the Holder Representative (in his capacity as the Company Stockholder Representative under the Merger Agreement) is indemnified pursuant to Section 3.9(d) of the Merger Agreement.
“Permitted Transfer” means (subject at all times to Section 2.02) a transfer of one or more CVRs (a) upon death by will or intestacy; (b) by instrument to an inter vivos or testamentary trust in which the CVRs are to be passed to beneficiaries upon the death of the trustee; (c) made pursuant to a court order; (d) made by operation of law (including a consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other entity; (e) in the case of CVRs payable to a nominee, from a nominee to a beneficial owner (and, if applicable, through an intermediary) or from such nominee to another nominee for the same beneficial owner, in each case as allowable by the Depository Trust Company; or (f) as provided in Section 2.07.
“Person” means any natural person, firm, limited liability company, general or limited partnership, association, corporation, unincorporated organization, company, joint venture, trust, Governmental Authority or other entity.
“Pivotal Clinical Trial” means a clinical trial of an Acquired Product that either: (a) would satisfy the requirements of 21 C.F.R. 312.21(c) or corresponding foreign regulations; or (b) is intended (as of the time the Clinical Trial is initiated) to obtain sufficient data to support the filing of a Regulatory Approval application for such Acquired Product. Pivotal Clinical Trial may include (i) a Clinical Trial that is designed to satisfy the requirements of both 21 C.F.R. 312.21(b) and 21 C.F.R. 312.21(c) or corresponding foreign regulations, or (ii) a Clinical Trial that is designed to satisfy the requirements of 21 C.F.R. 312.21(b) that is subsequently optimized or expanded to satisfy the requirements of 21 C.F.R. 312.21(c) or to provide sufficient data to support the filing of a Regulatory Approval application for such Acquired Product, as supported by a Regulatory Authority’s formal meeting minutes, IRB approval or comparable documents.
| - 5 - |
“Regulatory Approval” means all approvals of each applicable Regulatory Authority necessary for the commercial marketing and sale of a product in a country (including any required pricing or reimbursement approvals).
“Regulatory Authority” means any federal, national, multinational, state, provincial, or local regulatory agency, department, bureau, or other Governmental Authority with authority over the testing, manufacture, use, storage, import, promotion, marketing, or sale (including pricing and reimbursement approval) of any pharmaceutical or biologic product in any country or territory.
“Rights Agent” means the Rights Agent named in the first paragraph of this Agreement, until a successor Rights Agent shall have become such pursuant to the applicable provisions of this Agreement, and thereafter “Rights Agent” shall mean such successor Rights Agent.
“Subsidiary” means, with respect to a Person, a corporation or other entity of which more than 50% of the voting power of the equity securities or equity interests is owned, directly or indirectly, by such Person.
“Successful Completion” means, with respect to an Acquired Product, that statistically significant results have been generated from a Pivotal Clinical Trial for such Acquired Product, which results meet or exceed the primary endpoint(s) and secondary endpoint(s) set forth in the protocol, as evidenced by the clinical trial report prepared by the principal investigator for the Pivotal Clinical Trial. Should the specific conditions of Successful Completion not be met, however, and the FDA or the EMA, or the other similar foreign Regulatory Authority nonetheless grants Regulatory Approval of an Acquired Product for the indication agreed in the ESC approved development plan as may be amended or modified, then Successful Completion shall be deemed to have achieved upon receipt of such Regulatory Approval.
Article
2
CONTINGENT VALUE RIGHTS
Section 2.01. Holders of CVRs; Appointment of Rights Agent.
(a) As contemplated by the Merger Agreement:
(i) upon the effectiveness of the Reverse Split (and prior to the Effective Time), each Holder holding fractional shares of Company Common Stock (after giving effect to the Reverse Split) shall receive, among other things, one (1) CVR for each share of Company Common Stock that was converted into such fractional share (and not aggregated into a whole number of shares held by the applicable holder) pursuant to such Reverse Split; and
(ii) pursuant to the Merger Agreement, each Holder shall be entitled to a number of CVRs equal to the denominator in the Reverse Split for each share of Company Common Stock, if any, that is issued and outstanding and held by such Holder (after giving effect to the Reverse Split) as of immediately prior to the First Effective Time.
| - 6 - |
(b) The initial Holders shall be determined pursuant to the terms of the Merger Agreement and this Agreement, and a list of the initial Holders shall be furnished to the Rights Agent by or on behalf of Parent in accordance with this Agreement.
(c) Parent hereby appoints the Rights Agent to act as rights agent for Parent in accordance with the terms and conditions set forth in this Agreement, and the Rights Agent hereby accepts such appointment.
Section 2.02. Nontransferable.
CVRs may not be sold, assigned, transferred, pledged, encumbered or transferred or disposed of in any other manner, in whole or in part, other than pursuant to a Permitted Transfer, and, in the case of a Permitted Transfer, only in accordance with the terms of this Agreement and in compliance with (and to the extent permitted by) applicable United States federal and state securities laws, ASX Listing Rules and the terms and conditions hereto. Any attempted sale, assignment, transfer, pledge, encumbrance or disposition of CVRs, in whole or in part, in violation of this Section 2.02 or Section 2.03 shall be void ab initio and of no effect.
Section 2.03. No Certificate; Registration; Registration of Transfer; Change of Address.
(a) CVRs shall not be evidenced by a certificate or other instrument; provided however, the Holders shall receive evidence of issuance of the CVRs in the form of an account statement of other written documentation from the Rights Agent.
(b) The Rights Agent shall keep a register (the “CVR Register”) for the purposes of (i) identifying the Holders of CVRs and (ii) registering CVRs and Permitted Transfers thereof. The CVR Register will be created, and CVRs will be distributed, pursuant to written instructions to the Rights Agent from Parent. In furtherance of Section 2.04(e), the CVR Register shall specify each CVR that is a Cash-Out CVR. For the avoidance of doubt, any CVRs payable to a Company Stockholder as Merger Consideration will not be deemed outstanding or included in the CVR Register unless and until such Company Stockholder completes the exchange procedures set forth in Section 3.7(b) of the Merger Agreement.
(c) Without limiting the restriction on transferability set forth in Section 2.02, every request made to transfer a CVR must be in writing and accompanied by a written instrument of transfer and other requested documentation in form reasonably satisfactory to the Rights Agent, duly executed by the registered Holder or Holders thereof, or by the duly appointed legal representative, personal representative or survivor of such Holder or Holders, setting forth in reasonable detail the circumstances relating to the transfer demonstrating that such proposed transfer is a Permitted Transfer. Upon receipt of such written notice, the Rights Agent shall, subject to its reasonable determination that the transfer instrument is in proper form and the transfer is a Permitted Transfer and otherwise complies with the other terms and conditions of this Agreement, register the transfer of the applicable CVRs in the CVR Register and notify Parent of the same. Subject to Section 2.07, all duly and validly transferred CVRs registered in the CVR Register shall be the valid obligations of Parent, evidencing the same right, and entitling the transferee to the same benefits and rights under this Agreement, as those held by the transferor. No transfer of a CVR shall be valid unless and until registered in the CVR Register in accordance with this Agreement.
| - 7 - |
(d) A Holder may make a written request to the Rights Agent to change such Holder’s address of record in the CVR Register. Such written request must be duly executed by such Holder. Upon receipt of such written notice, the Rights Agent shall promptly record the change of address in the CVR Register.
Section 2.04. Payment Procedures.
(a) If any Milestone is achieved during the Milestone Period, then, in each case, on a date that is no later than thirty (30) days following the achievement of such Milestone, Parent will deliver to the Rights Agent (i) a notice (a “Milestone Notice”) indicating (A) the achievement of such Milestone, and (B) a calculation of the amount of cash and/or number of Parent Ordinary Shares, as applicable, payable as the applicable Milestone Payment, including, if applicable, the amount of any Permitted Deductions from such Milestone Payment and the portion of any Milestone Payment that will be paid in cash in lieu of Parent Ordinary Shares pursuant to Section 2.04(e), and (ii) for payment to the Holders, cash and/or shares equal to the applicable Milestone Payment (in each case less any applicable withholding Tax, if any).
(b) The Rights Agent shall promptly, and in no event later than ten (10) Business Days after receipt of a Milestone Notice, send each Holder at its address set forth in the CVR Register a copy of such Milestone Notice. At the time the Rights Agent sends a copy of such Milestone Notice to the Holders, the Rights Agent shall also pay to each Holder, subject to any applicable withholding Tax and Section 2.04(e), the applicable Milestone Payment (the portion of such Milestone Payment which each Holder is entitled to receive shall be equal to (i) (A) the applicable Milestone Payment divided by (B) the aggregate number of CVRs registered in the CVR Register at such time, multiplied by (ii) the number of CVRs held by such Holder as reflected on the CVR Register). For the avoidance of doubt, none of Parent, the Company or any of their Affiliates will have any further liability in respect of the relevant Milestone Payments upon delivery of such Milestone Payment in accordance with this Section 2.04 to the Rights Agent. For clarity, no Milestone Payment shall be payable more than once.
(c) Parent shall be entitled to deduct and withhold, or cause to be deducted and withheld, from each Milestone Payment otherwise payable pursuant to this Agreement, such amounts as it is required to deduct and withhold with respect to any such deliveries and payments under the United States Internal Revenue Code of 1986, as amended, or any provision of state, local, provincial or foreign Law. To the extent that amounts are so deducted and withheld, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the Holder in respect of which such deduction and withholding was made.
| - 8 - |
(d) Any portion of a Milestone Payment that remains undistributed to the Holders six (6) months after applicable date of payment of such Milestone Payment to the Rights Agent (including by means of invalid addresses on the CVR Register) will be delivered by the Rights Agent to Parent or a person nominated in writing by Parent (with written notice thereof from Parent to the Rights Agent), and subject to this Section 2.04(d), such property shall be deemed forfeited by the applicable Holders and become the property of Parent. The Rights Agent shall promptly notify the Holder Representative in the event that any undistributed amount is delivered to Parent or its nominee. To the extent all such undistributed payment(s) exceed $50,000 in the aggregate (whether payable in cash or stock), upon written notice by the Holder Representative, Parent and Rights Agent shall cause such amounts to be reallocated and distributed to the other CVR Holders in accordance with their respective pro rata shares of the aggregate number of CVRs registered in the CVR Register, excluding the CVRs to which such undistributed payments were otherwise payable.
(e) Notwithstanding anything herein to the contrary, with respect to the amount of any Milestone Payments which would, but for this Section 2.04(e), be payable in Parent Ordinary Shares in accordance with definition of “Milestone Payment,” such portion of such Milestone Payment payable in respect of (i) Reverse Split CVRs, (ii) CVRs held by Cash-Out CVR Holders or (iii) CVRs that have been transferred pursuant to a Permitted Transfer (other than a Permitted Transfer of the nature described in clause (e) of the definition of “Permitted Transfer”) (any such CVRs as described in clauses (i) through (iii), “Cash-Out CVRs”) shall, in each case, be paid in cash in lieu of any Parent Ordinary Shares.
Section 2.05. No Fractional Shares. Parent shall not be required to issue fractional Parent Ordinary Shares upon payment of CVRs, and no certificates or scrip for any such fractional shares shall be issued. If more than one CVR shall be payable at the same time with respect to the same Holder, the number of full Parent Ordinary Shares which shall be issuable upon the payment thereof shall be computed on the basis of the aggregate number of Parent Ordinary Shares issuable upon the payment of such CVRs. If any fraction of a share of Parent Ordinary Shares would, except for the provisions of this Section 2.05, be issuable on the payment of any CVRs, Parent shall pay in cash the dollar amount (rounded to the nearest whole cent, with numbers of cents ending with .5 or more being rounded up to the nearest whole cent), without interest, determined by multiplying such fraction by the Milestone Share Price.
Section 2.06. No Voting, Dividends or Interest; No Equity or Ownership Interest.
(a) CVRs shall not have any voting or dividend rights (whether fixed or at the discretion of the directors of Parent), and interest shall not accrue on any amounts payable in respect of CVRs.
(b) CVRs shall not represent any equity or ownership interest in Parent, any constituent company to the Merger or any of their respective Affiliates.
(c) CVRs shall not confer any right (i) to a return of capital, whether in a winding up, upon a reduction of capital or otherwise; (ii) to participate in the surplus profit or assets of Parent upon a winding up; or (iii) to participate in new issues of securities such as bonus issues or entitlement issues.
(d) The rights of a Holder in respect of the CVRs are solely limited to those expressly included in this Agreement.
| - 9 - |
Section 2.07. Ability to Abandon CVR.
A Holder may at any time, at such Holder’s option, abandon all of such Holder’s remaining rights in a CVR by transferring such CVR to Parent without consideration therefor. Nothing in this Agreement shall prohibit Parent or any of its Affiliates from offering to acquire or acquiring any CVRs from the Holders for consideration, in private transactions or otherwise, in its sole discretion. Any CVRs acquired by Parent or any of its Affiliates shall be automatically deemed extinguished and no longer outstanding for purposes of Article 5 and Section 6.04 hereunder, but shall still be deemed as outstanding for purposes of calculating the aggregate number of CVRs registered in the CVR Register under Section 2.04.
Article 3
THE RIGHTS AGENT
Section 3.01. Certain Duties and Responsibilities of Rights Agent.
The Rights Agent shall not have any liability for any actions taken or not taken in connection with this Agreement, except to the extent such liability arises as a result of the willful misconduct, bad faith or gross negligence of the Rights Agent.
Section 3.02. Certain Rights of Rights Agent.
(a) The Rights Agent undertakes to perform such duties and only such duties as are specifically set forth in this Agreement, and no implied covenants or obligations shall be read into this Agreement against the Rights Agent.
(b) The Rights Agent may rely and shall be protected in acting or refraining from acting upon any resolution, certificate, statement, instrument, opinion, report, notice, request, direction, consent, order or other paper or document believed by it in good faith to be genuine and to have been signed or presented by the proper party or parties.
(c) Whenever the Rights Agent shall deem it desirable that a matter be proved or established prior to taking, suffering or omitting any action hereunder, the Rights Agent may, in the absence of bad faith, gross negligence or willful misconduct on its part, rely upon an Officer’s Certificate.
(d) The Rights Agent may engage and consult with counsel of its reasonable selection and the written advice or opinion of such outside counsel shall be full and complete authorization and protection in respect of any action taken, suffered or omitted by it hereunder in good faith and in reliance thereon.
(e) Any permissive rights of the Rights Agent hereunder shall not be construed as a duty.
| - 10 - |
(f) The Rights Agent shall not be required to give any note or surety in respect of the execution of such powers or otherwise in respect of such powers.
(g) Parent agrees to indemnify the Rights Agent for, and to hold the Rights Agent harmless from and against, any loss, liability, damage or expense (“Loss”) suffered or incurred by the Rights Agent and arising out of or in connection with the Rights Agent’s performance of its obligations under this Agreement, including the reasonable costs and expenses of defending the Rights Agent against any claims, charges, demands, actions or suits arising out of or in connection with such performance, except to the extent such Loss shall have been determined by a court of competent jurisdiction to have resulted from the Rights Agent’s gross negligence, bad faith or willful misconduct. Parent’s obligations under this Section 3.02(g) to indemnify the Rights Agent shall survive the resignation or removal of any Rights Agent and the termination of this Agreement.
(h) In addition to the indemnification provided under Section 3.02(g), but without duplication, Parent agrees (i) to pay the fees of the Rights Agent in connection with the Rights Agent’s performance of its obligations hereunder, as agreed upon in writing by the Rights Agent and Parent on or prior to the date of this Agreement, and (ii) to reimburse the Rights Agent promptly upon demand for all reasonable and documented out-of-pocket expenses, including all Taxes (other than income, receipt, franchise or similar Taxes) and governmental charges, incurred by the Rights Agent in the performance of its obligations under this Agreement.
Section 3.03. Resignation and Removal; Appointment of Successor.
(a) The Rights Agent may resign at any time by giving written notice thereof to Parent specifying a date when such resignation shall take effect, which notice shall be sent at least 60 days prior to the date so specified, but in no event shall such resignation become effective until a successor Rights Agent has been appointed and accepted such appointment in accordance with Section 3.04.
(b) Parent shall have the right to remove the Rights Agent at any time by specifying a date when such removal shall take effect, but no such removal shall have become effective until a successor Rights Agent has been appointed and accepted such appointment in accordance with Section 3.04. Notice of such removal shall be given by Parent to the Rights Agent, which notice shall be sent at least 60 days prior to the date so specified.
(c) If the Rights Agent shall resign, be removed or become incapable of acting, Parent shall promptly appoint a qualified successor Rights Agent. The successor Rights Agent so appointed shall, forthwith upon its acceptance of such appointment in accordance with this Section 3.03(c) and Section 3.04, become the Rights Agent for all purposes hereunder.
(d) Parent shall give notice of each resignation or removal of the Rights Agent and each appointment of a successor Rights Agent through the facilities of DTC in accordance with DTC’s procedures and/or by mailing written notice of such event by first-class mail to the Holders. Each notice shall include the name and address of the successor Rights Agent. If Parent fails to send such notice within ten (10) Business Days after acceptance of appointment by a successor Rights Agent, the successor Rights Agent shall cause the notice to be transmitted at the expense of Parent. Failure to give any notice provided for in this Section 3.03, however, shall not affect the legality or validity of the resignation or removal of the Rights Agent or the appointment of the successor Rights Agent, as the case may be.
(e) Notwithstanding anything to the contrary in this Section 3.03, unless consented to in writing by the Holder Representative, Parent shall not appoint as a successor Rights Agent any Person that is not a transfer agent of national reputation or the corporate trust department of a commercial bank.
| - 11 - |
Section 3.04. Acceptance of Appointment by Successor.
Every successor Rights Agent appointed hereunder shall, at or prior to such appointment, execute, acknowledge and deliver to Parent and to the retiring Rights Agent an instrument accepting such appointment and a counterpart of this Agreement, and thereupon such successor Rights Agent, without any further act, deed or conveyance, shall become vested with all the rights, powers, trusts and duties of the Rights Agent; provided that upon the request of Parent or the successor Rights Agent, such resigning or removed Rights Agent shall execute and deliver an instrument transferring to such successor Rights Agent all the rights, powers and trusts of such resigning or removed Rights Agent.
Article 4
COVENANTS; OTHER AGREEMENTS
Section 4.01. List of Holders.
Parent shall furnish or cause to be furnished to the Rights Agent the names and addresses of the Holders within forty-five (45) Business Days following the Closing Date.
Section 4.02. Commercially Reasonable Efforts.
Commencing upon the Closing Date and continuing until the earlier of expiration of the Milestone Period or the achievement of all Milestones, Parent shall use Commercially Reasonable Efforts to (a) develop at least one Acquired Product in at least one Major Market Country, including using Commercially Reasonable Efforts to finalize the clinical trial report within three (3) months from database lock for the related Pivotal Clinical Trial (for clarity, completion of database lock within such time period is not guaranteed, and is subject to a number of factors including without limitation receipt of Protocol compliant and GCP compliant data from trial investigators, institutions, ethics bodies, regulators and third parties); and (b) commercialize at least one Acquired Product in the Major Market Countries after receipt of Regulatory Approval in any such country.
Section 4.03. Audit Rights.
(a) Until the earlier of achievement of the Net Sales Milestone or the expiration of the Milestone Period, upon reasonable advance written notice from the Holder Representative, Parent shall permit an independent certified public accounting firm of nationally recognized standing mutually agreed by the Holder Representative and Parent (the “Independent Accountant”) to have access at reasonable times during normal business hours to the books and records of Parent and its Affiliates as may be reasonably necessary to evaluate and verify Parent’s calculation of the Net Sales Milestone hereunder; provided that (i) such Holder Representative (and the Independent Accountant) enter into customary confidentiality agreements reasonably satisfactory to Parent with respect to the confidential information of Parent or its Affiliates to be furnished pursuant to this Section 4.03 and (ii) such access does not unreasonably interfere with the conduct of the business of Parent or any of its Affiliates. The Independent Accountant will keep all books and records of Parent and its Affiliates strictly confidential, and will provide only a report of the results of its findings to Holder Representative. The reasonable, documented, out-of-pocket fees charged by such accounting firm (to the extent consistent with a previously agreed budget at the time of engagement by such Independent Accountant) shall be borne by the Holder Representative. The Independent Accountant shall provide Parent with a copy of all disclosures made to the Holder Representative. The decision of such accounting firm shall be final, conclusive and binding on Parent, Holder Representative and the Holders, shall be nonappealable and shall not be subject to further review, absent manifest error. The audit rights set forth in this Section 4.03(a) may not be exercised by the Holder Representative more than once; provided however, that if the Independent Accountant determines in its audit that the actual amount of Net Sales as of the date the Independent Accountant began its audit pursuant to this Section 4.03(a) is more than 10% greater than the amount Parent calculated Net Sales to be as of such date, the Holder Representative may exercise these audit rights a second time no sooner than 12 months after the completion of the first audit.
| - 12 - |
(b) If, in accordance with the procedures set forth in Section 4.03(a), the Independent Accountant concludes that the Net Sales Milestone should have been paid but was not paid when due, Parent shall promptly, and in any event within thirty (30) days of the date the Independent Accountant delivers to Parent the Independent Accountant’s written report, pay each Holder the applicable portion of the Net Sales Milestone Amount (to the extent not paid on a subsequent date), plus interest at the thirty (30) day U.S. dollar “prime rate” effective for the date such payment was due, as reported by Bloomberg, from when such Milestone should have been paid, as applicable, to the date of actual payment, as applicable; provided that, for clarity, such adjusted Net Sales Milestone Amount shall otherwise be paid pursuant to the procedures set forth in Section 2.04.
Section 4.04. Executive Steering Committee.
(a) Effective as of the Closing Date, an Executive Steering Committee (the “ESC”), shall be deemed established, which ESC shall continue until the date that is six (6) months after the Closing Date, at which time the ESC shall be automatically deemed dissolved. The ESC shall be composed of four members, with two members to be appointed by Parent and two members to be appointed by the Company, in each case no later than the Closing Date. Parent shall designate one of the ESC members as the “ESC Chair.” Each ESC member shall have executed a confidentiality agreement reasonably acceptable to Parent. The ESC will meet once every other month. The ESC Chair will send a draft agenda for each meeting to the other members, and each of the members may, with the reasonable approval of the ESC Chair, invite individuals who are not ESC members to participate in ESC meetings (provided that such individuals have executed a confidentiality agreement with the party that invited it). The ESC Chair shall record minutes of each meeting and promptly distribute them to the ESC members.
(b) The ESC’s primary responsibility will be to review and approve a development and commercialization plan with respect to the Acquired Products (the “Acquired Product Plan”). Parent will prepare and deliver to the ESC a draft of the Acquired Product Plan, and, at its regularly scheduled meetings and any special meetings agreed to, any attended by, all four members of the ESC, the ESC will review, discuss and provide comments to Parent with respect to such Acquired Product Plan, and the ESC will be responsible for approving the final Acquired Product Plan.
(c) The unanimous approval of the ESC will be required with respect to all matters within the scope of the ESC’s authority. If the ESC cannot reach unanimous agreement, then (i) such matter shall be referred to the Holder Representative and the Chief Executive Officer of Parent, and such persons shall negotiate in good faith to resolve any such dispute in a mutually satisfactory manner for thirty (30) days after the referral of the applicable matter to them (or such longer period of time to which the Chief Executive Officer of Parent and Holder Representative may mutually agree) and (ii) if Parent and the Holder Representative fail to reach unanimous agreement within the thirty (30) day period described in the prior clause (i), then Parent shall have the final decision-making authority with respect to any such matters.
(d) Notwithstanding anything to the contrary, the ESC will have no authority to (i) amend, modify or waive compliance with this Agreement or the Merger Agreement or any terms hereof or thereof, or (ii) resolve any dispute concerning the validity, interpretation, construction of, or breach of this Agreement, and, for clarity, the ESC will not have any decision-making authority with respect to any matters except as expressly set forth in this Section 4.04.
(e) Nothing herein shall be deemed to affect the ownership of any intellectual property rights, including Parent’s sole ownership of all intellectual property and other rights with respect to the Acquired Products acquired by virtue of the Merger as well as any intellectual property developed with respect thereto during the course of this Agreement.
| - 13 - |
Article
5
AMENDMENTS
Section 5.01. Amendments Without Consent of Holders.
(a) Without the consent of any Holders or Holder Representative, Parent and the Rights Agent, at any time and from time to time, may enter into one or more amendments hereto, for any of the following purposes:
(i) to evidence the appointment of another Person as a successor Rights Agent and the assumption by any successor Rights Agent of the covenants and obligations of the Rights Agent herein in accordance with the provisions hereof;
(ii) to add to the covenants of Parent such further covenants, restrictions, conditions or provisions as Parent shall consider to be for the protection of the Holders;
(iii) to cure any ambiguity, to correct or supplement any provision herein that may be defective or inconsistent with any other provision herein or in the Merger Agreement, or to make any other provisions with respect to matters or questions arising under this Agreement;
(iv) as may be necessary or appropriate to ensure that CVRs are not subject to registration under the Securities Act, the Exchange Act or any applicable state or foreign securities laws;
(v) any other amendment hereto which would provide any additional rights or benefits to the Holders or Holder Representative or that does not adversely affect the legal rights or intended economic benefits under this Agreement of the Holders or Holder Representative.
(b) Promptly after the execution by Parent and the Rights Agent of any amendment pursuant to the provisions of this Section 5.01, Parent shall mail or otherwise transmit (or cause the Rights Agent to mail or otherwise transmit) a notice thereof through the facilities of DTC in accordance with DTC’s procedures and/or by first class mail to the Holders at their addresses as set forth on the CVR Register, setting forth in general terms the substance of such amendment.
Section 5.02. Amendments with Consent of Holders or Holder Representative.
(a) In addition to any amendments to this Agreement that may be made by Parent without the consent of any Holder or the Rights Agent pursuant to Section 5.01, with the consent of the Holder Representative (which may be granted or withheld in its sole discretion) acting on behalf of the Holders, Parent and the Rights Agent may enter into one or more amendments hereto for the purpose of adding, eliminating or changing any provisions of this Agreement, even if such addition, elimination or change is adverse to the interests of the Holders.
(b) Promptly after the execution by Parent and the Rights Agent of any amendment pursuant to the provisions of this Section 5.02 (but prior to the effectiveness of such amendment), Parent shall mail or transmit (or cause the Rights Agent to mail or transmit) a notice thereof by first class mail to the Holder Representative and to the Holders at their addresses as set forth on the CVR Register, setting forth in general terms the substance of such amendment. Any amendment to this Agreement made pursuant to this Section 5.02 shall become effective automatically upon the mailing or transmittal of such notice.
Section 5.03. Execution of Amendments.
Subject to the consent rights of the Holder Representative provided in Section 5.02, this Agreement may be amended or modified in whole or in part, by a duly authorized agreement in writing executed by Parent and the Rights Agent. In executing any amendment permitted by this Article 5, the Rights Agent shall be entitled to receive, and shall be fully protected in relying upon, an opinion of counsel selected by Parent stating that the execution of such amendment is authorized or permitted by this Agreement.
| - 14 - |
Section 5.04. Effect of Amendments.
Upon the execution of any amendment under this Article 5, this Agreement shall be modified in accordance therewith, such amendment shall form a part of this Agreement for all purposes and the Holder Representative and every Holder shall be bound thereby.
Article
6
MISCELLANEOUS
Section 6.01. Notices to Rights Agent, Parent and Holder Representative.
All notices and other communications among the parties shall be in writing and shall be deemed to have been duly given (a) when delivered in person, (b) when delivered after posting in the United States mail having been sent registered or certified mail return receipt requested, postage prepaid, (c) when delivered by FedEx or other nationally recognized overnight delivery service, or (d) when delivered by email, with affirmative confirmation of delivery (i.e., an electronic record of the sender that the email was sent to the intended recipient thereof without an “error” or similar message that such email was not received by such intended recipient), addressed as follows:
If to Parent or the Company:
Telix
Pharmaceuticals Limited
55 Flemington Road
North Melbourne, Victoria, 3051, Australia
| Attention: | Lena Moran-Adams |
| Email: | [●] |
with copies (which shall not constitute notice) to:
Wilmer
Cutler Pickering Hale and Dorr LLP
7 World Trade Center
250 Greenwich Street
New York, New York 10007
| Attention: | Christopher D. Barnstable-Brown |
| Jason L. Kropp | |
| Email: | Chris.Barnstable-Brown@wilmerhale.com |
| Jason.Kropp@wilmerhale.com |
If to the Rights Agent:
[_______]
[Address]
Attention: [____]
Email: [____]
| - 15 - |
If to the Holder Representative:
David H. Clarke
Email: [●]
with a copy (which shall not constitute notice) to:
Christopher Nelson
Email: [●]
or to such other address as the Person to whom notice is given may have previously furnished to the others in writing in the manner set forth above.
Section 6.02. Notice to Holders.
All notices, requests and communications required to be given to the Holders shall be given (unless otherwise herein expressly provided) in writing and mailed, first-class postage prepaid, to each Holder affected by such event, at his, her or its address set forth in the CVR Register, not later than the latest date, and not earlier than the earliest date, prescribed for the giving of such notice. In any case where notice to the Holders is given by mail, neither the failure to mail such notice, nor any defect in any notice so mailed, to any particular Holder shall affect the sufficiency of such notice with respect to other Holders.
Section 6.03. Entire Agreement.
This Agreement and the Merger Agreement constitute the entire agreement between the parties with respect to the subject matter of this Agreement and supersede all prior agreements and understandings, both written and oral, among or between any of the parties with respect to the subject matter of this Agreement.
Section 6.04. Successors and Assigns.
Except as expressly set forth in this Section 6.04, no party hereto shall assign this Agreement or any part hereof without the prior written consent of the other parties, except that Parent or the Company may transfer or assign their respective rights and obligations under this Agreement, in whole or from time to time in part, to (a) any acquiror of all or substantially all of Parent’s or the Company’s business or assets that assumes Parent’s obligations, duties and covenants under this Agreement to the extent not already effected by operation of law or (b) one (1) or more of their Affiliates (and any such Affiliate assignee may thereafter assign, in its sole discretion and without the consent of any other party, any or all of its rights, interests and obligations hereunder to one or more additional Affiliate assignees; provided that, in the case of an assignment by Parent to any of its Affiliates, such assignment shall not relieve Parent of any of its obligations hereunder. The Rights Agent may not assign this Agreement without Parent’s consent. Any attempted assignment of this Agreement or any of such rights in violation of this Section 6.04 shall be void ab initio and of no effect. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns.
| - 16 - |
Section 6.05. Benefits of Agreement.
Nothing in this Agreement, express or implied, shall give to any Person (other than the parties hereto, the Holders and their permitted successors and assigns hereunder) any benefit or any legal or equitable right, remedy or claim under this Agreement or under any covenant or provision herein contained, all such covenants and provisions being for the sole benefit of the parties hereto, the Holders and their permitted successors and assigns. The Holders shall have no rights hereunder except as are expressly set forth herein. For the avoidance of doubt, no Holder shall have any right to enforce or otherwise assert a claim with respect to this Agreement; all such rights and claims shall only be brought by the Holder Representative on behalf of all such Holders.
Section 6.06. Governing Law.
This Agreement, and all claims or causes of action based upon, arising out of, or related to this Agreement or the transactions contemplated hereby, shall be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to principles or rules of conflict of laws to the extent such principles or rules would require or permit the application of Laws of another jurisdiction.
Section 6.07. Jurisdiction.
Each of the parties hereto (a) consents to submit itself to the exclusive jurisdiction of the Court of Chancery of the State of Delaware or, solely if such court lacks subject matter jurisdiction, the United States District Court sitting in the State of Delaware, with respect to any dispute arising out of, relating to or in connection with this Agreement or the transactions contemplated hereby, (b) agrees that it will not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from any such court and (c) agrees that it will not bring any action arising out of, relating to or in connection with this Agreement or the transactions contemplated hereby, in any court other than any such court. The parties irrevocably and unconditionally waive any objection to the laying of venue of any Action arising out of this Agreement or the transactions contemplated hereby in the chancery courts of the State of Delaware or in any Federal court located in the State of Delaware, and hereby further irrevocably and unconditionally waive and agree not to plead or claim in any such court that any such Legal Proceeding brought in any such court has been brought in an inconvenient forum. Each of the parties hereto hereby agrees that service of any process, summons, notice or document by U.S. registered mail to the respective addresses set forth in Section 6.01 shall be effective service of process for any proceeding arising out of, relating to or in connection with this Agreement or the transactions contemplated hereby.
Section 6.08. WAIVER OF JURY TRIAL.
EACH PARTY ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS AGREEMENT IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES, AND THEREFORE EACH SUCH PARTY HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN ANY LITIGATION ARISING OUT OF, RELATING TO OR IN CONNECTION WITH THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) EACH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATION OF THIS WAIVER, (III) EACH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) EACH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION.
| - 17 - |
Section 6.09. Severability.
If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any rule of Law, or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of this Agreement is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible in a mutually acceptable manner in order that this Agreement be effected as originally contemplated to the fullest extent possible.
Section 6.10. Counterparts; Effectiveness.
This Agreement may be executed in counterparts, each of which shall be deemed to be an original, but all of which, taken together, shall constitute one and the same agreement. This Agreement or any counterpart may be executed and delivered by facsimile, electronic mail (including ..pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000 (e.g., www.docusign.com)) or other transmission method, each of which shall be deemed an original. This Agreement shall become effective when each party hereto shall have received a counterpart hereof signed by the other party hereto. Until and unless each party has received a counterpart hereof signed by the other party hereto, this Agreement shall have no effect and no party shall have any right or obligation hereunder (whether by virtue of any other oral or written agreement or other communication).
Section 6.11. Termination.
This Agreement shall be terminated and of no force or effect, and the parties hereto shall have no liability hereunder (other than to the extent of any obligations which expressly survive or provide for performance following termination), upon the earlier to occur of (a) the payment of all Milestone Payments and (b) the expiration of the Milestone Period. The termination of this Agreement will not affect or limit the right of Holders to receive the Milestone Payments under this Agreement to the extent earned prior to the termination of this Agreement, and the provisions applicable thereto will survive the expiration or termination of this Agreement.
Section 6.12. Holder Representative.
The Holders hereby irrevocably appoint the Holder Representative as the representative, attorney-in-fact and agent of the Holders for all purposes in connection with the transactions contemplated by this Agreement and any other matters ancillary hereto and in any litigation or arbitration involving this Agreement, the CVR or the matters contemplated hereby. In connection therewith, the Holder Representative is authorized to do or refrain from doing all further acts and things, and to execute all such documents as the Holder Representative shall deem necessary or appropriate, and shall have the power and authority to take such actions and have such rights, roles and responsibilities, and the rights of Holders to act other than through the Holder Representative shall be so limited, in each case by application of the provisions of Section 3.9 of the Merger Agreement with respect to the Company Stockholder Representative to the Holder Representative under this Agreement, the CVR and the matters contemplated hereby, mutatis mutandis.
Section 6.13. Legal Holidays.
In the event that the day on which any Milestone Payment is due shall not be a Business Day, then, notwithstanding any provision of this Agreement to the contrary, any payment required to be made in respect of the CVRs on or prior to such date need not be made on or prior to such date, but may be made on the next succeeding Business Day with the same force and effect as if made on the last day on which such Milestone Payment is due.
Section 6.14. Construction.
(a) The words “hereof,” “herein,” “hereby,” “herewith” and words of similar import shall, unless otherwise stated, be construed to refer to this Agreement as a whole and not to any particular provision of this Agreement, and article, section and paragraph references are to the articles, sections and paragraphs of this Agreement unless otherwise specified. Whenever the words “include,” “includes” or “including” are used in this Agreement they shall be deemed to be followed by the words “without limitation.” The words describing the singular number shall include the plural and vice versa, words denoting either gender shall include both genders and words denoting natural persons shall include all Persons and vice versa. The phrases “the date of this Agreement,” “the date hereof,” “of even date herewith” and terms of similar import, shall be deemed to refer to the date set forth in the preamble to this Agreement. Any reference in this Agreement to a date or time shall be deemed to be such date or time in New York City, unless otherwise specified or the context otherwise requires. The parties have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the parties and the Company and no presumption or burden of proof shall arise favoring or disfavoring any Person by virtue of the authorship of any provision of this Agreement.
(b) The descriptive headings herein are inserted for convenience of reference only and are not intended to be part of or to affect the meaning or interpretation of this Agreement.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK; SIGNATURE PAGE FOLLOWS]
| - 18 - |
IN WITNESS WHEREOF, each of the parties has caused this Agreement to be executed on its behalf by its duly authorized officers as of the day and year first above written.
| Telix Pharmaceuticals Limited | ||
| By: | ||
| Name: | ||
| Title: | ||
| QSAM Biosciences, Inc. | ||
| By: | ||
| Name: | ||
| Title: | ||
| David H. Clarke | ||
| By: | ||
| Name: | ||
| Title: | ||
| [ ] | ||
| By: | ||
| Name: | ||
| Title: | ||
[Signature Page to Contingent Value Rights Agreement]

February 7th, 2024
PRIVATE & CONFIDENTIAL
For the Board of Directors of QSAM Biosciences, Inc. (OTC:QSAM)
9442 Capital of Texas Hwy N, Plaza 1, Suite 500, Austin, TX | 78759 | United States
We understand that QSAM Biosciences, Inc. (OTC:QSAM), a publicly traded company that is a Delaware corporation (“QSAM”), is considering entering into an Agreement and Plan of Merger (the “Merger Agreement”) with Telix Pharmaceuticals Limited (ASX:TLX), a publicly traded company that trades on the Australian Securities Exchange (ASX).
| ■ | Upon signing of the Term Sheet, TLX agreed to pay QSAM an upfront Collaboration and Option Fee of $2.0M USD (approximately AU$3.1 million) to advance development efforts based on mutually agreed goals and to provide sixty days of exclusivity pending completion of diligence and execution of a Merger Agreement. TLX has also agreed to pay an additional $500,000 towards closing expenses of the Merger. | |
| ■ | At closing, TLX will pay a total purchase price of $33.1M USD in equity through the issuance of fully paid ordinary TLX shares (the “Purchase Price Consideration”), which together with the Collaboration and Option Fee and assumption of closing expenses is the “Initial Consideration” of $35.6M USD. |
| ○ | Prior to the Closing of the Merger, the Company expects to effectuate a reverse split of its common stock, currently estimated to be in the range of 1000:1 to 2000:1. Fractional shares after the reverse split will be exchanged for a cash value equal to the Initial Consideration, less closing fees and expenses, divided by the fully diluted capitalization of QSAM prior to the reverse split (the “Fractional Share Cash-out Amount”). | |
| ○ | The Purchase Price Consideration (less the Fractional Share Cash-out Amount) shall be paid as an aggregate number of TLX shares equal to the applicable Purchase Price Consideration divided by the 10-day volume weighted average price (VWAP) of TLX stock in US Dollars (based on a mutually agreed currency exchange rate methodology agreed in the QSAM Merger Agreement) on the Australian Stock Exchange at the date of execution of the Merger Agreement. |
| ■ | Other Transaction terms: |
| ○ | The merger is expected to qualify as a tax-free reorganization under the U.S. Tax Code. | |
| ○ | The TLX shares for the Initial Consideration will be issued pursuant an exemption from registration requirements under U.S. securities laws, and as a result may not be sold for a period of six months after issuance. | |
| ○ | Following closing, TLX will also pay up to $90.0M USD in contingent clinical and commercial milestone payments in cash or equity (at TLX’s election), subject to achievement and satisfactory completion of milestones, through a Contingent Value Rights (“CVR”) structure. | |
| ○ | If the proposed Acquisition of QSAM does not close, the Collaboration and Option Fee will be converted to QSAM common stock at US$6.70 per share. |
The Board of Directors has retained Newbridge Securities Corporation to render an opinion as to whether, on the date of November 14th, 2023, the Purchase Price Consideration to be paid by TLX for QSAM is fair, from a financial point of view, to QSAM’s common stockholders (the “Opinion”). Newbridge used the date of November 14th, 2023, because that is the date that QSAM and TLX signed and made public a Term Sheet which contains substantially the same terms as in the definitive Merger Agreement.
Investment Advisory Services offered through Newbridge Financial Services Group, Inc. an SEC Registered Investment Advisor
1200 North Federal Highway, Suite 400, Boca Raton, FL 33432 | Telephone: 561.395.1220 Fax: 561.229.1531
www.newbridgesecurities.com
Newbridge’s analysis is focused on the “Initial Consideration”, and not the future value to shareholders that may be gained through the Contingent Value Rights. These are deferred payments that may not occur, and therefore not included into our calculations.
We have not been requested to opine to, and our Opinion does not in any manner address, the underlying business decision of QSAM to enter into the Merger Agreement. Our Opinion does not address the relative merits of entering into the Merger Agreement as compared to any alternative business strategy that might exist for QSAM.
Newbridge, as part of its investment banking business, is regularly engaged in the valuation of businesses and their securities in connection with mergers and acquisitions, going private transactions, related-party transactions, negotiated underwritings, secondary distributions of listed and unlisted securities, debt restructurings, private placements, and valuations for corporate and other purposes. We do not perform tax, accounting or legal services, nor do we render such advice.
Newbridge will receive a fee and reimbursement of its expenses for such services. In addition, QSAM has agreed to indemnify Newbridge for certain liabilities arising out of its engagement, including the rendering of this Opinion.
Newbridge has not participated in, or provided advice with respect to, the pricing determination, structuring or negotiation of the Merger Agreement.
In the ordinary course of business, Newbridge, certain customer accounts held at Newbridge, and certain of our affiliates, as well as investment funds in which we or our affiliates may have financial interests, may acquire, hold or sell, long or short positions, or trade or otherwise effect transactions, in equity, debt, and other securities and financial instruments (including bank loans and other obligations) of, or investments in QSAM.
In connection with the review and analysis performed to render our Opinion, among other things, we have undertaken the following:
| ■ | Considered our assessment of general economic, market and financial conditions as well as our experience in connection with similar transactions, and business and securities valuations generally; | |
| ■ | Reviewed various drafts of the Merger Agreement; | |
| ■ | Reviewed QSAM’s publicly available last eight fiscal quarters of historical financial results, (Q4-2021 -Q3-2023) as well as certain publicly available information concerning the trading of, and the trading market for, the ordinary shares of QSAM since January 2022; | |
| ■ | Reviewed publicly available financial information of QSAM filed with the U.S. Securities & Exchange Commission, including its Form 10-Ks and 10-Qs, and certain reports on material events filed on Forms 8-K between January 1st, 2022, through February 2nd, 2024; | |
| ■ | Reviewed publicly available financial information of TLX filed with the ASX, including its release of new corporate presentations, new announcements, and its trading history between January 1st, 2022, through February 2nd, 2024; | |
| ■ | Conducted discussions with QSAM’s management team to better understand QSAM’s recent business history, and near-term financials; | |
| ■ | Performed a Public Company Comparable analysis of similar companies to QSAM, that included variables such as companies trading on the OTC Markets, in the Biotechnology or Pharmaceutical sector, and had early-stage clinical drug assets focused on treating Cancer, to derive equity values; and | |
| ■ | Performed a Premiums Paid Transaction analysis that reviewed the variance between purchase prices paid and the market capitalization 1-day prior to the public announcement of public companies in the Biotech/Pharma sector, wherein an outside party purchased over 50% of the company’s equity. The average premium paid for this dataset in 2023 was then compared to that of TLX acquiring QSAM. |
Investment Advisory Services offered through Newbridge Financial Services Group, Inc. an SEC Registered Investment Advisor
1200 North Federal Highway, Suite 400, Boca Raton, FL 33432 | Telephone: 561.395.1220 Fax: 561.229.1531
www.newbridgesecurities.com
In forming our Opinion, we have had full access to, and full cooperation from, the management team of QSAM to ask questions and receive answers. Our Opinion is solely and necessarily based on economic, financial and market conditions as they exist and can be evaluated as of the date hereof.
In connection with our review and analyses and in arriving at our Opinion, we have assumed and relied upon the accuracy and completeness of the financial and other information provided to us or publicly available and have not attempted to verify independently any such information.
With respect to certain financial information, including financial analyses and projections, relating to the business and prospects of QSAM provided to us, we have assumed that the financial information has been reasonably prepared on a basis reflecting best currently available estimates and good faith judgments of the management team of QSAM as to the future financial performance of the Company without and subsequent to entering into the Merger Agreement.
This Opinion is solely for the use of the Board of Directors of QSAM Biosciences, Inc. (OTC:QSAM), and is not to be publicly disclosed, used, excerpted, reproduced or disseminated, quoted or referred to at any time, in any manner or for any purpose, without the prior written consent of Newbridge Securities Corporation, except that this Opinion may be reproduced in full in, and references to this Opinion and to Newbridge and its relationship with QSAM may be included in, filings made by QSAM with the U.S. Securities & Exchange Commission and by Telix Pharmaceuticals Limited (ASX:TLX) with the Australian Securities Exchange Ltd., as well as any proxy statement or similar disclosure document delivered to the stockholders of QSAM and TLX.
We have tried to apply objective measures of value in rendering our Opinion. You understand, however, that such a valuation necessarily is based on some subjective interpretations of value. We understand that we are not obligated to review our Opinion due to events and fluctuating economic conditions occurring subsequent to the date of this Opinion.
Based upon and subject to the foregoing, it is our Opinion that, on the date of November 14th, 2023, the Purchase Price Consideration to be paid by TLX for QSAM is fair, from a financial point of view, to QSAM’s common stockholders (the “Opinion”).
Sincerely,
| Newbridge Securities Corporation | |
| /s/ Chad Champion | |
| Chad D. Champion | |
| Senior Managing Director | |
| Head of Equity Capital Markets & Investment Banking |
Investment Advisory Services offered through Newbridge Financial Services Group, Inc. an SEC Registered Investment Advisor
1200 North Federal Highway, Suite 400, Boca Raton, FL 33432 | Telephone: 561.395.1220 Fax: 561.229.1531
www.newbridgesecurities.com
DGCL SECTION 262
§ 262. Appraisal rights.
(a) Any stockholder of a corporation of this State who holds shares of stock on the date of the making of a demand pursuant to subsection (d) of this section with respect to such shares, who continuously holds such shares through the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, who has otherwise complied with subsection (d) of this section and who has neither voted in favor of the merger, consolidation, conversion, transfer, domestication or continuance nor consented thereto in writing pursuant to § 228 of this title shall be entitled to an appraisal by the Court of Chancery of the fair value of the stockholder’s shares of stock under the circumstances described in subsections (b) and (c) of this section. As used in this section, the word “stockholder” means a holder of record of stock in a corporation; the words “stock” and “share” mean and include what is ordinarily meant by those words; the words “depository receipt” mean a receipt or other instrument issued by a depository representing an interest in 1 or more shares, or fractions thereof, solely of stock of a corporation, which stock is deposited with the depository; the words “beneficial owner” mean a person who is the beneficial owner of shares of stock held either in voting trust or by a nominee on behalf of such person; and the word “person” means any individual, corporation, partnership, unincorporated association or other entity.
(b) Appraisal rights shall be available for the shares of any class or series of stock of a constituent, converting, transferring, domesticating or continuing corporation in a merger, consolidation, conversion, transfer, domestication or continuance to be effected pursuant to § 251 (other than a merger effected pursuant to § 251(g) of this title), § 252, § 254, § 255, § 256, § 257, § 258, § 263, § 264, § 266 or § 390 of this title (other than, in each case and solely with respect to a converted or domesticated corporation, a merger, consolidation, conversion, transfer, domestication or continuance authorized pursuant to and in accordance with the provisions of § 265 or § 388 of this title):
(1) Provided, however, that no appraisal rights under this section shall be available for the shares of any class or series of stock, which stock, or depository receipts in respect thereof, at the record date fixed to determine the stockholders entitled to receive notice of the meeting of stockholders, or at the record date fixed to determine the stockholders entitled to consent pursuant to § 228 of this title, to act upon the agreement of merger or consolidation or the resolution providing for the conversion, transfer, domestication or continuance (or, in the case of a merger pursuant to § 251(h) of this title, as of immediately prior to the execution of the agreement of merger), were either: (i) listed on a national securities exchange or (ii) held of record by more than 2,000 holders; and further provided that no appraisal rights shall be available for any shares of stock of the constituent corporation surviving a merger if the merger did not require for its approval the vote of the stockholders of the surviving corporation as provided in § 251(f) of this title.
(2) Notwithstanding paragraph (b)(1) of this section, appraisal rights under this section shall be available for the shares of any class or series of stock of a constituent, converting, transferring, domesticating or continuing corporation if the holders thereof are required by the terms of an agreement of merger or consolidation, or by the terms of a resolution providing for conversion, transfer, domestication or continuance, pursuant to § 251, § 252, § 254, § 255, § 256, § 257, § 258, § 263, § 264, § 266 or § 390 of this title to accept for such stock anything except:
a. Shares of stock of the corporation surviving or resulting from such merger or consolidation, or of the converted entity or the entity resulting from a transfer, domestication or continuance if such entity is a corporation as a result of the conversion, transfer, domestication or continuance, or depository receipts in respect thereof;
b. Shares of stock of any other corporation, or depository receipts in respect thereof, which shares of stock (or depository receipts in respect thereof) or depository receipts at the effective date of the merger, consolidation, conversion, transfer, domestication or continuance will be either listed on a national securities exchange or held of record by more than 2,000 holders;
c. Cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a. and b. of this section; or
d. Any combination of the shares of stock, depository receipts and cash in lieu of fractional shares or fractional depository receipts described in the foregoing paragraphs (b)(2)a., b. and c. of this section.
(3) In the event all of the stock of a subsidiary Delaware corporation party to a merger effected under § 253 or § 267 of this title is not owned by the parent immediately prior to the merger, appraisal rights shall be available for the shares of the subsidiary Delaware corporation.
(4) [Repealed.]
(c) Any corporation may provide in its certificate of incorporation that appraisal rights under this section shall be available for the shares of any class or series of its stock as a result of an amendment to its certificate of incorporation, any merger or consolidation in which the corporation is a constituent corporation, the sale of all or substantially all of the assets of the corporation or a conversion effected pursuant to § 266 of this title or a transfer, domestication or continuance effected pursuant to § 390 of this title. If the certificate of incorporation contains such a provision, the provisions of this section, including those set forth in subsections (d), (e), and (g) of this section, shall apply as nearly as is practicable.
(d) Appraisal rights shall be perfected as follows:
(1) If a proposed merger, consolidation, conversion, transfer, domestication or continuance for which appraisal rights are provided under this section is to be submitted for approval at a meeting of stockholders, the corporation, not less than 20 days prior to the meeting, shall notify each of its stockholders who was such on the record date for notice of such meeting (or such members who received notice in accordance with § 255(c) of this title) with respect to shares for which appraisal rights are available pursuant to subsection (b) or (c) of this section that appraisal rights are available for any or all of the shares of the constituent corporations or the converting, transferring, domesticating or continuing corporation, and shall include in such notice either a copy of this section (and, if 1 of the constituent corporations or the converting corporation is a nonstock corporation, a copy of § 114 of this title) or information directing the stockholders to a publicly available electronic resource at which this section (and, § 114 of this title, if applicable) may be accessed without subscription or cost. Each stockholder electing to demand the appraisal of such stockholder’s shares shall deliver to the corporation, before the taking of the vote on the merger, consolidation, conversion, transfer, domestication or continuance, a written demand for appraisal of such stockholder’s shares; provided that a demand may be delivered to the corporation by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs the corporation of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such stockholder’s shares. A proxy or vote against the merger, consolidation, conversion, transfer, domestication or continuance shall not constitute such a demand. A stockholder electing to take such action must do so by a separate written demand as herein provided. Within 10 days after the effective date of such merger, consolidation, conversion, transfer, domestication or continuance, the surviving, resulting or converted entity shall notify each stockholder of each constituent or converting, transferring, domesticating or continuing corporation who has complied with this subsection and has not voted in favor of or consented to the merger, consolidation, conversion, transfer, domestication or continuance, and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this section, of the date that the merger, consolidation or conversion has become effective; or
(2) If the merger, consolidation, conversion, transfer, domestication or continuance was approved pursuant to § 228, § 251(h), § 253, or § 267 of this title, then either a constituent, converting, transferring, domesticating or continuing corporation before the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, or the surviving, resulting or converted entity within 10 days after such effective date, shall notify each stockholder of any class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation who is entitled to appraisal rights of the approval of the merger, consolidation, conversion, transfer, domestication or continuance and that appraisal rights are available for any or all shares of such class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation, and shall include in such notice either a copy of this section (and, if 1 of the constituent corporations or the converting, transferring, domesticating or continuing corporation is a nonstock corporation, a copy of § 114 of this title) or information directing the stockholders to a publicly available electronic resource at which this section (and § 114 of this title, if applicable) may be accessed without subscription or cost. Such notice may, and, if given on or after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, shall, also notify such stockholders of the effective date of the merger, consolidation, conversion, transfer, domestication or continuance. Any stockholder entitled to appraisal rights may, within 20 days after the date of giving such notice or, in the case of a merger approved pursuant to § 251(h) of this title, within the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days after the date of giving such notice, demand in writing from the surviving, resulting or converted entity the appraisal of such holder’s shares; provided that a demand may be delivered to such entity by electronic transmission if directed to an information processing system (if any) expressly designated for that purpose in such notice. Such demand will be sufficient if it reasonably informs such entity of the identity of the stockholder and that the stockholder intends thereby to demand the appraisal of such holder’s shares. If such notice did not notify stockholders of the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, either (i) each such constituent corporation or the converting, transferring, domesticating or continuing corporation shall send a second notice before the effective date of the merger, consolidation, conversion, transfer, domestication or continuance notifying each of the holders of any class or series of stock of such constituent, converting, transferring, domesticating or continuing corporation that are entitled to appraisal rights of the effective date of the merger, consolidation, conversion, transfer, domestication or continuance or (ii) the surviving, resulting or converted entity shall send such a second notice to all such holders on or within 10 days after such effective date; provided, however, that if such second notice is sent more than 20 days following the sending of the first notice or, in the case of a merger approved pursuant to § 251(h) of this title, later than the later of the consummation of the offer contemplated by § 251(h) of this title and 20 days following the sending of the first notice, such second notice need only be sent to each stockholder who is entitled to appraisal rights and who has demanded appraisal of such holder’s shares in accordance with this subsection and any beneficial owner who has demanded appraisal under paragraph (d)(3) of this section. An affidavit of the secretary or assistant secretary or of the transfer agent of the corporation or entity that is required to give either notice that such notice has been given shall, in the absence of fraud, be prima facie evidence of the facts stated therein. For purposes of determining the stockholders entitled to receive either notice, each constituent corporation or the converting, transferring, domesticating or continuing corporation may fix, in advance, a record date that shall be not more than 10 days prior to the date the notice is given, provided, that if the notice is given on or after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, the record date shall be such effective date. If no record date is fixed and the notice is given prior to the effective date, the record date shall be the close of business on the day next preceding the day on which the notice is given.
(3) Notwithstanding subsection (a) of this section (but subject to this paragraph (d)(3)), a beneficial owner may, in such person’s name, demand in writing an appraisal of such beneficial owner’s shares in accordance with either paragraph (d)(1) or (2) of this section, as applicable; provided that (i) such beneficial owner continuously owns such shares through the effective date of the merger, consolidation, conversion, transfer, domestication or continuance and otherwise satisfies the requirements applicable to a stockholder under the first sentence of subsection (a) of this section and (ii) the demand made by such beneficial owner reasonably identifies the holder of record of the shares for which the demand is made, is accompanied by documentary evidence of such beneficial owner’s beneficial ownership of stock and a statement that such documentary evidence is a true and correct copy of what it purports to be, and provides an address at which such beneficial owner consents to receive notices given by the surviving, resulting or converted entity hereunder and to be set forth on the verified list required by subsection (f) of this section.
(e) Within 120 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, the surviving, resulting or converted entity, or any person who has complied with subsections (a) and (d) of this section and who is otherwise entitled to appraisal rights, may commence an appraisal proceeding by filing a petition in the Court of Chancery demanding a determination of the value of the stock of all such stockholders. Notwithstanding the foregoing, at any time within 60 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, any person entitled to appraisal rights who has not commenced an appraisal proceeding or joined that proceeding as a named party shall have the right to withdraw such person’s demand for appraisal and to accept the terms offered upon the merger, consolidation, conversion, transfer, domestication or continuance. Within 120 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, any person who has complied with the requirements of subsections (a) and (d) of this section, upon request given in writing (or by electronic transmission directed to an information processing system (if any) expressly designated for that purpose in the notice of appraisal), shall be entitled to receive from the surviving, resulting or converted entity a statement setting forth the aggregate number of shares not voted in favor of the merger, consolidation, conversion, transfer, domestication or continuance (or, in the case of a merger approved pursuant to § 251(h) of this title, the aggregate number of shares (other than any excluded stock (as defined in § 251(h)(6)d. of this title)) that were the subject of, and were not tendered into, and accepted for purchase or exchange in, the offer referred to in § 251(h)(2) of this title)), and, in either case, with respect to which demands for appraisal have been received and the aggregate number of stockholders or beneficial owners holding or owning such shares (provided that, where a beneficial owner makes a demand pursuant to paragraph (d)(3) of this section, the record holder of such shares shall not be considered a separate stockholder holding such shares for purposes of such aggregate number). Such statement shall be given to the person within 10 days after such person’s request for such a statement is received by the surviving, resulting or converted entity or within 10 days after expiration of the period for delivery of demands for appraisal under subsection (d) of this section, whichever is later.
(f) Upon the filing of any such petition by any person other than the surviving, resulting or converted entity, service of a copy thereof shall be made upon such entity, which shall within 20 days after such service file in the office of the Register in Chancery in which the petition was filed a duly verified list containing the names and addresses of all persons who have demanded appraisal for their shares and with whom agreements as to the value of their shares have not been reached by such entity. If the petition shall be filed by the surviving, resulting or converted entity, the petition shall be accompanied by such a duly verified list. The Register in Chancery, if so ordered by the Court, shall give notice of the time and place fixed for the hearing of such petition by registered or certified mail to the surviving, resulting or converted entity and to the persons shown on the list at the addresses therein stated. The forms of the notices by mail and by publication shall be approved by the Court, and the costs thereof shall be borne by the surviving, resulting or converted entity.
(g) At the hearing on such petition, the Court shall determine the persons who have complied with this section and who have become entitled to appraisal rights. The Court may require the persons who have demanded an appraisal for their shares and who hold stock represented by certificates to submit their certificates of stock to the Register in Chancery for notation thereon of the pendency of the appraisal proceedings; and if any person fails to comply with such direction, the Court may dismiss the proceedings as to such person. If immediately before the merger, consolidation, conversion, transfer, domestication or continuance the shares of the class or series of stock of the constituent, converting, transferring, domesticating or continuing corporation as to which appraisal rights are available were listed on a national securities exchange, the Court shall dismiss the proceedings as to all holders of such shares who are otherwise entitled to appraisal rights unless (1) the total number of shares entitled to appraisal exceeds 1% of the outstanding shares of the class or series eligible for appraisal, (2) the value of the consideration provided in the merger, consolidation, conversion, transfer, domestication or continuance for such total number of shares exceeds $1 million, or (3) the merger was approved pursuant to § 253 or § 267 of this title.
(h) After the Court determines the persons entitled to an appraisal, the appraisal proceeding shall be conducted in accordance with the rules of the Court of Chancery, including any rules specifically governing appraisal proceedings. Through such proceeding the Court shall determine the fair value of the shares exclusive of any element of value arising from the accomplishment or expectation of the merger, consolidation, conversion, transfer, domestication or continuance, together with interest, if any, to be paid upon the amount determined to be the fair value. In determining such fair value, the Court shall take into account all relevant factors. Unless the Court in its discretion determines otherwise for good cause shown, and except as provided in this subsection, interest from the effective date of the merger, consolidation, conversion, transfer, domestication or continuance through the date of payment of the judgment shall be compounded quarterly and shall accrue at 5% over the Federal Reserve discount rate (including any surcharge) as established from time to time during the period between the effective date of the merger, consolidation or conversion and the date of payment of the judgment. At any time before the entry of judgment in the proceedings, the surviving, resulting or converted entity may pay to each person entitled to appraisal an amount in cash, in which case interest shall accrue thereafter as provided herein only upon the sum of (1) the difference, if any, between the amount so paid and the fair value of the shares as determined by the Court, and (2) interest theretofore accrued, unless paid at that time. Upon application by the surviving, resulting or converted entity or by any person entitled to participate in the appraisal proceeding, the Court may, in its discretion, proceed to trial upon the appraisal prior to the final determination of the persons entitled to an appraisal. Any person whose name appears on the list filed by the surviving, resulting or converted entity pursuant to subsection (f) of this section may participate fully in all proceedings until it is finally determined that such person is not entitled to appraisal rights under this section.
(i) The Court shall direct the payment of the fair value of the shares, together with interest, if any, by the surviving, resulting or converted entity to the persons entitled thereto. Payment shall be so made to each such person upon such terms and conditions as the Court may order. The Court’s decree may be enforced as other decrees in the Court of Chancery may be enforced, whether such surviving, resulting or converted entity be an entity of this State or of any state.
(j) The costs of the proceeding may be determined by the Court and taxed upon the parties as the Court deems equitable in the circumstances. Upon application of a person whose name appears on the list filed by the surviving, resulting or converted entity pursuant to subsection (f) of this section who participated in the proceeding and incurred expenses in connection therewith, the Court may order all or a portion of such expenses, including, without limitation, reasonable attorney’s fees and the fees and expenses of experts, to be charged pro rata against the value of all the shares entitled to an appraisal not dismissed pursuant to subsection (k) of this section or subject to such an award pursuant to a reservation of jurisdiction under subsection (k) of this section.
(k) Subject to the remainder of this subsection, from and after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, no person who has demanded appraisal rights with respect to some or all of such person’s shares as provided in subsection (d) of this section shall be entitled to vote such shares for any purpose or to receive payment of dividends or other distributions on such shares (except dividends or other distributions payable to stockholders of record at a date which is prior to the effective date of the merger, consolidation, conversion, transfer, domestication or continuance). If a person who has made a demand for an appraisal in accordance with this section shall deliver to the surviving, resulting or converted entity a written withdrawal of such person’s demand for an appraisal in respect of some or all of such person’s shares in accordance with subsection (e) of this section, either within 60 days after such effective date or thereafter with the written approval of the corporation, then the right of such person to an appraisal of the shares subject to the withdrawal shall cease. Notwithstanding the foregoing, an appraisal proceeding in the Court of Chancery shall not be dismissed as to any person without the approval of the Court, and such approval may be conditioned upon such terms as the Court deems just, including without limitation, a reservation of jurisdiction for any application to the Court made under subsection (j) of this section; provided, however that this provision shall not affect the right of any person who has not commenced an appraisal proceeding or joined that proceeding as a named party to withdraw such person’s demand for appraisal and to accept the terms offered upon the merger, consolidation, conversion, transfer, domestication or continuance within 60 days after the effective date of the merger, consolidation, conversion, transfer, domestication or continuance, as set forth in subsection (e) of this section. If a petition for an appraisal is not filed within the time provided in subsection (e) of this section, the right to appraisal with respect to all shares shall cease.
(l) The shares or other equity interests of the surviving, resulting or converted entity to which the shares of stock subject to appraisal under this section would have otherwise converted but for an appraisal demand made in accordance with this section shall have the status of authorized but not outstanding shares of stock or other equity interests of the surviving, resulting or converted entity, unless and until the person that has demanded appraisal is no longer entitled to appraisal pursuant to this section.